Abstract
Habitat loss and fragmentation, and biological invasions are widely considered the most significant threats to global biodiversity. While marine invasions have already shown dramatic impacts around the world’s coasts, many of these habitats are becoming increasingly urbanized, resulting in fragmentation of natural landscape worldwide. This study developed in Madeira (NE Atlantic) aims to understand the synergistic interactions between fragmentation and biological invasions using submerged experimental settlement panels in the field for 3 months. We fragmented crustose coralline habitats, decreasing patch size without an overall habitat loss, and determined its effects on the patterns of abundance of marine fouling organisms across limiting assemblages with or without the presence of non-indigenous species (NIS, considered invaded and non-invaded systems in this study). The presence of crustose coralline algae suppressed the recruitment of some NIS (Parasmitina alba and Botrylloides niger). Our results also showed that the abundance of NIS (e.g. B. niger) could be prompted in highly fragmented habitats, colonizing bare substrates very efficiently. Overall, evidence indicates that fragmentation events modulate biotic interactions and consequently determine the structure of the fouling communities. Future research should address both processes when analyzing biotic resistance to invasion in urban marine habitats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Habitat loss and fragmentation, climate change and biological invasions are considered the major anthropogenic stressors threatening biodiversity in marine ecosystems worldwide, imposing significant and permanent changes to the ecology of coastal communities (Myers et al. 2000; Thompson et al. 2002; Fahrig 2003; Reid et al. 2005; Airoldi and Beck 2007; Hutchison 2008; Cole et al. 2012).
Coastal urbanization, a process modifying marine and coastal ecosystems (Airoldi et al. 2015, 2021), affects the complex interactions among biotic and abiotic processes (Matias et al. 2015). Urbanization can be associated with habitat fragmentation, an umbrella term describing the process by which habitat loss results in the division of large, continuous habitats into smaller remnants, isolated from each other by a matrix of different habitats (Didham 2010). There is a longstanding debate within the conservation research community based on the principle postulating that a single patch of habitat holds more species than several small patches of the same total area (the so-called SLOSS debate, Diamond 1975). In coastal areas, fragmentation can alter the quality and connectivity of habitats (Wilcove et al. 1986; Collinge 1996; Gray 1997; Fahrig 2003; Wilson et al. 2016), and through different mechanisms affect the distribution and abundance of organisms, community structure and ecosystem processes (Didham 2010; Dugan et al. 2011; Smoothey 2013; Benedetti-Cecchi and Trussell 2014; Martins et al. 2016; Cacabelos et al. 2016a, b; Bertocci et al. 2017). The relative importance of these mechanisms has raised considerable discussion, highlighting the relevance of clearly discriminating direct versus indirect causal relationships among patch and landscape variables. While habitat fragmentation is a landscape‐level phenomenon, patch‐level processes (patch area, edge effects and patch shape complexity) can only be understood within a landscape context (isolation and matrix structure) (Didham 2010).
Biological invasions represent a severe threat to marine ecosystems (Halpern et al. 2008; Molnar et al. 2008), and can be exacerbated by fragmentation (Haddad et al. 2015). A successful invasion depends on traits associated with the non-indigenous species (NIS) involved in the invasion process, its propagule pressure (i.e., the number and frequency with which larvae, seeds, juveniles, or adults of a species, arrive at a recipient native community over time) and the invasibility of the recipient community (Vitousek et al. 1997; Lonsdale 1999; Mack et al. 2000; Canning-Clode 2015). According to Elton (1958) the uptake of available resources and the occupation of niches are complete in a species-rich community, preventing invasions. Multiple mechanisms must be considered when investigating biotic resistance hypotheses in marine systems (Caselle et al. 2018). For example, biodiversity loss can facilitate several processes (e.g. provision of bare space) to promote the settlement and expansion of successful invaders (Stachowicz and Byrnes 2006). While disturbed ecosystems can reduce persistence against human-induced impacts (Hooper et al. 2005), ‘healthy’ native communities have shown greater resistance to invasion (Levine and D'Antonio 1999; Arenas et al. 2006; Giakoumi and Pey 2017). There has been considerable research effort on detection of invasive alien species (EU 2014; Tsiamis et al. 2019), but not much experimental work has been performed to better understand NIS impacts in coastal benthic communities (but see Katsanevakis et al. 2014, and references therein). Further research is still needed to advance the current understanding of which factors determine invasion success (with most studies focused on species traits and both biotic and abiotic characteristics, e.g. Arenas et al. 2006), the underlying processes and mechanisms and how invasions influence biodiversity patterns, with evidence suggesting that propagule pressure is of paramount importance (Simberloff 2009; Brown and Barney 2021).
The different components of global environmental change are often studied and managed independently, but simultaneously multiple stressors may produce synergistic or antagonistic effects (Didham et al. 2007; Crain et al. 2008). Habitat fragmentation strongly interacts with other components of global environmental change, including species invasions, habitat‐use intensification and climate change (Hutchison 2008; Didham 2010). Habitat loss and fragmentation may enhance the spread of biological invasions worldwide, partially due to the available bare space provided by the loss of native species, consequences of climate change or the increasing urbanization of coastal environments (With 2004; Megina et al. 2013). Several studies focused on mitigating the effects of loss and fragmentation of coastal urban areas have been developed in recent years, with promising results (e.g. Bulleri 2005; Bishop et al. 2017). The capacity of a fragmented habitat to sustain biodiversity and ecosystem services will hinge upon the total amount and quality of habitat left in fragments, their degree of connectivity, and how they are affected by other human-induced stressors such as invasive species (Haddad et al. 2015). However, little is known on the effects of fragmentation in marine systems (but see Moschella et al. 2005; Goodsell et al. 2007), or how the alteration of landscape structure might promote NIS spread, as well as its ecological consequences (With 2004).
In this context, this study aims to assess the effect of biological invasions in structuring benthic communities and the consequences of fragmentation and generation of free base space on forcing interactions among neighbouring assemblages. Both stressors were simultaneously manipulated to examine their isolated and combined effects on recruitment patterns, and macrofouling assemblages' invasibility. According to theory, fragmented habitats are expected to hold fewer species than a single large patch, while assemblages with NIS in the neighbourhood, i.e. more exposed to invasion, are expected to be more readily colonized by NIS. On the other hand, considering fragmentation as a ‘landscape level’ disturbance (Hobbs and Huenneke 1992; With 2004), and since disturbance generally promotes invasion (With 2002; Hutchison 2008), we hypothesize that fragmented assemblages will show a higher invasibility than unfragmented (i.e. undisturbed) assemblages.
