Abstract
While digging and foraging, the non-indigenous green crab (Carcinus maenas) creates a landscape of distinctive pits or depressions in the sediment. Despite their visibility and widespread occurrence in Atlantic Canada and elsewhere, the community influence and persistence of this disturbance remain undocumented. This study addressed this gap in our knowledge using two approaches. First, in both sandy and muddy habitats, we monitored fresh feeding pits (disturbed sediments) for up to 9–11 days after their formation, recording their sediment properties and diversity and density of invertebrate fauna, and comparing these characteristics to those of ambient (undisturbed) sediments in similar habitat. Second, we quantified local-scale invertebrate diversity and density in feeding pits and ambient sediments in muddy habitat only, at three other sites within a Marine Protected Area (MPA). Grain size did not differ between disturbed and ambient sediments and did not change over time within habitats. We also found no significant differences in invertebrate diversity and density between disturbed and undisturbed sandy sediments. In contrast, the invertebrate fauna differed significantly between disturbed and ambient muddy sediments, particularly during the first 4 days after disturbance. Feeding pits in muddy sediments also took twice longer to fill up than pits in sandy sediments. These results were consistent with the comparison of disturbed and undisturbed muddy sediments in the MPA: at least at the local scale, the foraging by this invader significantly altered community structure. Ambient sediments had a higher number of species and nearly twice as many invertebrates compared to disturbed sediments. Overall, our results suggest that visual evidence of green crab feeding pits in muddy sediments can be used as a fairly reliable predictor of local-scale changes in invertebrate communities. The persistence of these local-scale changes depends on the type of habitat in which the disturbance takes place.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Some coastal predators have impacts that go beyond their own prey (Wilson 1991) and involve the recurrent alteration of the habitat (e.g. Volkenborn et al. 2009; Lee 2010; Pacheco et al. 2013). By digging, they disturb the matrix of sediment as well as displace or cause mortality of non-targeted species or remove and expose their prey to other predators (Botto and Iribarne 1999; Escapa et al. 2004). In the process, they also open up opportunities for other individuals to colonize and establish in areas previously not available to them (DePatra and Levin 1989; Dernie et al. 2003; Flach 2003). In fact, the effects of these bioturbators on habitats and communities can be multiple (Reise 2002; Alvarez et al. 2015). Beyond potential changes to sediment characteristics (e.g. Le Calvez 1987; Palanques et al. 2001), disturbance due to feeding may increase food and invertebrate patchiness (e.g. Griffiths et al. 2006) or make more uniform food or infaunal distributions (Thrush et al. 1991). The European green crab (Carcinus maenas), a widespread coastal invader, is among the predators that are able to alter community structure while feeding (Schratzberger and Warwick 1999; Gregory and Quijón 2011). In their search for prey, this species digs into the sediment and creates characteristic depressions (hereafter “feeding pits”) that remain visible for several days (Audet et al. 2003). A “landscape” of feeding pits is widespread in areas where the green crab is abundant and is likely to be a good indication of community alteration considering this species’ broad diet and high feeding rates (e.g. Elner and Hughes 1978; Cohen et al. 1995; Pickering and Quijón 2011).
In addition to its effects on individual prey, green crab feeding is known to have significant effects on meio-benthic (Cohen et al. 1995; Schratzberger and Warwick 1999) and macro-benthic communities (Gregory and Quijón 2011). However, no studies have focused on the impact of feeding pits on infaunal communities nor on the persistence of these changes to the sediment over time. The question of whether visual evidence, such as feeding pits, can predict the occurrence of an impact on native invertebrate communities has not been addressed. Recolonization of sediments following disturbance by shellfish harvesting (Dernie et al. 2003; Griffiths et al. 2006) or by feeding activities of large epibenthic predators such as rays and horseshoe crabs (e.g. Thrush et al. 1991) is well documented. However, recolonization or short-term changes following smaller disturbances such as those created by green crabs are not. After the creation of a feeding pit, community changes should be expected to occur due to the temporary absence of at least some prey and the provision of open, under-populated patches of sediment (e.g. Volkenborn et al. 2009). The type of sediment influences the feeding rate of some decapod predators (e.g. the blue crab, Callinectes sapidus; Lipcius and Hines 1986), and in consequence, it may also influence the speed of recovery from disturbance (Dernie et al. 2003) or the short-term variation in community structure. For instance, unstable, shifting habitats such as coarse sandy habitats are expected to recover faster from disturbance than more stable muddy habitats (Thrush et al. 1991; Dernie et al. 2003). This difference is not trivial considering the uneven distribution of different sediment types and the ongoing spread of populations of green crab into new locations and habitats in many parts of the world
This study combines two approaches to study the influence of green crab feeding pits on intertidal invertebrate communities located in Prince Edward Island (hereafter PEI), southern Gulf of St. Lawrence, Canada. First, it describes short-term community changes following the visual detection of green crab feeding pits in muddy and sandy habitats during a period of up to 11 consecutive days. Second, it documents local-scale differences between freshly disturbed (feeding pits) and undisturbed muddy sediments in three other intertidal communities. A priori expectations included potential differences in composition and abundance as a result of green crab feeding and sediment disturbance (cf. Levin 1984). In addition, native species mobility (cf. Frid 1989; Gray and Elliott 2009) or the presence of opportunistic species following disturbance was also expected to contribute to changes in community composition.
Methods
Study area
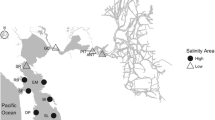
The study of short-term variation following green crab disturbance was conducted in two locations (Fig. 1), characterized by different grain size composition: one was located in Basin Head (primarily muddy sediments) and one in Souris River estuary (sandy sediments). The sites are located ~6 km apart and have similar tidal ranges (approximately 1.4 m during spring tide conditions). During low tide, the upper limit of the feeding pits in both areas becomes exposed for up to 2.5 h. Height differences between those upper level and their corresponding low tide levels were approximately 0.5 m (see Gregory and Quijón 2011; Lutz-Collins and Quijón 2014 for a detailed description of each study area). Water temperature and salinity were relatively uniform among sites and most of their variation was primarily due to changes associated with tidal cycles (Sharp et al. 2003; DFO 2009). Both areas are also known to support green crab populations that have been well established for over a decade (Audet et al. 2003).
