Abstract
Some key aspects of the reproductive strategy of the brown trout (Salmo trutta fario L.) in the Yadong River, Tibet, including spawning season, age at sexual maturity, fecundity and egg size, have been studied. The majority of the samples were less than 215 mm and age ranged from 1 to 4 in both sexes, indicating that the majority of the fish were younger and the pressure by overfishing was high. The spawning periodicity was determined to be between the end of October and January, mainly in November and December. The ratio of male to female brown trout population (1.29:1 with P > 0.05) suggested no sex significant differences, although males were significantly more abundant than females in October (P < 0.0001) on monthly basis. Age and size of males and females at maturity was different and males matured earlier than females. Fecundity was markedly correlated with their body weight (P < 0.001, r = 0.9255), standard length (P < 0.01, r = 0.8879), and gonad weight (P < 0.001, r = 0.9366). The mean size of mature eggs in the spawning season was: 4.0 ± 0.45 mm and tended to increase along with the female spawners size (P < 0.001, r = 0.9641). Further researches about the brown trout population in the Yadong River should be conducted on issues such as artificial reproduction, culture, conservation, management, and restocking.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The brown trout (Salmo trutta), family Salmonidae, is indigenous to Europe, North Africa, and western Asia (MacCrimmon et al. 1970), but it is now found world-wide (Klemetsen et al. 2003). The reproductive traits of brown trout have been described in geographically different populations previously (Hegge et al. 1991; Crisp 1994; Sorensen et al. 1995; Garcia-Vazquez et al. 2001; Nicola and Almodovar 2002; Pender and Kwak 2002; Alp et al. 2003; Estay et al. 2004; Olsen and Vollestad 2005; Rubin et al. 2005; Arslan and Aras 2007).
The brown trout (Salmo trutta fario L.) was first reported from the Yadong River, Tibet, in 1962 (Zhang and Wang 1962) although it was probably introduced into the river more than a hundred years ago. The population of this brown trout has decreased because of water pollution, overexploitation and damming for waterpower. Studies of such trout from the Yadong River were limited to their morphology (Zhang and Wang 1962) and our recent study of their embryonic development (Hao et al. 2006). Given the local economic importance of this fish (the most important economic source of local fishery and travel industry), the reproductive biology of the brown trout from the Yadong River, Tibet, has not hitherto been studied thoroughly.
The major objective of this study is to reveal the reproductive traits of the wild brown trout population in the Yadong River, Tibet, by investigating spawning season, age and size at sexual maturity, fecundity and egg size. We believe that such studies are deemed vitally important for the management of the local fishery and environmental protection.
Methods and materials
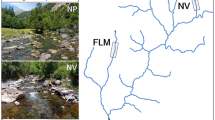
Sampling locations for brown trout were the mid-section of the Yadong River (Fig. 1), that originates from the south slope of Himalayas and flows into Brahmaputra River, India. The river length is over 60 km. The width and depth varied from 5 to 16 m and from 20 cm to 2.5 m, respectively. It runs throughout a sharp and narrow valley. There is bushy vegetation in both sides. The river bed is covered by boulders and cobbles. Water temperature fluctuated between 0°C (in winter) and 15°C (in summer). The water flow was between 1 and 12 m3 s−1 during the study period. The sampling region has altitudes from around 2,700 to 3,700 m above sea level. No fishes were captured above altitude 3,700 m.
We gathered data to investigate size and age at maturity for both male and female, spawning periodicity, sex ratios and fecundity. Brown trout samples were collected by using casting net (1 cm mesh size) for about 20 km along the riversides of the Yadong River from August 1999 to January 2003. Four hundred sixty-two individuals were collected. They were measured for total length (TL) and standard length (SL), with an accuracy of 0.5 mm (putting fish into a box and measuring the length with steel tape), body weight (Wb) with an accuracy of 0.5 g (weighing fish with an electronic balance). Three hundred two individuals were sacrificed and sex was determined visually or by microscopic examination of the gonads. After the gonads were weighted (Wg) with an accuracy of 0.1 g (weighing sample with an electronic balance), they were preserved in 95% ethanol and Bouin’s solution, respectively. The other 160 individuals were released after measuring the TL, SL and Wb. The developmental status of the gonads was investigated in six different stages, in which stage I was not observed in the current study; stage II and III were defined as immature, consisting of very young individuals; stage IV and V were considered as mature and stage VI was considered as post-mature (Yin 1995). In the laboratory, total egg number and average egg size (egg diameter, D) were determined for each female from a random sample of 20 eggs to nearest 0.1 mm with a digital micrometer for three times. Scales were used for estimating age (Rifflart et al. 2006). All specimens were preserved in 10% formalin and kept in the Museum of Freshwater Fishes, Institute of Hydrobiology, Chinese Academy of Sciences (IHB, CAS).