Methods
Natural assemblages in the study area
Macrobenthic community structure in the shallow rocky subtidal of Madeira are mainly affected by wave exposure, sedimentation, depth and grazing by the sea urchin Diadema africanum Rodríguez, Hernández, Clemente & Coppard, 2008 (Bianchi et al. 1998; Alves et al. 2001; Friedlander et al. 2017; Gizzi et al. 2020). Spatial variation among morphofunctional groups is mainly correlated with variation in the density of sea urchins (Sangil et al. 2018). When the density of sea urchins is high (> 2–2.5 ind. m−2), subtidal areas previously covered by erect, fleshy algae are transformed into unproductive overgrazed habitats dominated by crustose coralline algae (CCA), impoverished communities often termed ‘urchin barrens’ (Hernández et al. 2008; Friedlander et al. 2017; Sangil et al. 2018). A similar phenomenon has been described in other Macaronesian archipelagos and worldwide (see Tuya et al. 2004 and references therein), probably due to increased inshore fishing pressure. Impoverished communities such as these coralline barrens can also be exposed to fragmentation due to physical or biological processes, such as urbanization (habitat modifications occurring at a large spatial scale) or grazing by sea urchins (occurring at smaller spatial scale). Sea urchins can be continually scraping CCA, consuming the surficial layers along with any microalgal films and macroalgal recruits (Chapman 1981), or even ‘scrape’ the substrate, as has been described for Arbacia lixula (Linnaeus, 1758), that has a robust Aristotle’s lantern consistently with its preference for crustose algae as food (Bonaviri et al. 2011).
Building synthetic assemblages
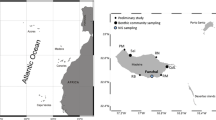
To test the effect of invasions and fragmentation on the forcing of interactions among fouling organisms, we created synthetic assemblages containing bare rock, crustose coralline algae (CCA) and mature marina assemblages with a relevant NIS component, intending to mimic the invasion process. In advance, and to get mature assemblages containing NIS, 7 × 7 × 2 cm basalt panels, the dominant natural volcanic rock in Madeira island, were suspended upside-down 1 m deep from wharves of Quinta do Lorde marina for 14 months (from April 2018 to June 2019) to be fouled with mature assemblages (marina-assemblages with a relevant NIS component, hereafter named ‘Invaded’). After 14 months in the field, assemblages colonizing these basalt panels were composed by 62.0 ± 19.6% (mean percent cover ± SE, n = 10) of NIS.
In June 2019, small basalt boulders bearing CCA species were collected from natural rock pools in Quinta do Lorde (NW Madeira Island, Portugal) and transported to be cut by a professional stonecutter. Rock pieces were cut into the experimental size (10 units of 7 × 7 × 2 cm and 40 units of 3.5 × 3.5 × 2 cm) and maintained in the mesocosm system and laboratory facilities of MARE—Marine and Environmental Research Centre, located at Quinta do Lorde Marina, for 24 h until the start of the experiment.
Experimental assemblages were created and attached to main 14 × 14 × 0.3 cm PVC panels with a high-quality adhesive sealant (T-REX Power Turbo, SMX® Polymer-Soudal, 20 min.) and suspended from wharves. Therefore, they consisted of PVC plates where one 7 × 7 × 2 cm or four 3.5 × 3.5 × 2 cm pieces of rock containing CCA were glued, depending on corresponding unfragmented or fragmented treatment. In the case of small rock pieces, they were glued to the four corners of the main PVC panel. Half of the PVC panels, corresponding to the ‘invaded’ treatment, contained 7 × 7 cm marina-assemblages with a relevant NIS component. Empty basalt panels were used to cover the remaining free space in the PVC panels and complete the configuration (see Fig. 1 and A1.). Synthetic assemblages were then suspended at approx. 50–70 cm depth upside down from wharves in a randomly located position in June 2019.
Sampling synthetic assemblages
In September 2019, three months after deployment, panels were retrieved from the field and sampled. For this, all fouling organisms were identified to the lowest possible taxonomic level with the aid of a stereomicroscope Leica S8APO, based on scientific literature and then assigned to the categories of ‘native’, ‘non-indigenous species’ (NIS), ‘cryptogenic’ (sensu Carlton 1996) or ‘unresolved’ (unable to identify to species level) based on scientific literature (e.g., Canning-Clode et al. 2013; Chainho et al. 2015; Marchini et al. 2015; Gestoso et al. 2017; Ramalhosa et al. 2019, 2021) and several current databases (AquaNIS Editorial Board 2015; Ahyong et al. 2018; Fofonoff et al. 2018; WoRMS Editorial Board 2020). All NIS, and not only those included in the marina assemblages’ were sampled to include the biological succession on bare rocks and calcareous crusts, which would be influenced by the particular species first arriving at the substratum, an array of direct and indirect species interactions, and physical-environmental change, that dictates that the fouling assemblage generated will follow a range of trajectories with potentially variable endpoints (Jenkins and Martins, 2010).
High-quality photographs were taken from each panel with an Olympus TG-4 camera and analysed using Coral Point Count's image analysis software (CPCe 4.1, Kohler and Gill 2006). In each image, cells containing the unfragmented (one square 7 × 7 cm) and fragmented calcareous crusts (CCA) (four squares 3.5 × 3.5 cm) were selected, and 60 or 15 random points were deployed per cell, respectively (i.e. commensurately with area), resulting in a matrix of 60 randomly distributed points per panel. Similar image analysis was used for sampling the bare rock, and, thus, cells 7 × 7 cm or 3.5 × 3.5 cm were sampled close to unfragmented or fragmented CCA (see Fig. 1 for details). Fouling organisms were visually identified beneath each point, deployed on calcareous crusts and bare rock, up to the highest achievable taxonomic resolution. Organisms present but not falling underneath cross points were recorded as rare and then assigned an arbitrary score of 1%. Obtained data were used to determine the cover of each identified taxa and bare space for specific cells of each panel, total percent cover, and Shannon diversity index.