The chosen sites had clear visual signs of green crab foraging activity in the intertidal zone, i.e. large number of feeding pits. Relative abundance of green crabs (crabs trap−1 day−1) in each area (Basin Head and Souris) were considered fairly similar: estimations conducted before and after the study (June to August) with wire mesh traps baited with Atlantic mackerel (Scomber scombrus) and deployed for 24 h are presented in Table 1. Average number of green crab was as high as 41.5 and 37.4 crabs trap−1 day−1 in muddy (Basin Head) and sandy (Souris) sediments, respectively. The overall size range of these crabs was 20–79 mm CW. With regard to the number of feeding pits, counts conducted in the areas that were subsequently used for monitoring (10 × 30 m areas; see below) indicated that muddy sediments had almost twice as many feeding pits than sandy sediments. The diameter of 30 randomly selected pits ranged between ~3 and 7 cm and their depth 0.5–4.5 cm (Table 1).
The collection of samples for the monitoring of feeding pits was conducted during spring low tide conditions in July 2008 while the collection of samples for the snapshot comparison of disturbed and undisturbed sediments was conducted in August 2008. No substantial temporal changes in infaunal numbers were detected between the beginning and end of the study in both locations (July–August 2008). Thus, potential alterations due to seasonal events such as settlement/recruitment were not expected to severely influence the samples collected during this study. Harvester’s digging for shellfish (clamming) creates pits somewhat similar to those created by green crab foraging and could be considered a source of confusion. However, clamming is not allowed in Basin Head, one of the few Marine Protected Areas in the region. In Souris River, where clamming is allowed, no evidence of it was observed in or around the study site during the daily visits to monitor the feeding pits.
Sampling design and processing
Areas measuring 10 × 30 m located parallel to the low tide level were identified at a site with muddy sediments (Basin Head) and a site with sandy sediments (Souris). All the depressions found in these areas that were visually attributable to green crab foraging pits (~3–7 cm diameter) were carefully tagged with thin straws of a standard colour and no longer considered for sampling. During low tide (LT) on the following day (day 1), all new (freshly dug) feeding pits (>100 on each area) were tagged with straws of a different colour and were considered suitable for sampling. At each site, sediment samples were taken from randomly selected feeding pits (n = 6) using a 7-cm-diameter cylinder (38.5 cm2) inserted 5 cm into the sediment. Based on Lutz-Collins and Quijón (2014), these samples were considered representative of the infaunal communities of these sites. For comparison, six additional samples of the same size were taken simultaneously near each foraging pit in visually undisturbed sediments. Early during the process of recolonization, the collection of 5-cm-deep samples from disturbed sediments might have included relatively deep layers of sediment, known to be less populated than those located near the surface (e.g. Quijón and Jaramillo 1996). We did not consider this a bias, as in our opinion this reflects the nature of the sediment disturbance we were studying; the sampling of 5-cm-deep samples was consistent between disturbed and undisturbed sediments from both types of habitats.
Using straws of distinctive colours, the tagging protocol (to distinguish new feeding pits) continued at each site for up to 11 days. During each day, another six random feeding pit samples were collected from the pits that were labelled on day 1 and labelled as “2 day old”, “3 day old”, etc. As in day 1, these samples were matched with others collected from undisturbed sediments located in the immediate vicinity. No feeding pit or undisturbed sediment was sampled twice. Sediment samples for grain size analysis were collected in a less intensive timeline and a lower replication level (n = 4): Small sediment samples collected with a 2-cm-diameter cylinder (0–5 cm deep) were taken during days 1, 3, 5 and during the last day of the monitoring in each of the habitats (days 11 and 9 in muddy and sandy habitats, respectively). These samples were kept frozen until their analysis in the laboratory. In parallel, feeding pit sediment filling rates were visually estimated and recorded as empty (0 % of the pit was filled with sediment), 25, 50, 75 and 100 % full (completely filled).
For the spatial comparison of disturbed and undisturbed sediments, three additional 10 × 30 m separate areas were located in muddy locations within Basin Head and all the feeding pits were tagged with coloured straws. Following the protocol described above, initial feeding pits were not considered for sampling, but those found the following day (freshly dug) pits and undisturbed sediments located nearby were sampled (n = 8 in each area). This sampling was conducted on day 1 only.
Samples collected for grain size analysis were unfrozen and processed using running distilled water and standard sieves to separate three sediment fractions: mud (<63 μm), sand (63 μm—2 mm) and gravel (>2 mm) (see Anderson et al. 1981). Samples collected for infauna analysis were sieved through a 500-μm mesh and the residual preserved in 70 % ethanol stained with Rose Bengal. All the organisms retained were sorted under stereomicroscope and identified to the species level using standard keys for the region (e.g. Bousfield 1973; Appy et al. 1980; Bromley and Bleakney 1984). Only one taxon, juvenile Capitellids, could not be identified at the species level due to the difficulty in discerning morphological features in these small organisms (see Lutz-Collins and Quijón 2014).
Data analysis
Grain size data and data about infaunal richness and abundance (total densities, densities of polychaetes, bivalves and several individual species) were used as response variables to assess differences between feeding pits and ambient sediments. Data on filling rates were considered semi-quantitative and were not used for statistical comparisons. For the study of short-term variation following disturbance, separate one-way ANOVAs assessing daily differences between feeding pits and ambient sediments were conducted for each of the response variables described above. Data were transformed when necessary to satisfy ANOVA assumptions (Sokal and Rohlf 2011). In the case of grain size data, comparisons refer to four dates only: days 1, 3, 5 and final day. Summaries of p-values from all the ANOVA comparisons (including Two-way ANOVAs described below) are presented in separate tables, as part of an Electronic Supplementary Appendix to this article.