Spawning season was estimated from the gonadsomatic index (GSI) and gonad development status. Fecundity also was quantified as the GSI. The GSI was calculated from the equation: GSI = (Wg / Wb) × 100, where Wg is the gonad weight (gram) and Wb is the body weight (gram). Body weight, standard length, gonad weight and fecundity relationships were determined from the equations according to Alp et al. (2003):
Where F is the number of eggs (fecundity), SL, Wb, and Wg are the standard length (millimeter), body weight (gram) and gonad weight (gram), respectively, and p and q are constant parameters in linear regression analysis and pl = e p.
The data analysis was conducted using Statistic 6.0 and Excel. The number of fish was given for each test unless the whole data set was used. The chi-squared test was also performed to compare the sex ratio. One-way ANOVA and Duncan’s multiple comparison test were used to determine the difference of GSI. An independent t test was used to compare fecundities of different populations.
Results
Of the 462 individuals examined, 170 were males, 132 were females and 160 were not determined before they were released into the river. The standard length frequency distributions of these individuals were summarized in Fig. 2. Males ranged from 103.0 to 364.0 mm, with a mean standard length of 188.91 ± 52.259 mm and females ranged from 93.0 to 362.0 mm, with a mean standard length of 192.60 ± 68.581 mm. The majority of the samples (accounting for 68.40%) were comprised of individuals between 120.0–215.0 mm in length group (Fig. 2). Age ranged between 1 and 4 years old in males and females. The group of age 2 was dominant in both sexes (71.2% and 54.5% in male and in female, respectively).
Seasonal distribution of the maturity stages is shown in Table 1. Immature individuals (stage II and III) were observed in most months. Females with maturing ovaries (stage IV) appeared from June to December whereas males with maturing testis (stage IV) were seen from June to October. Fewer female fish were observed with mature ovaries (stage V) in October–December compared with male with mature testis (stage V) observed at the same seasons. The monthly distribution of the maturity stages confirmed that brown trout began to mature during the autumn (only one sample in October) and spawned mainly between November and December, although some individuals were observed to spawn in January (data not shown).
For the 302 specimens examined for sex determination, 33.3% females and 45.3% males were sexually mature. For females ages at first maturity were 3 to 4 years old, whereas for males ages at first maturity were about 2 to 3 years old. The youngest female mature brown trout was 3 years old, 242 mm standard length, in the Yadong River, whereas the youngest male mature fish was 2 years old, 168 mm standard length (Table 2).
In the current study, the sex ratio (males/females) of the Yadong brown trout population was 1.29:1 (chi-squared test, P > 0.05) and this is not significantly different from 1:1 ratio. The monthly changes of sex ratios were illustrated in Fig. 3. The sex ratios ranged from 1.2:1 to 4.43:1 in spawning seasons. There were no significant differences in sex ratios on monthly basis, with an exception in October (4.43:1, chi-squared test, P < 0.0001).
GSI values between October and December were significantly higher (P < 0.05) than other months (Fig. 4). The GSI in female brown trout ranged from 0.89 to 17.66. The maximum GSI value in females was observed in December, whereas the minimum value was in April. These results showed that sexual development accelerated in autumn (September, October and November) and reached the maximum in December.
Fecundity varied from 312 to 1,655 with a mean of 946.35 ± 363.07 based on 23 females. The size of eggs from 23 spawners ranged from 3.3 to 4.5 mm with a mean 4.0 ± 0.45 mm. The size of mature eggs trended to increase along with the size of the female spawners (Fig. 5). Some significant correlations were found between fecundity and body weight (P < 0.001), standard length (P < 0.01) and gonad weight (P < 0.001) (Fig. 6). These linear relationships may be expressed by the following regression equations:
Discussion
In the Yadong River, the standard length of the majority of the samples were less than 215 mm and age ranged from 1 to 4 years old in both sexes, indicating that the majority of the fish were younger and the pressure of overfishing was high. A similar situation has been cited for overfished brown trout populations elsewhere (Almodovar and Nicola 1999; Almodovar et al. 2002; Arslan and Aras 2007).