Diagram shows the synthetic assemblages, the areas sampled, and the number of random points, after the three months, they were suspended from wharves of Quinta do Lorde marina. Framed in green are points sampled in calcareous crusts, and those sampled on bare rock in blue (see photographs of synthetic assemblages in Supp. Mat.)
Data analysis
Changes in univariate data, namely abundance (total percent cover of sessile taxa, including both algae and calcified filter feeders, as well as covers of specific status) and diversity index, were analysed using permutational analysis of variance (PERMANOVA) based on Euclidean distances of untransformed data (Anderson 2001). To evaluate NIS settlement success on experimental assemblages/treatments, those categorized as cryptogenic and unresolved were pooled with native species (NCU) for the statistical analysis, as a more conservative approach (e.g., Gestoso et al. 2018; Ramalhosa et al. 2019). Analyses were based on a three-way model, including fragmentation (2 levels: unfragmented and fragmented), invasion (2 levels: uninvaded and invaded), and substrate (2 levels: calcareous crust and bare rock), all of them fixed and orthogonal, with five replicates. In addition, a test for homogeneity of multivariate dispersions (PERMDISP) was performed to complement PERMANOVA (Anderson 2017), and transformations were applied when necessary. Whenever PERMANOVA showed a significant factor or a significant interaction of factors (p < 0.05), pair-wise comparisons were done to explore differences among all pairs of levels of the selected factor.
Changes in the structure of assemblages, encompassing richness and abundance, were analysed using PERMANOVA performed on square-root transformed data and based on Bray–Curtis similarity matrix (Anderson 2001) and including three factors mentioned above. Non-metric multidimensional scaling (nMDS) was used to visualize multivariate results. To detect what taxa contributed most to similarity within and dissimilarity among groups, an analysis of similarity percentages (SIMPER) was carried out. Analyses of variance based on Euclidean distances was later used to test for differences in the main contributors to these similarities following previously described analysis for univariate data. The software PERMANOVA + for PRIMER (PRIMER-E Ltd, Plymouth, UK) was used for analyses.
Results
After 3 months suspended from wharves, and due to forcing interactions post fragmentation, the total percentage cover of macrofouling showed significantly lower abundance at unfragmented than on fragmented assemblages (Table 1, Fig. 2). The same effect, although barely non-significant, was observed on the diversity index counting on native, cryptogenic and unresolved status species (Table 1, Fig. 2). Thus, although invaded treatments have greater cover and biodiversity of biofouling organisms than non-invaded treatments, invasion treatment did not significantly affect the percentage cover of benthic organisms or biodiversity (Table 1). On the other hand, the percentage cover of total macrofouling and native, cryptogenic, and species with unresolved status was significantly lower on calcareous crust substrate than bare rock (Table 1, Fig. 2).
Mean (+ SE, per panel) total cover of fouling organisms across fragmentation (A) and substrates (Bare = Bare rock, CCA = Calcareous crust) (B), diversity index of native, cryptogenic and unresolved species (NCU) across fragmentation levels (C) and the cover of NCU species across substrates (D) (n = 5)
The factor ‘substrate’ affected the structure of macrofouling assemblages significantly, with assemblages settled on bare rock differing from those settled on the calcareous crust (Table 2, Fig. 3). SIMPER analysis revealed that while Spirorbis sp., Salmacina dysteri and the NIS Botrylloides niger, Parasmittina alba and Distaplia corolla recruited more on bare rock, the bryozoan Crisia sp. presented greater cover on top of the calcareous crust (Table 6). The structure of macrofouling assemblage also changed significantly with ‘fragmentation’, but depending on the ‘invasion’ level (i.e. a significant interaction Fr x Inv, Table 2, Fig. 3). The variability among replicates did not significantly contribute to these differences (PERMDISP = 0.561, p > 0.05). SIMPER analysis indicated that NIS contribution to unfragmented and uninvaded habitats was dominated by Cradoscrupocellaria bertholletii and B. niger (accumulating 33.9% similarity) (Table 7, showing up to 75% similarity, dissimilarities are indicated in Table 8). The polychaete Spirorbis sp. was especially abundant in these unfragmented treatments, contributing for more than 37% to the similarity in both invaded and uninvaded treatments. The NIS D. corolla and P. alba contributed to 19% similarity in unfragmented and invaded habitats. In contrast, in the case of fragmented habitats, NIS contribution to similarity within treatments was higher in invaded than in uninvaded treatments, with both B. niger and D. corolla contributing up to 33% similarity in invaded treatment versus previously cited NIS and P. alba accounting for 19.5% cumulative similarity in uninvaded treatment.
Non-metric multidimensional scaling (nMDS) representing the assemblage structure between fragmentation (squares vs. circles) and invasion levels (full vs. empty figures) across substrate levels (Bare = Bare rock, CCA = Calcareous crust). nMDS is based Bray–Curtis similarities on square-root transformed (for further details, see Table 2)
Univariate analysis performed on the percentage cover of these key species clarifies these results. Spirorbis sp. showed significantly higher abundance on unfragmented than fragmented habitats (Table 3, Fig. 4a). It was also negatively affected by calcareous crust, which recruited much lower individuals than bare rock (Fig. 4b). The same substrate effect was observed for S. dysteri and P. alba (Table 3, Figs. 4c and d, respectively). On the other hand, percentage cover of NIS C. bertholletii and D. corolla were affected by invasion, but C. bertholletii showed greater abundance on uninvaded habitats, whereas D. corolla showed the inverse trend (Table 3, Fig. 4e and f, respectively). B. niger and Crisia sp. were both affected by fragmentation, but inconsistently across invasion levels. The NIS B. niger was more abundant in invaded treatments on fragmented habitats, while the opposite tendency was observed on unfragmented conditions. Crisia sp. showed the opposite trend, and while it was more abundant on uninvaded treatments of fragmented habitats, its cover was higher on invaded and unfragmented treatments (Table 3, Fig. 4g and h, respectively).