Information about species composition and density was also used to conduct multivariate analyses using PRIMER-5 (Plymouth Routines In Multivariate Ecological Research). Patterns of community structure were visualized using multidimensional scaling (MDS) plots, and analyses of similarity (ANOSIM) were used to test for differences between disturbed and undisturbed sediments. SIMPER analyses were also used to identify those species that were the most important drivers of the dissimilarity between disturbed and undisturbed sediments. Multivariate analyses were applied to data from the days in which most differences between disturbed and undisturbed sediments were detected with the ANOVAs (i.e. days 1–4 in muddy sediments). These analyses were not applied to sandy sediments due to the lack of significant and consistent differences between disturbed and undisturbed sediments (see Results).
For spatial comparisons of disturbed and undisturbed sediments, two-way ANOVAs were conducted for each of the three sites separately. This ANOVA model included the following explanatory variables: “pit location” (to take into consideration spatial differences among pairs of pit/ambient samples), “disturbance” (pit versus ambient) and their interaction. Multivariate analyses (MDS, ANOSIM and SIMPER) were also applied for this data set to test for significant community level differences between disturbed and undisturbed sediments at each site.
Results
Short-term variation in muddy and sandy habitats
Muddy and sandy sediment was clearly different in terms of grain size characteristics, and these differences remained similar (unchanged) overtime (see Table 2). None of the statistical comparisons between disturbed and undisturbed sediments detected significant differences (all p values >0.293) in any of the grain size categories. With regard to sediment filling rates in the muddy habitat, filling rate in an average feeding pit was near 70 % by day 5 and over 90 % by the end of the monitoring (day 11). In the sandy habitat, the filling rate in an average feeding pit was near 80 % by day 3 and 100 % by day 5 (Table 2).
In the muddy habitat, the average number of taxa collected from ambient sediments was generally higher than from disturbed sediments (Fig. 2). This difference persisted up to 4 days after the disturbance event (p < 0.05 in one-way comparisons conducted on days 1–4). Total density and total number of polychaetes followed the same trend, with significantly higher densities in ambient than in disturbed sediments up to day 4 (total density) and up to day 5 (polychaetes). Total densities declined by day 6: polychaete density was at its lowest in both disturbed and undisturbed sediments, but the reduction was more severe in undisturbed sediments; polychaete disturbed–undisturbed differences became non-significant (p > 0.05) (Fig. 2). Subsequently, some significant differences were again detected at least once during day 8 (total density) or days 8–9 (polychaetes). In contrast, the total number of bivalves did not significantly differ between ambient and disturbed sediments at any time during the daily monitoring of feeding pits (Fig. 2).
Average (±SE) taxa richness, total density, and densities of polychaetes and bivalves in the muddy sediments (Basin Head) during the 11 days following the detection of a disturbance. Feeding pits (n = 6) and ambient sediments (n = 6) are represented with filled and open symbols, respectively. Asterisks identify days when significant differences between disturbed and ambient sediments were detected
The four most abundant species in the muddy habitat (out of 34) showed different temporal patterns. The spionid polychaetes Pygospio elegans and Streblospio benedicti exhibited higher densities in ambient sediments than in disturbed sediments, particularly during the first 4 days following disturbance, but due to high levels of variation, several of these differences were not significant (Fig. 3). The soft-shell clam (Mya arenaria) and the polychaete Eteone heteropoda did not show any clear, persistent difference between disturbed and undisturbed sediments, and only in the latter case, there was some evidence of higher density values in ambient sediments during the sampling period (Fig. 3).
Average (±SE) density of four representative species in the muddy sediments (Basin Head) during the 11 days following the detection of a disturbance. Feeding pits (n = 6) and ambient sediments (n = 6) are represented with filled and open symbols, respectively. Asterisks identify days when significant differences between disturbed and ambient sediments were detected
In the sandy habitat, the average number of taxa was similar to the number recorded in the muddy habitat (roughly between 5 and 9), but the densities were higher, particularly due to the higher number of bivalves (Fig. 4). The number of taxa, the total density and the density of polychaetes and bivalves did change over time, but did not exhibit any significant differences between ambient and disturbed sediments (Fig. 4). With respect to the most abundant species (out of 39 taxa), significant differences between disturbed and ambient sediments were only observed at day 1 for the mud snail Hydrobia totteni and the spionid polychaete Streblospio benedicti. In both cases, densities in ambient sediments were almost double the densities measured in disturbed sediments. The same occurred on days 4–5 for Hydrobia totteni (p < 0.05; Fig. 5).
Average (±SE) taxa richness, total density, and densities of polychaetes and bivalves in the sandy sediments (Souris estuary) during the 9 days following the detection of a disturbance. Feeding pits (n = 6) and ambient sediments (n = 6) are represented with filled and open symbols, respectively. Asterisks identify days when significant differences between disturbed and ambient sediments were detected
Average (±SE) density of four representative species in the sandy sediments (Souris estuary) during the 9 days following the detection of a disturbance. Feeding pits (n = 6) and ambient sediments (n = 6) are represented with filled and open symbols, respectively. Asterisks identify days when significant differences between disturbed and ambient sediments were detected
A comparison of species composition and abundance was conducted with an MDS plot at the muddy habitat, in which a substantial number of significant differences were detected, particularly during days 1–4 (Figure 6). Differences in community structure were significant (ANOSIM’s p = 0.001). SIMPER analyses to determine which species were the most influential in driving the dissimilarity between disturbed and undisturbed sediments are summarized in Table 3. Pygospio elegans, Streblospio benedicti, Mya arenaria and Eteone heteropoda accounted for 66 % of the dissimilarity between disturbed and undisturbed sediments. Based on a large majority of the comparisons made (see Figs. 4 and 5), community structure in disturbed and undisturbed sandy sediments was similar.