In the present study, age and size at maturity was different between the males and females in the Yadong River and males matured earlier than females, since females have higher energetic to mature (Euzenat et al. 1999). Similarly, there was considerable variation in age and size at maturity in brown trout among different populations in previous studies (Lobon-Cervia et al. 1986; Hegge et al. 1991; Olsen and Vollestad 2005). According to Olsen and Vollestad (2005), age and size at maturity varied between the sexes and males usually matured earlier than females, which was also agreeable with the early studies (Johnson 1989). There was evidence showing that within an area that a correlation between size and age was a good predictor of maturation, but for a given age and size a salmon parr was more likely to become sexually mature if it came from a high-altitude site (Baum et al. 2004). Some studies showed males may attain maturity from less than 100 mm in length at age 1 year old (Dellefors and Faremo 1988; L’Abée-Lund et al. 1990). Alp et al. (2003) reported that age of sexual maturity of brown trout living in Fırnız Stream was between 2 and 3 years old, the smallest mature male and female fishes were 174 and 178 mm, respectively. Recently, Arslan and Aras (2007) described that male and female brown trout reached maturity at the age of 1.99 and 3.19, and when they were 141 and 172 mm in the Anuri Stream while those in the Cenker Stream attained at age of 1.99 and 3.22 when they were 140 and 173 mm length, respectively.
The sex ratio males to females of Yadong River brown trout population was 1.29:1 (P > 0.05), suggesting no significant difference. In general, the sex ratio is expected to be 1:1 in closed populations(Nikolsky 1963), though some factors (such as food availability, spawning season, spawning ground and so on) could influence sex ratio (Alp et al. 2005). Arslan and Aras (2007) also reported that the sex ratios for brown trout were 0.94:1 and 0.97:1 in the Anuri and the Cenker streams, respectively, indicating a significant difference in the numbers of males and females. However, males were significantly more numerous than females in October (P < 0.0001) on monthly basis in our study. For the case, the limited number of samples was insufficient to draw a reliable conclusion.
Our data displayed that the spawning periodicity was between the end of October and January (autumn–winter period), mainly concentrated in November and December, being consistent with the general pattern described. As has been documented in the literature on this subject, the spawning time in brown trout occurs during the autumn–winter period (Hobbs 1937; Needham et al. 1945; Horton 1961; Thomas 1964; Hopkins 1970). Some more recent reports also indicate similar spawning periodicity (Pender and Kwak 2002; Alp et al. 2003; Estay et al. 2004; Rubin et al. 2005; Arslan and Aras 2007).
This study observed positive correlations between female length, body weight, gonad weight with fecundity in Yadong brown trout populations. Similarly, high correlation was also found between female size and salmonid fecundity in relation to fish length in an earlier study (Taube 1976). Nicola and Almodovar (2002) also described a significant relationship between female length and fecundity in brown trout populations in seven study streams in Spain. According to Olsen and Vollestad (2003), fecundity increased with fish length and there was no consistent difference in fecundity, based on comparing all eight populations. Estay et al. (2004) also reported that analyses of female body weight during spawning and total fecundity over three seasons revealed a positive linear correlation, that is heavier females produced more eggs. However, the fecundity for Yadong brown trout was different (higher or lower) from other reports (Nicola and Almodovar 2002; Alp et al. 2003; Olsen and Vollestad 2003; Estay et al. 2004; Arslan and Aras 2007), possibly caused by different factors like altitude, water temperature, age, and food availability.
In addition, this study showed the size of mature eggs tended to increase along with the female spawners size. Variation in egg mass and fecundity among the populations studied may result largely from selectively different environmental factors, of which water temperature was considered the most probable selective factor responsible for this variation (Jonsson and Jonsson 1999). However, egg size was strongly influenced by environmental factors, and individual fish produces eggs of different size at different reproductive conditions (Kamler 1992; Chambers and Waiwood 1996). Variation in egg size among salmonid populations may represent local adaptations (Fleming and Grossm 1990). There were some evidences that such variation was heritable (Gall 1975; Gjerde 1986), but egg size was also a plastic trait and it can be influenced by maternal feeding conditions (Jonsson and Jonsson 1999) and maternal size (L’Abée-Lund and Hindar 1990; Heath et al. 1999).