Discussion
In this study, we investigated the role of the fragmentation of crustose coralline habitats in determining the diversity patterns (i.e. species richness and abundance) of marine fouling organisms across assemblages with or without the presence of non-indigenous species in Madeira Island. Despite limitations related to the increase of richness and percentage cover in invaded treatments, which could mask invasion effects, our results indicate that both fragmentation and substrate affected the abundance of fouling species, with the structure of fouling assemblages differing strongly between bare rock and calcareous crust. Moreover, fragmentation and invasion may interact and affect the composition of assemblages by modulating the recruitment and successful establishment of species.
Although the effects on some species were mostly negligible, opposite responses were observed on some non-indigenous species (NIS) versus native species (e.g. B. niger vs Crisia sp.), with previously invaded habitats facilitating NIS spread after fragmentation. Different NIS can aid one another in different ways, with numerous idiosyncratic interactions described in the literature (e.g. Simberloff and Von Holle 1999 and references therein). On some occasions, interactions may be synergistic, and these interactions among invaders may accelerate impacts on native ecosystems—an invasional ‘meltdown’ process (Simberloff and Von Holle 1999). Facilitation can be significant among invasive species and occur between invasive and native species, where the invader may act as either the facilitated or the facilitating species (see Gallien and Carboni 2016 and references therein). The relative importance of competition and facilitation is likely to vary along environmental gradients associated with disturbance (Gallien and Carboni 2016), forcing interactions among species such as the fragmentation event performed in the present study.
Sea urchin barren grounds, habitats dominated by encrusting coralline algae, are considered stable-state systems (Chapman 1981; Filbee-Dexter and Scheibling 2014), and various feedback mechanisms have been cited to enable them to persist or resist to minor disturbances. Firstly, sea urchins themselves can contribute to the resilience of these systems, preventing kelp recruitment by continuously scraping calcareous crusts (Chapman 1981; Filbee-Dexter and Scheibling 2014). However, the persistence of the coralline crustose algae in our experimental panels is not related to this mechanism as they were suspended from wharves, and therefore inaccessible to benthic fauna like sea urchins. On the other hand, as chemical cues, some characteristics of the crustose coralline algae can induce fouling or contrarily suppress settlement of marine organisms larval and spores. Furthermore, larvae can use those biotic cues to select attachment sites. For example, in the north shore of Moorea, French Polynesia, Price (2010) found corals recruited more frequently to one species of CCA, experiencing increased growth and survivorship on top of ‘preferred’ CCA. Other authors detected similar responses, showing that CCA can reduce settlement of potential competitors (see Bulleri et al. 2002, and references therein) or structure coral reef communities through suppressions of macroalgae (Vermej et al. 2011). In the present study, we found suppression in recruiting some species on top of CCA compared to those colonizing the neighbouring bare rock. More importantly, some of them, such as P. alba and B. niger, are categorized as NIS, supporting the capacity of CCA as key players in determining the colonization process of macrofoulers and, consequently, on the biotic resistance or resilience of these systems. Thus, our findings suggest that the mechanism that helps prevent recruitment on top of calcareous crusts may be highly relevant in determining the stability and invasibility of these systems (Gestoso et al. 2017).
Habitat fragmentation positively affected the native biodiversity and total cover of the sampled fouling assemblages. Our result contrasts with broader literature as fragmentation: (i) does not affect the number of species nor the structure of benthic assemblages (Matias et al. 2015 and references therein), or (ii) leads to lower abundances (biomass) and species richness, in agreement with the ‘SL > SS’ principle postulated by Diamond (1975), proposing that a single large patch of habitat (SL) holds more species than several small patches (SS) of the same total area). Although species richness has been a standard measure of diversity in disturbance studies, as species losses may be coupled with immigration, a global decrease in species richness does not necessarily result in local decreases in species richness (Elo et al. 2016). The mechanism where a disturbance event (such as fragmentation) affects species diversity depends on both community assembly processes (e.g. dispersal) and on whether disturbance disrupts the processes or not (Elo et al. 2016). For example, building coastal defences results in the loss and fragmentation of sedimentary habitats, and their replacement by artificial rocky habitats that become colonised by algae and marine animals (Moschella et al. 2005).
While habitat loss typically occurs concurrently with habitat fragmentation (Collinge 2009), and the impact of edge proximity is exacerbated by fragmentation (showed for vegetated habitats, see Colomer and Serra 2021 for references), we follow the proxy of fragmentation established in Matias et al. (2015), i.e., decreasing patch size without an overall habitat loss. For example, Matias et al. (2015) found that fragmentation did not significantly affect the assemblages of macroinvertebrates, suggesting that fragmentation effects may be limited when associated with habitat reduction. However, these effects may well be positive through habitat complexity enhancement (Bertolini et al. 2020), determining species composition and predation risks while altering effects that have been frequently analysed in vegetated habitats (Colomer and Serra 2021). Changes in connectivity among patches depend on their spatial scale and configuration, the organisms’ perception of changes in spatial patterns, the surrounding matrix and dispersal among patches (see Matias et al. 2015 and references therein). Fragmentation increases the edge-to-area ratio of patches, potentially affecting the intensity of the wave action and the local nearshore hydrodynamics and biota recruitment, and although there is a growing body of literature on the responses of animals to increases in edge habitat (i.e. ‘edge effects’), no consistent evidence have been found about the net effect in aquatic systems (Boström et al. 2011). In addition, abiotic effects at these edges can also create abrupt changes in the transition zone between the fragment and surrounding matrix habitats (as widely explored in terrestrial environments or marine seagrass meadows, Cadenasso et al. 2003, Colomer and Serra 2021). In the present study, the structural conditions at the CCA side do not strongly differ from adjacent bare rocks. Even so, the forcing of interactions among assemblages after habitat fragmentation could influence the obtained positive response, as in the case of Spirorbis sp., favoured in fragmented treatments, or B. niger, favoured in fragmented treatments when NIS are present but favoured in unfragmented treatments when they are absent. Many species avoid edge habitats, while others have their proliferation favoured by less predation and/or increased resource availability (Wirth et al. 2008), depending on if resources are concentrated around edged or divided between habitats (Ries and Sisk 2004), and can therefore exert a direct influence on the benthic community.