Multidimensional scaling plots (MDS) illustrating the level of similarity among muddy samples (Basin Head) based on species composition and density. Ambient and disturbed sediments are identified with open and filled symbols, respectively. Dashed lines identify significant differences between the corresponding groups of samples based on ANOSIM results (p < 0.05). The analyses include samples from days 1–4
Spatial comparison of disturbed and undisturbed muddy sediments
The average number of taxa was consistently lower in disturbed sediments (associated to feeding pits) than in ambient sediments (Fig. 7), although these differences were significant only in one of the three sites surveyed. Total densities, total number of polychaetes and densities of the spionid polychaetes Pygospio elegans and Streblospio benedicti were all higher in ambient than in disturbed sediments (in most cases twice as many), and at the three sites these differences were significant (Fig. 7). Similarly, the largest-sized polychaete in the community, the rag worm Nereis diversicolor, showed higher densities in ambient sediments, but these differences were only significant in two of the three sites (Fig. 7). The number of juvenile capitellids showed significant differences on a single site while the total number of bivalves was not significantly different between disturbed and ambient sediments (Fig. 7).
The analysis of species composition and abundance in muddy sediments (multidimensional scaling plot, MDS; Fig. 8) identified significant differences between communities associated with disturbed and undisturbed sediments (Fig. 8; ANOSIM p = 0.001). SIMPER analyses identified the following four species as those that were most influential in driving the dissimilarity between disturbed and undisturbed sediments: Pygospio elegans, Streblospio benedicti, juvenile capitellids and Nereis diversicolor (Table 4). Together, these species accounted for almost 70 % of the dissimilarity between disturbed and ambient sediments.
Multidimensional scaling plot (MDS) illustrating the level of similarity among samples from three muddy sites based on species composition and density. Ambient and disturbed sediments are identified with open and filled symbols, respectively. Dashed lines identify significant differences between the corresponding groups of samples based on ANOSIM results (p < 0.05)
Discussion
Invasive species whose mobility, habits and feeding behaviour affect multiple features of their native habitats also have the potential to play significant roles in their expanded range of distribution. The green crab, for example, is an eager consumer of an array of species associated with natural or artificial shellfish beds (e.g. Le Calvez 1987; Cohen et al. 1995). This invader is also able to disrupt essential habitat by digging in the sediment, uprooting or consuming eelgrass (Davis et al. 1998; Malyshev and Quijón 2011), or consuming large amounts of reef-forming American oysters (Pickering and Quijón 2011). In less structured habitats such as mud or sand, only a few species of decapods such as the shrimp Callianassa kraussi or the burrowing crab Neohelice (Chasmagnathus) granulata alter the habitat in a consistent way (Pillay et al. 2007; Volkenborn et al. 2009; Alvarez et al. 2015). The European green crab belongs to that group because it is able to create relatively stable feeding pits (that can persist for a number of days), while digging and foraging for prey (Audet et al. 2003; this study). We must be cautious though, as green crabs have also been observed feeding on bivalves and hydrobiid snails and leaving no visible trails or pits in the sediment. This suggests that predation on certain prey not always implies the creation of a persistent trail or pit in the sediment. Despite the lack of temporal changes in grain size characteristics and potentially in sediment nutrient quality (the mud fraction typically reflects organic content) in disturbed areas, we did find significant changes in infaunal community structure. Considering the ongoing spread of this invasive species in Atlantic Canada and elsewhere, our results have implications for the local-scale impact of green crabs on infaunal communities associated with muddy sediments.
Short-term variation from disturbance at muddy and sandy sites
Shortly after the detection of feeding pits in muddy sediments, the total abundance and the total number of polychaetes were both significantly lower in disturbed than in undisturbed sediments. In general, these results are in line with those previously described for sediments affected by other sources of disturbance (e.g. Pearson and Rosenberg 1978; Gray et al. 2002; Magni et al. 2009): the alteration of the sedimentary habitat induces mortality or displaces organisms and thus promotes the creation of a landscape of partially defaunated patches of sediment. Several related studies have described a disproportional increase in the number of opportunistic species following disturbance (e.g. VanBlaricom 1982; Oliver and Slattery 1985). For example, Thistle (1980) and Savidge and Taghon (1988) described an aggregation of opportunistic organisms in their disturbed plots, following increased deposition of fine particles and organics. However, unlike those studies, we did not observe a build up in fine sediments or an increase in opportunistic species. Although we detected short-term changes in abundance and community structure, those changes did not follow the typical recovery trend that follows defaunation of disturbed sediments (e.g. Pearson and Rosenberg 1978). In fact, organisms associated with disturbed sediments remained at low densities or had inconsistent short-term variations. Although it is clear that green crab predation is not the only factor playing a role here, the changes detected and their persistence over time (~4 d) are consistent with the results of short-term experimental manipulations conducted in similar habitats (e.g. Quijón and Snelgrove 2008). Such experiments involved the inclusion of green crabs within cages for periods of one week and found similar community changes to those detected by day 4 in our daily monitoring study.