Surprisingly, we found that habitat fragmentation negatively affected NIS cover in uninvaded systems, mainly because of the great abundance of C. bertholletii in unfragmented (and uninvaded) systems. Many studies provide clear evidence of substantial and typically degrading impacts of habitat fragmentation on biodiversity and ecological processes across world environments (see, e.g. Hagen et al. 2012; Haddad et al. 2015; Pardini et al. 2017). Habitat fragmentation implies decreasing habitat size and connectivity, although having the same habitat across fragmentation levels. Thus, specific disturbance patterns would benefit good colonizers, predicted to spread better in landscapes where disturbances are small and dispersed (i.e., fragmented habitats) (With 2004). Some taxa colonizing the fragmented habitats are successful invaders of natural communities (as botryllids, Sheets et al. 2016). Although not analysed in this study, the deployment time can also have a relevant role in the succession of assemblages. For example, although spatially variable across Madeira island, settlement of B. niger showed maximum values on bare plates deployed in April in the study area (compared to January or September deployments, Ramalhosa et al. 2021).
In addition, fragmentation can affect particular species interactions and marine food webs (Hagen et al. 2012). For instance, fragments of surviving coral surrounded by reef pavement and coral rubble created by coral bleaching can have consequences for top-down control as average food chains shorten, generalist species proliferate, and phase shifts may occur (Hughes 1994). Our results indicate that habitat fragmentation negatively affected the system by increasing the NIS potential for spreading. We found different effects of habitat fragmentation across invasion levels (i.e. a significant interaction), which became more apparent in the fragmented habitats exposed to invaders. Inadequate dispersers may spread better in landscapes in which disturbances are concentrated in space, whereas good dispersers (as typically invasive species are) are predicted to spread better in landscapes, where disturbances are small and dispersed (i.e., fragmented habitats) (With 2004). It is important to highlight that our experiment mimicked the invasion process by taxa with NCU status at panel scale, and our experimental results must be interpreted with caution. If invasive species spread primarily through disturbed landscape areas, fragmentation can have more substantial consequences in invaded habitats, as previously established NIS can proliferate in fragments (see e.g. B. niger behaviour). When unfragmented, the system should have more chances to activate a kind of biotic resistance against NIS dispersion, and therefore invasion could be controlled (remember that NIS on unfragmented and invaded systems accounted for 3.65 average percent cover and 19.5% similarity, whereas it rose to 4.8 average percent cover and 33% similarity in fragmented and invaded habitats (Av ab., sq root transformed data). Accordingly, we assume that our result was likely attributed to the strong effect of fragmentation on biotic resistance, in agreement with previously reported effects of other global stressors known to reduce population sizes and biodiversity and that is exacerbated by fragmentation (see Haddad et al. 2015 and references therein).
In regions experiencing anthropogenic alteration, particularly habitat fragmentation and biotic homogenization, such as coastal habitats, critical connectivity thresholds may be required to maintain the ecological integrity of native communities (Howeth 2017). This study represents an additional contribution to the general understanding of interactive effects between specific global change drivers, namely habitat fragmentation and biological invasions. This experiment revealed changes only 3-months after fragmentation, but understanding the relationship between short and long-term dynamics is a substantial challenge that ecologists must tackle, mainly in the current human scenarios where fragmentation and biological invasions will continue (Haddad et al. 2015). Our findings suggest that both fragmentation and invasion perspectives synergistically aid in managing biological invasions and conservation actions on marine ecosystems. Both processes deserve consideration when analysing biotic resistance to invasion in urban marine habitats. The change in the importance of NIS across an invasion gradient suggests that conservation priorities for unfragmented habitats should be established when considering management issues. Using Madeira Island as a model system, this study contributes to a better understanding of the ecology of invasive spread across fragmented landscapes.
Availability of data and material
Data available on request from the authors.
References
Ahyong S, Costello MJ, Galil BS, Gollash S, Hutchins P, et al. (2018) World Register of Introduced Marine Species (WRiMS). Accessed at http://www.marinespecies.org/introduced on 2020–06.
Airoldi L, Beck MW (2007) Loss, status and trends for coastal marine habitats of Europe. Oceanogr Mar Biol Annu Rev 45:345–405
Airoldi L, Turon X, Perkol-Finkel S, Rius M, Biologiche S (2015) Corridors for aliens but not for natives: effects of marine urban sprawl at a regional scale. Divers Distrib 21:755–768
Airoldi L, Beck MW, Firth LB, Bugnot AB, Steinberg PD et al (2021) Emerging solutions to return nature to the urban ocean. Ann Rev Mar Sci 13:445–477
Alves FMA, Chícharo LM, Serrão E, Abreu AD (2001) Algal cover and sea urchin spatial distribution at Madeira Island (NE Atlantic). Scientia Marina 65(4):383–392. https://doi.org/10.3989/scimar.2001.65n4383
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46
Anderson MJ (2017) Permutational multivariate analysis of variance (PERMANOVA). Wiley StatsRef Stat Ref Online:1–15
AquaNIS-Editorial-Board (2015) Information system on Aquatic Non-Indigenous and Cryptogenic Species. World Wide Web electronic publication. www.corpi.ku.lt/databases/aquanis. Version 2.36+. Accessed 2020–06
Arenas F, Sánchez I, Hawkins SJ, Jenkins SR (2006) The invasibility of marine algal assemblages: role of functional diversity and identity. Ecology 87:2851–2861
Benedetti-Cecchi L, Trussell GC (2014) Intertidal rocky shores. In: Bertness M, Bruno J, Silliman B, Stachowicz J (eds) Marine community ecology and conservation. Sinauer Associates, INC, pp 203–225
Bertocci I, Arenas F, Cacabelos E, Martins GM, Seabra MI et al (2017) Nowhere safe? Exploring the influence of urbanization across mainland and insular seashores in continental Portugal and the Azorean Archipelago. Mar Pollut Bull 114:644–655
Bertolini C, Montgomery W, O’Connor NE (2020) Edge effects are not linked to key ecological processes in a fragmented biogenic reef. Estuaries Coasts 43:708–721
Bianchi CN, Morri C, Sartoni G, Wirtz P (1998) Sublittoral epibenthic communities around Funchal. Bol Mus Mun Funchal 5:59–80
Bishop M, Mayer-Pinto M, Airoldid L, Firth LB, Morris RL, Loke LHL, Hawkins SJ, Naylor LA, Coleman RA, Chee SY, Dafforn KA (2017) Effects of ocean sprawl on ecological connectivity: impacts and solutions. J Exp Mar Biol Ecol 492:7–30
Bonaviri C, Fernandez TV, Fanelli G, Badalamenti F, Gianguzza P (2011) Leading role of the sea urchin Arbacia lixula in maintaining the barren state in southwestern Mediterranean. Mar Biol 158:2505–2513
Boström C, Pittman SJ, Simenstad C, Kneib RT (2011) Seascape ecology of coastal biogenic habitats: advances, gaps, and challenges. Mar Ecol-Prog Ser 427:191–217
Brown BL, Barney JN (2021) Rethinking biological invasions as a metacommunity problem. Front Ecol Evol. https://doi.org/10.3389/fevo.2020.584701
Bulleri F (2005) Experimental evaluation of early patterns of colonisation of space on rocky shores and seawalls. Mar Environ Res 60:355–374
Bulleri F, Bertocci I, Micheli F (2002) Interplay of encrusting coralline algae and sea urchins in maintaining alternative habitats. Mar Ecol Prog Ser 243:101–109
Cacabelos E, Martins GM, Thompson R, Prestes ACL, Azevedo MN et al (2016a) Material type and roughness influence structure of intertidal communities on coastal defenses. Mar Ecol 37:801–812
Cacabelos E, Martins GM, Thompson R, Prestes ACL, Azevedo MN et al (2016b) Factors limiting the establishment of canopy-forming algae on artificial structures. Estuar Coast Shelf Sci 181:277–283
Cadenasso ML, Pickett STA, Weathers KC, Jones CG (2003) A framework for a theory of ecological boundaries. Bioscience 53:750–758
Canning-Clode J (2015) Biological invasions in changing ecosystems. vectors, ecological impacts, management and predictions. De Gruyter, Berlin, p 488
Canning-Clode J, Fofonoff P, McCann L, Carlton JT, Ruiz G (2013) Marine invasions on a subtropical island: fouling studies and new records in a recent marina on Madeira Island (Eastern Atlantic Ocean). Aquat Invasions 8:261–270
Carlton JT (1996) Biological invasions and cryptogenic species. Ecology 77(6):1653–1655
Caselle JE, Davis K, Marks L (2018) Marine management affects the invasion success of a non - native species in a temperate reef system in California, USA. Ecol Lett 21:43–53
Chainho P, Fernandes A, Amorim A, Ávila SP, Canning-Clode J et al (2015) Non-indigenous species in Portuguese coastal areas, coastal lagoons, estuaries and islands. Estuar Coast Shelf Sci 167:199–211
Chapman ARO (1981) Stability of sea urchin dominated barren grounds following destructive grazing of kelp in St. Margarets Bay, eastern Canada. Mar Biol 62:307–311
Cole VJ, Johnson LG, McQuaid CD (2012) Effects of Patch-Size on Populations of Intertidal Limpets, Siphonaria spp., in a Linear Landscape. PLoS One 7:e52076
Collinge SK (1996) Ecological consequences of habitat fragmentation: Implications for landscape architecture and planning. Landsc Urban Plan 36:59–77
Collinge SK (2009) Ecology of fragmented landscapes, Johns Hopk. Baltimore
Colomer J (2021) Serra T (2021) The world of edges in submerged vegetated marine canopies: from patch to canopy scale. Water 13:2430
Crain CM, Kroeker K, Halpern BS (2008) Interactive and cumulative effects of multiple human stressors in marine systems. Ecol Lett 11:1304–1315
Diamond JM (1975) The island dilemma: lessons of modern biogeographic studies for the design of natural reserves. Biol Cons 7:129–146
Didham RK (2010) Ecological consequences of habitat fragmentation. Encyclopedia of life sciences. John Wiley & Sons, Chichester
Didham R, Tylianakis JM, Gemmell NJ, Rand TA, Ewers RM (2007) Interactive effects of habitat modification and species invasion on native species decline. Trends Ecol Evol. https://doi.org/10.1016/j.tree.2007.07.001
Dugan JE, Airoldi L, Chapman MG, Walker SJ, Schlacher T (2011) Estuarine and coastal structures: environmental effects, A focus on shore and nearshore structures. Treatise Estuar Coast Sci 8:17–41
Elo M, Kareksela S, Haapalehto T, Vuori H, Aapala K et al (2016) The mechanistic basis of changes in community assembly in relation to anthropogenic disturbance and productivity. Ecosphere 7(4):e01310
Elton CS (1958) The ecology of invasions by animals and plants. Methuen, London
EU (2014) Regulation (EU) No 1143/2014 of the European Parliament and of the Council of 22 October 2014 on the prevention and management of the introduction and spread of invasive alien species
Fahrig L (2003) Effects of habitat fragmentation on biodiversity. Annu Rev Ecol Evol Syst 34:487–515
Filbee-Dexter K, Scheibling RE (2014) Sea urchin barrens as alternative stable states of collapsed kelp ecosystems. Mar Ecol Prog Ser 495:1–25
Fofonoff PW, Ruiz GM, Steves B, Simkanin C, Carlton JT (2018) National Exotic Marine and Estuarine Species Information System. http://invasions.si.edu/nemesis/. Accessed June 2020
Friedlander AM, Ballesteros E, Clemente S, Goncalves EJ, Estep A et al (2017) Contrasts in the marine ecosystem of two Macaronesian islands: a comparison between the remote Selvagens Reserve and Madeira Island. PLoS One 12:e0187935
Gallien L, Carboni M (2016) The community ecology of invasive species: where are we and what’s next? Ecography 40:335–352
Gestoso I, Ramalhosa P, Oliveira P, Canning-Clode J (2017) Marine protected communities against biological invasions : a case study from an offshore island. Mar Pollut Bull 119:72–80