Green crab feeding pits had no impact on communities associated with sandy sediments. Given that sandy habitats are exposed to a more intense current dynamics, these results support the notion that the impact of a disturbance depends on the background disturbance regime of a given habitat (cf. Dernie et al. 2003). In sandy habitats, recolonization occurs at faster rates because redistribution of organisms is more dynamic (Oliver and Slattery 1985). Indeed, differences in total densities between disturbed and undisturbed sediments were negligible 24 h after the creation of the pits. Unlike the results observed in the muddy habitat, polychaetes as a whole did not respond to green crab disturbance. Bivalves did not respond to disturbance in this habitat either, and only a couple of significant differences were detected in Hydrobia totteni’s abundance in day 1 and later on days 4 and 5. This contrast between habitats may be partly due to the higher number of bivalves in sandy sediments as opposed to the less prominent density of polychaetes. Thrush et al. (1991) studied the recovery of eagle ray feeding pits in New Zealand sandflats and compared recolonization between bivalve- and polychaete-dominated communities. The arguments raised by these authors are likely applicable to our results: communities with abundant bivalves recolonize feeding pits faster than communities with abundant polychaetes, where disturbance effects are longer lasting (Thrush et al. 1991). As indicated before, feeding on bivalves and some species of hydrobiids, particularly on those living near the surface of the sediment, may not always leave visible pits in the sediment.
Rate of recovery from disturbance can be also related to the rate of sediment infilling of the feeding pits (Thrush et al. 1991; Dernie et al. 2003). Although such a relationship might not be valid in every sedimentary habitat, it is likely applicable to our results. At the muddy site, a majority of the feeding pits remained clearly visible for a few days, whereas in the sandy habitat they were filled much more quickly. Our filling rate estimations, although semi-quantitative, do reflect those short-term changes. As indicated in other studies (cf. Hall 1994; Kaiser 1998), community structure differences also seem to reflect the pace of the changes taking place in muddy and sandy sediments. The rapid recovery reported in coarser sediments may be also related to individual species strategies for coping with different disturbance regimes. Small mudsnails (Hydrobia spp.), for example, are common on sandy sediments such as those studied here and lead fast recolonization processes (e.g. Norkko and Bonsdorff 1996). In contrast, small polychaete species rely on larval dispersal (undetectable with the sieves and the temporal scale of our study) and are relatively slow post-settler colonizers, both of which seem to drive the slower pace of recovery.
Despite the significant differences detected between disturbed and undisturbed sediments in the muddy habitat, there was considerable variation that was not necessarily accounted for by crab predation. Among other factors, such variation may be related to undetected variability in feeding pit size and depth (e.g. Smith and Brumsickle 1989). Green crab feeding pits were shallower than the semi-permanent burrows built by species such as the burrowing crab Neohelice (Chasmagnathus) granulata (e.g. Iribarne et al. 1997) and were far from uniform in size (~4–7 cm diameter). Another source of variation could be related to faster than expected rates of recolonization exhibited by some species, which potentially enhanced feeding pit densities shortly after their creation (see Thrush et al. 1991). As described above, individual species have various means to arrive and establish in a partially “vacant” patch (Levin 1984; DePatra and Levin 1989). One additional (unexpected) event that likely prevented the detection of clearer differences in the muddy habitat occurred on day 6, when a drop in the abundance of polychaetes took place in both undisturbed and disturbed sediments. A different source of variation or type of disturbance, likely a weather-related event (rain before day 6 sampling), may have altered the sequence of change of these sediments at this time. In addition, high levels of patchiness which are typical in this type of habitats may have also contributed to short-term changes like these. Although the level of replication used (n = 6) seemed appropriate to us, it is plausible that our samples that day were collected from patches of sediment naturally poor in infauna.
Spatial comparisons and implications
Results from the spatial comparison of disturbed and undisturbed sediments indicate that the digging and foraging by green crabs have a rapid (within 24 h) impact upon soft-sediment intertidal communities. Total density of invertebrates was reduced by as much as ~50 % in disturbed patches, an effect that, regardless of the predator’s nature (native or invasive), would qualify as “strong” in sedimentary communities (cf. Ólafsson et al. 1994). Such an effect is also substantial when compared with those resulting from manipulative experiments reported for other predators in the region (e.g. snow crab and toad crab; Quijón and Snelgrove 2005) or comparable species elsewhere (e.g. Thrush 1986; Ólafsson et al. 1994 for a review). Our results suggest that, at least in the short term and at the local scale, green crabs play a significant role in structuring communities in muddy habitats. Hence, depending on the prevalence of muddy sediments, and the number and spatial extent of green crab feeding pits, they may explain at least a portion of the spatial variation in infaunal abundance and structure. Likely, other sources of variation can also contribute to the changes detected here: condition of the crabs (Tummon Flynn et al. 2015), weather events, infaunal patchiness, nutrient limitation or sediment characteristics (e.g. Lutz-Collins and Quijón 2014).
As expected, species richness was lower in disturbed sediments, but this difference was not statistically significant at every site. Although predation is undeniably important for the structure of sedimentary assemblages (e.g. Le Calvez 1987; Lenihan and Micheli 2001), diversity declines are considerably less common than changes in prey population abundance, behaviour or size composition (e.g. Peterson 1979; Kvitek et al. 1992). Consistent with this, the abundance of polychaetes as a group or as individual species (e.g. Pygospio elegans and Streblospio benedicti) but not their diversity was lower in response to the digging by green crabs. In contrast, bivalves showed no response to green crab disturbance, despite the fact that they are known to be a preferred prey of this species (Elner and Hughes 1978; Grosholz and Ruiz 1996; Jamieson et al. 1998). This unexpected result may be explained by the size and density of the bivalves. At all three sites bivalves were very small (≤1 mm SL; P. Quijón, Unpublished) and less abundant than polychaetes. It is therefore reasonable to assume that they were not a preferred prey for green crabs of the size (adults) that leaves noticeable feeding pits. Accordingly, green crabs may have dig for spionid polychaetes or other species larger in size that were far more abundant than the small bivalves. This is also consistent with the results gathered from sandy sediments, where the mobility of Gemma gemma and Hydrobia totteni may have resulted in inconsistent (not significant) differences. And as indicated before, crab feeding on these species may in some cases leave no trails or clear pits behind.