Gestoso I, Ramalhosa P, Canning-Clode J (2018) Biotic effects during the settlement process of non-indigenous species in marine benthic communities. Aquat Invasions 13:247–259
Giakoumi S, Pey A (2017) Assessing the effects of marine protected areas on biological invasions: a global review. Front Mar Sci. https://doi.org/10.3389/fmars.2017.00049
Gizzi F, Jiménez J, Schafer S, Castro N, Costa S, Lourenço S, José R, Canning-Clode J, Monteiro J (2020) Before and after a disease outbreak: tracking a keystone species recovery from a mass mortality event. Mar Environ Res 156:1–8
Goodsell PJ, Chapman MG, Underwood AJ (2007) Differences between biota in anthropogenically fragmented habitats and in naturally patchy habitats. Mar Ecol Prog Ser 351:15–23
Gray JS (1997) Marine biodiversity: patterns, threats and conservation needs. Biodivers Conserv 6:153–175
Haddad NM, Brudvig LA, Clobert J, Davies KF, Gonzalez A et al (2015) Habitat fragmentation and its lasting impact on earth’s ecosystems. Sci Adv 1:1–9
Hagen M, Kissling WD, Rasmussen C, De Aguiar MAM, Brown LE et al (2012) Biodiversity, species interactions and ecological networks in a fragmented world. Adv Ecol Res 46:89–210
Halpern B, Walbridge S, Selkoe KA, Kappel CV, Micheli F et al (2008) A global map of human impact on marine ecosystems. Science 319:948–952
Hernández JC, Clemente S, Sangil C, Brito A (2008) The key role of the sea urchin Diadema antillarum in controlling macroalgae assemblages throughout the Canary Islands (eastern subtropical Atlantic): an spatio-temporal approach. Mar Environ Res 66(2):259–270
Hobbs RJ, Huenneke LF (1992) Disturbance, diversity, and invasion: implications for conservation. Conserv Biol 6:324–337
Hooper DU, Chapin FS III, Ewel JJ, Hector A, Inchausti P et al (2005) Effects of biodiversity on ecosystem functioning: a consensus of current. Ecol Monogr 75:3–35
Howeth JG (2017) Native species dispersal reduces community invasibility by increasing species richness and biotic resistance. J Anim Ecol 86:1380–1393
Hughes TP (1994) Catastrophes, phase-shifts, and large-scale degradation of a Caribbean coral-reef. Science 265:1547–1551
Hutchison MAS (2008) Interactions between habitat fragmentation and invasions: factors driving exotic plant invasions in native forest remnants, West Coast. University of Canterbury, New Zealand
Jenkins SR, Martins GM (2010) Succession on hard substrata. In: Dürr S, Thomason JC (eds) Biofouling. Blackwell Publishing Ltd
Katsanevakis S, Wallentinus I, Zenetos A, Leppäkoski E, Çinar ME (2014) Impacts of invasive alien marine species on ecosystem services and biodiversity: a pan-European review. Aquat Invasions 9:391–423
Kohler K, Gill SM (2006) Coral Point Count with Excel extensions (CPCe): a Visual Basic program for the determination of coral and substrate coverage using random point count methodology. Comput Geosci 32:1259–1269
Levine JM, D’Antonio CM (1999) Elton revisited: a review of evidence linking diversity and invasibility. Oikos 80:15–26
Lonsdale WM (1999) Global patterns of plant invasions and the concept of invasibility. Ecology 80:1522–1526
Mack RN, Simberloff D, Lonsdale WM, Evans H, Clout M, Bazzaz F (2000) Biotic invasions: causes, epidemiology, global consequences and control. Ecol Appl 10:689–710
Marchini A, Galil BS, Occhipinti-Ambrogi A (2015) Recommendations on standardizing lists of marine alien species: lessons from the Mediterranean Sea. Mar Pollut Bull 101:267–273
Martins GM, Neto AI, Cacabelos E (2016) Ecology of a key ecosystem engineer on hard coastal infrastructure and natural rocky shores. Mar Environ Res 113:88–94
Matias MG, Arenas F, Rubal M, Pinto IS (2015) Macroalgal composition determines the structure of benthic assemblages colonizing fragmented habitats. PLoS One 10:e0142289
Megina C, González-Duarte MM, López-González PJ, Piraino S (2013) Harbours as marine habitats: hydroid assemblages on sea-walls compared with natural habitats. Mar Biol 160:371–381
Molnar JL, Gamboa RL, Revenga C, Spalding MD (2008) Assessing the global threat of invasive species to marine biodiversity. Front Ecol Environ 6:485–492
Moschella PS, Abbiati M, Aberg P, Airoldi L, Anderson JM et al (2005) Low-crested coastal defence structures as artificial habitats for marine life: using ecological criteria in design. Coast Eng 52:1053–1071
Muricy and Tailor 2011 (listed twice), Herdman, 1886 and Huxley, 1855 are not references but authors of species. Checked in WORMS database. https://www.marinespecies.org/aphia.php?p=taxdetails&id=573180
Myers N, Mittermeier R, Mittermeier C, da Fonseca G, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858
Pardini R, Nichols E, Püttker T (2017) Biodiversity response to habitat loss and fragmentation. In: Encyclopedia of the Anthropocene. Elsevier Inc., pp 1–11
Price N (2010) Habitat selection, facilitation, and biotic settlement cues affect distribution and performance of coral recruits in French Polynesia. Oecologia 163:747–758
Ramalhosa P, Gestoso I, Duarte B, Caçador I (2019) Metal pollution affects both native and non-indigenous biofouling recruitment in a subtropical island system. Mar Pollut Bull 141:373–386
Ramalhosa P, Gestoso I, Rocha RM, Lambert G, Canning-Clode J (2021) Ascidian biodiversity in the shallow waters of the Madeira Archipelago: Fouling studies on artificial substrates and new records. Reg Stud Mar Sci 43:101672
Reid WV, Mooney HA, Cropper A, Capistrano D, Carpenter SR et al. (2005) Ecosystems and Human Well-Being. Millennium Ecosystem Assessment Synthesis Report
Ries L, Sisk T (2004) A predictive model of edge effects. Ecology 85:2917–2926
Sangil C, Martins GM, Carlos J, Alves F, Neto AI et al (2018) Shallow subtidal macroalgae in the North-eastern Atlantic archipelagos (Macaronesian region): a spatial approach to community structure. Eur J Phycol 53:83–98
Sheets EA, Cohen CS, Ruiz GM, Rocha RM (2016) Investigating the widespread introduction of a tropical marine fouling species. Ecol Evol 6:2453–2471
Simberloff D (2009) The role of propagule pressure in biological invasions. Ann Rev Ecol Evol Syst 40:81–102. https://doi.org/10.1146/annurev.ecolsys.110308.120304