Lack of predator effects on bivalves and a few other species may be also related to top-down indirect effects. Predators such as green crabs displace or prey heavily on relatively large prey including mobile predatory organisms (e.g. the ragworm Nereis diversicolor; Rosa et al. 2008; Volkenborn et al. 2009). By targeting these organisms, they may have cascading effects on other fractions of the community (Ambrose 1984; Ronn et al. 1988; Quijón and Snelgrove 2008). Nereid polychaetes, for example, are known to have the ability to reduce densities of smaller polychaetes and bivalves (Hiddink et al. 2002), so their consumption by green crabs may therefore have a positive effect on smaller species. Alternatively, passive transport due to currents and tides is an important facilitator of feeding pit recolonization that has been demonstrated for small bivalves such as gem clams (Gemma gemma) (Grant 1981; Commito et al. 1995).
Our results are consistent with previous reports from field experiments (Quijón and Snelgrove 2008), which also found evidence that green crab feeding was a structural force in muddy habitats, where feeding pits lasted longer and differences were significant. In sandy sediments, the lack of effects we found in this study is not consistent with some other results: Gregory and Quijón (2011) conducted cage experiments in which green crabs did alter communities associated with sandy sediments. The changes in density observed in those experiments may indeed reflect the effects of recurrent (potentially daily) feeding pit formation. However, we prefer to be cautious since that particular study only focused on larger size infauna (>2 mm in size) and so may not be fully comparable to the communities studied here.
Altogether our results suggest that although feeding pits occur in most unstructured habitats, their actual impact and persistence depends considerably on the type of sediment. Community composition, which is heavily dependent on habitat characteristics (cf. Snelgrove and Butman 1994; Gray and Elliott 2009), also drives the rate of change in each habitat type and suggests that those associated with sandy sediments are more resilient. These habitat-related differences must be highlighted considering the continued spread of green crab populations in some parts of the region (e.g. PEI and Newfoundland) (Pickering and Quijón 2011; Blakeslee et al. 2010) as well as elsewhere. After all, it is in areas most recently invaded where green crabs seem more aggressive (Rossong et al. 2012) and their impacts more obvious (e.g. Garbary et al. 2014; Gehrels et al. in press). In this changing scenario, the scope and frequency of feeding pits creation, and their potential impact on muddy habitats, are likely to increase. The occurrence of feeding pits is associated with local community change in muddy habitats, at least in the short term. So the presence of numerous feeding pits may be used as preliminary evidence of ongoing local community changes on that habitat.
References
Alvarez MF, Addino M, Iribarne O, Botto F (2015) Combined engineering effects of clams and crabs on infaunal assemblages and food availability in intertidal systems. Mar Ecol Progr Ser 540:57–71
Ambrose WG Jr (1984) Role of predatory infauna in structuring marine soft-bottom communities. Mar Ecol Progr Ser 17:109–115
Anderson FE, Black L, Mayer LM, Watling LE (1981) A temporal and spatial study of a mudflat texture. Northeast Geol 3:184–191
Appy TD, Linkletter EL, Dadswell MJ (1980) A guide to the marine flora and fauna of the Bay of Fundy: Annelida: Polychaeta. Fish Mar Serv Tech Rep 920:1–123
Audet D, Davis DS, Miron G, Moriyasu M, Benhalima K, Campbell R (2003) Geographical expansion of a nonindigenous crab, Carcinus maenas (L.) along the Nova Scotian shore into the southeastern Gulf of St. Lawrence, Canada. J Shellfish Res 22:255–262
Blakeslee AMH, McKenzie CH, Darling JA, Byers JE, Pringle JM, Roman J (2010) A hitchhiker’s guide to the Maritimes: anthropogenic transport facilitates long-distance dispersal of an invasive marine crab to Newfoundland. Divers Distrib 16:879–891
Botto F, Iribarne O (1999) Effect of the burrowing crab Chasmagnathus granulata (Dana) on the benthic community of a SW Atlantic coastal lagoon. J Exp Mar Biol Ecol 241:263–284
Bousfield EL (1973) Shallow-water gammaridean amphipoda of New England. Comstock Publishing Associates, Ithaca, p 312
Bromley JEC, Bleakney JS (1984) Keys to the fauna and flora of Minas Basin. National Research Council of Canada Report 24119
Cohen AN, Carlton JT, Fountain MC (1995) Introduction, dispersal and potential impacts of the green crab Carcinus maenas in San Francisco Bay, California. Mar Biol 122:225–237
Commito JA, Currier CA, Kane LR, Reinsel KA, Ulm IE (1995) Dispersal dynamics of the bivalve Gemma gemma in a patchy environment. Ecol Monogr 65:1–20
Davis RC, Short FT, Burdick DM (1998) Quantifying the effects of green crab damage to eelgrass transplants. Restor Ecol 6:297–302
DePatra K, Levin LA (1989) Evidence of the passive deposition of meiofauna into fiddler crab burrows. J Exp Mar Biol Ecol 125:173–192
Dernie KM, Kaiser MJ, Warwick RM (2003) Recovery rates of benthic communities following physical disturbance. J Anim Ecol 72:1043–1056
DFO (Department of Fisheries and Oceans) (2009) Ecological Assessment of Irish Moss (Chondrus Crispus) in Basin Head Marine Protected Area. DFO Can Sci Advis Sec Sci Advis Rep 2008/059
Elner RW, Hughes RN (1978) Energy maximization in the diet o the shore crab Carcinus maenas. J Anim Ecol 47:103–116
Escapa M, Iribarne O, Navarro D (2004) Effects of the intertidal burrowing crab Chasmagnathus granulatus on infaunal zonation patterns, tidal behavior, and risk of mortality. Estuaries 27:120–131
Flach EC (2003) The separate and combined effects of epibenthic predation and presence of macro-infauna on the recruitment success of bivalves in shallow soft-bottom areas on the Swedish west coast. J Sea Res 49:59–67
Frid CLJ (1989) The role of recolonization processes in benthic communities, with special reference to the interpretation of predator-induced effects. J Exp Mar Biol Ecol 126:163–171
Garbary DJ, Miller AG, Williams J, Seymour NR (2014) Drastic decline of an extensive eelgrass bed in Nova Scotia due to the activity of the invasive green crab (Carcinus maenas). Mar Biol 161:3–15
Gehrels H, Knysh KM, Boudreau M et al (2016) Hide and seek: habitat-mediated interactions between European green crabs and native mud crabs in Atlantic Canada. Mar Biol 163:152. doi:10.1007/s00227-016-2927-6
Grant J (1981) Sediment transport and distribution on intertidal sandflat: infaunal distribution and recolonization. Mar Ecol Progr Ser 6:249–255
Gray JS, Elliott M (2009) Ecology of marine sediments, 2nd edn. Oxford University Press, Oxford
Gray JS, Wu RS-S, Or YY (2002) Effects of hypoxia and organic enrichment on the coastal marine environment. Mar Ecol Progr Ser 238:249–279
Gregory GJ, Quijón PA (2011) The impact of a coastal invasive predator on infaunal communities: assessing the roles of density and a native counterpart. J Sea Res 66:181–186
Griffiths J, Dethier MN, Newsom N, Byers JE, Meyer JJ, Oyarzun F, Lenihan H (2006) Invertebrate community responses to recreational clam digging. Mar Biol 149:1489–1497
Grosholz ED, Ruiz GM (1996) Predicting the impact of introduced marine species: lessons from multiple invasions of the European green crab Carcinus maenas. Biol Conserv 78:59–66
Hall SJ (1994) Physical disturbance and marine benthic communities: life in unconsolidated sediments. Oceanogr Mar Biol Annu Rev 32:179–239
Hiddink JG, Marijnissen SAE, Troost K, Wolff WJ (2002) Predation on the 0-group and older year classes of the bivalve Macoma balthica: the interaction of size selection and intertidal distribution of epibenthic predators. J Exp Mar Biol Ecol 269:223–248
Iribarne O, Bortolus A, Botto F (1997) Between-habitat differences in burrow characteristics and trophic modes in the southwestern Atlantic burrowing crab Chasmagnathus granulata. Mar Ecol Prog Ser 155:137–145
Jamieson GS, Grosholz ED, Armstrong DA, Elner RW (1998) Potential ecological implications from the introduction of the European green crab, Carcinus maenas (Linneaus), to British Columbia, Canada, and Washington, USA. J Nat Hist 32:1587–1598
Kaiser MJ (1998) Significance of bottom-fishing disturbance. Conserv Biol 12:1230–1235
Kvitek RG, Oliver JS, DeGange AR, Anderson BS (1992) Changes in Alaskan soft-bottom prey communities along a gradient in sea otter predation. Ecology 73:413–428
Le Calvez JC (1987) Location of the shore crab Carcinus maenas L., in the food web of a managed estuary ecosystem: The Rance Basin (Brittany, France). Sci Mar 51:431–442
Lee WJ (2010) Intensive use of an intertidal mudflat by foraging adult American horseshoe crabs Limulus polyphemus in the Great Bay estuary, New Hampshire. Curr Zool 56:611–617
Lenihan HS, Micheli F (2001) Soft-sediment communities. In: Bertness M, Hay M, Gaines S (eds) Marine community ecology. Sinauer, Sunderland, pp 253–287
Levin LA (1984) Life history and dispersal patterns in a dense infaunal polychaete assemblage: community structure and response to disturbance. Ecology 65:1185–1200
Lipcius RN, Hines AH (1986) Variable functional responses of a marine predator in dissimilar homogeneous microhabitats. Ecology 67:1361–1371
Lutz-Collins V, Quijón PA (2014) Animal-sediment relationships in an Atlantic Canada marine protected area: richness, composition and abundance in relation to sediment food indicators. Mar Biol Res 10:577–588
Magni P, Tagliapietra D, Lardicci C, Balthis L, Castelli A, Como S, Frangipane G, Giordani G, Hyland J, Maltagliati F, Pessa G, Rismondo A, Tataranni M, Tomassetti P, Viaroli P (2009) Animal-sediment relationships: evaluating the ‘Pearson–Rosenberg paradigm’ in Mediterranean coastal lagoons. Mar Poll Bull 58:478–486
Malyshev A, Quijón PA (2011) Disruption of essential habitat by a coastal invader: new evidence of the effects of green crabs on eelgrass beds. ICES J Mar Sci 68:1852–1856
Norkko A, Bonsdorff E (1996) Population responses of coastal zoobenthos to stress induced by drifting algal mats. Mar Ecol Prog Ser 140:141–151
Ólafsson EB, Peterson CH, Ambrose WG Jr (1994) Does recruitment limitation structure populations and communities of macroinvertebrates in marine soft sediments: the relative significance of pre- and post-settlement processes. Oceanogr Mar Biol Annu Rev 32:65–109
Oliver JS, Slattery PN (1985) Destruction and opportunity on the sea floor: effects of gray whale feeding. Ecology 66:1965–1975
Pacheco AS, Thiel M, Uribe RA, Campos L, Riascos JM (2013) Effects of sympatric predatory crabs Romaleon polyodon and Cancer plebejus (Decapoda, Brachyura, Cancridae) on sublittoral macrobenthic communities. J Exp Mar Biol Ecol 443:147–154
Palanques A, Guileen J, Puig P (2001) Impact of bottom trawling on water turbidity and muddy sediment of an unfished continental shelf. Limnol Oceanogr 46:1100–1110
Pearson TH, Rosenberg R (1978) Macrobenthic succession in relation to organic enrichment and pollution in the marine environment. Oceanogr Mar Biol Annu Rev 16:229–311