Simberloff D, von Holle B (1999) Positive interactions of nonindigenous species: invasional meltdown? Biol Invasions 1:21–32
Smoothey AF (2013) Habitat-associations of turban snails on intertidal and subtidal rocky reefs. PLos ONE 8:e61257
Stachowicz JJ, Byrnes JE (2006) Species diversity, invasion success, and ecosystem functioning: disentangling the influence of resource competition, facilitation, and extrinsic factors. Mar Ecol Prog Ser 311:251–262
Thompson RC, Crowe TP, Hawkins SJ (2002) Rocky intertidal communities: past environmental changes, present status and predictions for the next 25 years. Environ Conserv 29:168–191
Tsiamis K, Palialexis A, Stefanova K, Ničević Ž, Skejić S et al (2019) Non-indigenous species refined national baseline inventories : a synthesis in the context of the European Union ’ s Marine Strategy Framework Directive. Mar Pollut Bull 145:429–435
Tuya F, Boyra A, Sanchez-Jerez P, Barbera C, Haroun RJ (2004) Relationships between rocky-reef fish assemblages, the sea urchin Diadema antillarum and macroalgae throughout the Canarian Archipelago. Mar Ecol Prog Ser 278:157–169
Vermeij MJA, Dailer ML, Smith CM (2011) Crustose coralline algae can suppress macroalgal growth and recruitment on Hawaiian coral reefs. Mar Ecol Prog Ser 422:1–7
Vitousek PM, D’Antonio CM, Loope LL, Rejmánek M, Westbrooks R (1997) Introduced species: a significant component of human-induced global change. N Z J Ecol 21:1–16
Wilcove DS, McLellan CH, Dobson AP (1986) Habitat fragmentation in the temperate zone. In: Conservation biology: the science of scarcity and diversity. Sinauer Associates, INC, Sunderland, MA: Sinauer, p 237–256
Wilson MC, Richard XC, Didham RK, Ding P, Holt RD et al (2016) Habitat fragmentation and biodiversity conservation : key findings and future challenges. Landsc Ecol 31:219–227
With KA (2002) The landscape ecology of invasive spread. Conserv Biol 16:1192–1203
With KA (2004) Assessing the risk of invasive spread in fragmented landscapes. Risk Anal 24:803–815
Wirth R, Meyer ST, Leal IR, Tabarelli M (2008) Plant herbivore interactions at the forest edge. In: Lüttge U, Beyschlag W, Murata J (eds) Progress in botany 69:423–448. Springer, Berlin Heidelberg.
WoRMS Editorial Board 2020. World Register of Marine Species. Available from https://www.marinespecies.org at VLIZ. Accessed 2020–06. doi:https://doi.org/10.14284/170
Acknowledgements
The authors are grateful to Janina Beltz and João Ladeira for their help during the preparation of the experimental units, and the two anonymous reviewers for their comments and suggestions, which greatly improved the quality of this work. This work was partially funded by projects MIMAR (MAC/4.6.d/066) and MIMAR+ (MAC2/4.6d/249), in the framework of INTERREG MAC 2014-2020 Programme. EC and IGG were financially supported by post-doctoral grants in the framework of the 2015 ARDITI Grant Programme Madeira 14-20 (Project M1420-09-5369-FSE-000002). I.G. is supported financially by a Maria Zambrano contract UCA under the grants call for the requalification of the Spanish university system 2021-2023, funded by the European Union—NextGenerationEU. PR was partially funded by the Project Observatório Oceânico da Madeira-OOM (M1420-01-0145-FEDER-000001), co-financed by the Madeira Regional Operational Programme (Madeira 14-20), under the Portugal 2020 strategy, through the European Regional Development Fund (ERDF) and currently funded by project MARE-Centro de Ciências do Mar e do Ambiente (UIDB/04292/2020). JCC is funded by national funds through FCT—Fundação para a Ciência e a Tecnologia, I.P., under the Scientific Employment Stimulus—Institutional Call—[CEECINST/00098/2018]. Finally, this study also had the support of Fundação para a Ciência e Tecnologia (FCT), through the strategic project [UIDB/04292/2020] granted to MARE. This is contribution 97 from the Smithsonian’s MarineGEO and Tennenbaum Marine Observatories Network.
Funding
This work was partially funded by projects MIMAR (MAC/4.6.d/066) and MIMAR + (MAC2/4.6d/249), in the framework of INTERREG MAC 2014–2020 Programme. EC and IGG were financially supported by post-doctoral grants in the framework of the 2015 ARDITI Grant Programme Madeira 14–20 (Project M1420-09–5369-FSE-000002). IGG is supported financially by a Maria Zambrano contract UCA under the grants call for the requalification of the Spanish university system 2021–2023, funded by the European Union—NextGenerationEU. PR was partially funded by the Project Observatório Oceânico da Madeira-OOM (M1420-01–0145-FEDER-000001), co-financed by the Madeira Regional Operational Programme (Madeira 14–20), under the Portugal 2020 strategy, through the European Regional Development Fund (ERDF) and currently funded by project MARE-Centro de Ciências do Mar e do Ambiente (UIDB/04292/2020). JCC is funded by national funds through FCT—Fundação para a Ciência e a Tecnologia, I.P., under the Scientific Employment Stimulus—Institutional Call—[CEECINST/00098/2018]. Finally, this study also had the support of Fundação para a Ciência e Tecnologia (FCT), through the strategic project [UIDB/04292/2020] granted to MARE. This is contribution 97 from the Smithsonian’s MarineGEO and Tennenbaum Marine Observatories Network.
Author information
Authors and Affiliations
Contributions
EC: Conceptualization, Investigation, Data curation, Formal analysis, Writing—original draft, Writing—review and editing. IG: Conceptualization, Methodology, Investigation, Data curation, Writing—review and editing. PR: Methodology, Investigation, Data curation, Writing—review and editing. JC-C: Funding acquisition, Writing—review and editing.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
See Fig. 5.
Photogras showing real synthetic assemblages (see Photographs in Fig. 1.)
Rights and permissions
About this article
Cite this article
Cacabelos, E., Gestoso, I., Ramalhosa, P. et al. Role of non-indigenous species in structuring benthic communities after fragmentation events: an experimental approach. Biol Invasions 24, 2181–2199 (2022). https://doi.org/10.1007/s10530-022-02768-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-022-02768-9