Peterson CH (1979) Predation, competitive exclusion, and diversity in the soft-sediment benthic communities of estuaries and lagoons. In: Livingston RJ (ed) Ecological processes in coastal and marine systems. Plenum Press, New York, pp 233–264
Pickering T, Quijón PA (2011) Potential effects of a non-indigenous predator in its expanded range: assessing green crab, Carcinus maenas (Linnaeus), prey preference in a productive shellfish area of Atlantic Canada. Mar Biol 158:2065–2078
Pillay D, Branch GM, Forbes AT (2007) Experimental evidence for the effects of the thalassinidean sandprawn Callianassa kraussi on macrobenthic communities. Mar Biol 152:611–618
Quijón PA, Jaramillo E (1996) Seasonal vertical distribution of the intertidal macroinfauna in an estuary of south-central Chile. Estuar Coast Shelf Sci 43:653–663
Quijón PA, Snelgrove PVR (2005) Differential regulatory roles of crustacean predators in a sub-arctic, soft-sediment system. Mar Ecol Progr Ser 285:137–149
Quijón PA, Snelgrove PVR (2008) Trophic complexity in marine sediments: new evidence from the Gulf of St. Lawrence. Mar Ecol Progr Ser 371:85–89
Reise K (2002) Sediment mediated species interactions in coastal waters. J Sea Res 48:127–141
Ronn C, Bonsdorff E, Nelson W (1988) Predation as a mechanism of interference within infauna in shallow brackish water soft bottoms: experiments with an infaunal predator Nereis diversicolor O.F. Muller. J Exp Mar Biol Ecol 116:143–157
Rosa S, Granadeiro JP, Vinagre C, França S, Cabral HN, Palmeirim JM (2008) Impact of predation on the polychaete Hediste diversicolor in estuarine intertidal flats. Estuar Coast Shelf Sci 78:655–664
Rossong M, Barrett T, Quijón PA, Snelgrove PVR, Mackenzie C, Locke A (2012) Regional differences in foraging behaviour and morphology of invasive green crab (Carcinus maenas) populations in Atlantic Canada. Biol Inv 14:659–669
Savidge WB, Taghon GL (1988) Passive and active components of colonization following two types of disturbance on and intertidal sandflat. J Exp Mar Biol Ecol 115:137–155
Schratzberger M, Warwick RM (1999) Impact of predation and sediment disturbance by Carcinus maenas (L.) on free-living nematode community structure. J Exp Mar Biol Ecol 235:255–271
Sharp G, Semple R, Connolly K, Blok R, Audet D, Cairns D, Courtenay S (2003) Ecological assessment of the Basin Head lagoon: a proposed marine protected area. Canadian manuscript report of fisheries and aquatic sciences, No 2641, p 78
Smith CR, Brumsickle SJ (1989) The effects of patch size and substrate isolation on colonization modes and rates in an intertidal sediment. Limnol Oceanogr 34:1263–1277
Snelgrove PVR, Butman CA (1994) Animal-sediment relationships revisited: cause versus effect. Oceanogr Mar Biol Annu Rev 32:111–177
Sokal RR, Rohlf FJ (2011) Biometry: the principles and practice of statistics in biological research. W. H. Freeman, New York, p 937
Thistle D (1980) The response of a harpacticoid copepod community to a small-scale natural disturbance. J Mar Res 38:381–395
Thrush SF (1986) Spatial heterogeneity in subtidal gravel generated by the pit-digging activities of Cancer pagurus. Mar Ecol Progr Ser 30:221–227
Thrush SF, Pridmore RD, Hewitt JE, Cummings VJ (1991) Impact of ray feeding disturbances on sandflat macrobenthos: do communities dominated by polychaetes or shellfish respond differently? Mar Ecol Progr Ser 69:245–252
Tummon Flynn P, Mellish C, Pickering TR, Quijón PA (2015) Effects of claw autotomy on green crab (Carcinus maenas) feeding rates. J Sea Res 103:113–119
VanBlaricom GR (1982) Experimental analysis of structural regulation in a marine sand community exposed to oceanic swell. Ecol Monogr 52:283–305
Volkenborn N, Robertson DM, Reise K (2009) Sediment destabilizing and stabilizing bio-engineers on tidal flats: cascading effects of experimental exclusion. Helgol Mar Res 63:27–35
Wilson WH Jr (1991) Competition and predation in marine soft-sediment communities. Annu Rev Ecol Syst 21:221–241
Acknowledgments
We thank J. Davidson and D. Giberson (UPEI) and K. Reise (AWI) in addition to an anonymous reviewer for their comments on earlier versions of this manuscript. The field and laboratory assistance of L. Rosenberg, M. Wadowski, A. Malyshev, J. Willis and M. Parent is also appreciated. This study was funded by a Natural Sciences and Engineering Research Council (NSERC) Discovery Grant and a MRG-UPEI grant both awarded to PAQ, and a NSERC PGS-M to VLC. RC was funded by the Canada Excellence Research Chairs Program. PAQ also wishes to acknowldege E. Jaramillo (UACH), who first instilled on him an interest on feeding pits and benthic ecology while working in southern Chile estuaries.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Informed consent
Consent was obtained from all participants in this study, and all required permissions were obtained to sample animals for the study.
Human and animal rights statement
All applicable international, national and/or institutional guidelines for the care and use of animals were followed. This study did not involve the use of human participants.
Additional information
Responsible Editor: F.T. Chan.
Reviewed by: K. Reise and an undisclosed expert.
This article is part of the Topical Collection on Invasive Species.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lutz-Collins, V., Cox, R. & Quijón, P.A. Habitat disruption by a coastal invader: local community change in Atlantic Canada sedimentary habitats. Mar Biol 163, 177 (2016). https://doi.org/10.1007/s00227-016-2947-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-016-2947-2