In conclusion, the majority of fish from the Yodong River were younger and age classes were simple, suggesting that this population is threatened, so some protective measures should be taken to prevent capturing the fish in spawning period (from October to January). Further studies should be conducted on artificial reproduction, culture, conservation, management, and restocking to improve our understanding of how to protect, restore and enhance brown trout population in that river.
References
Almodovar A, Nicola GG (1999) Effects of a small hydropower station upon brown trout Salmo trutta L. in the River Hoz Seca (Tagus basin, Spain) one year after regulation. Regul Rivers Res Mgmt 15:477–484
Almodovar A, Nicola GG, Suarez J (2002) Effects of fishery management on populations of brown trout Salmo trutta, in Central Spain. In: Collares-Pereira M, Coelho M, Cowx I (eds) Conservation of freshwater fishes: options for the future. Fishing News Books, Oxford, pp 337–345
Alp A, Kara C, Buyukcapar HM (2003) Reproductive biology of brown trout, Salmo trutta macrostigma Dumeril 1858, in a tributary of the Ceyhan River which flows into the eastern Mediterranean Sea. J Appl Ichthyol 19:346–351
Alp A, Kara C, Buyukcapar HM (2005) Age, growth and diet composition of the resident brown trout, Salmo trutta macrostigma Dumeril 1858, in Flrnlz Stream of the River Ceyhan, Turkey. Turk J Vet Anim Sci 29:285–295
Arslan M, Aras NM (2007) Structure and reproductive characteristics of two brown trout (Salmo trutta) populations in the Coruh river basin, north-eastern Anatolia, Turkey. Turk J Zool 31:185–192
Baum D, Laughton R, Armstrong JD, Metcalfe NB (2004) Altitudinal variation in the relationship between growth and maturation rate in salmon parr. J Anim Ecol 73:253–260
Chambers RC, Waiwood KG (1996) Maternal and seasonal differences in egg sizes and spawning characteristics of captive Atlantic cod, Gadus morhua. Can J Fish Aqua Sci 53:1986–2003
Crisp DT (1994) Reproductive investment of female brown trout, Salmo trutta L., in a stream and reservoir system in northern England. J Fish Biol 44:343–349
Dellefors C, Faremo U (1988) Early sexual maturation in males of wild sea trout, Salmo trutta L., inhibits smoltification. J Fish Biol 33:741–749
Estay FJ, Noriega R, Ureta JP, Martin W, Colihueque N (2004) Reproductive performance of cultured brown trout (Salmo trutta L.) in Chile. Aqua Res 35:447–452
Euzenat G, Fournel F, Richard A (1999) Sea trout (Salmo trutta L.) in Normandy and Picardy. In: Baglinière JL, Maisse G (eds) Biology and ecology of the brown trout and sea trout. Springer-Praxis Series in Aquaculture and Fisheries, Chichester, UK, pp 175–205
Fleming IA, Gross MR (1990) Latitudinal clines: a trade-off between egg number and size in Pacific salmon. Ecology 71:1–11
Gall GAE (1975) Genetics of reproduction in domesticated rainbow trout. J Anim Sci 40:19–28
Garcia-Vazquez E, Moran P, Martinez JL, Perez J, De Gaudemar B, Beall E (2001) Alternative mating strategies in Atlantic salmon and brown trout. J Heredity 92:146–149
Gjerde B (1986) Growth and reproduction in fish and shellfish. Aquaculture 57:37–55
Hao FH, Chen YF, Cai B (2006) Embryonic development of Salmo trutta fario from Yadong River, Tibet. J Fish China 30(3):289–296
Heath DD, Fox CW, Heath JW (1999) Maternal effect on offspring size: variation through early development of chinook salmon. Evolution 53:1605–1611
Hegge O, Dervo BK, Skurdal J (1991) Age and size at sexual maturity of heavily exploited Arctic char and brown trout in Lake Atnsjo, southeastern Norway. Trans Am Fish Soc 120(2):141–149
Hobbs DF (1937) Natural reproduction of quinat salmon, brown and rainbow trout in certain New Zealand waters. Fish Bull New Zealand 6:1–104
Hopkins CL (1970) Some aspects of the bionomic fish in the brown trout nursery stream. Fish Res Bull New Zealand 4:1–38
Horton PA (1961) The bionomics of brown trout in a Dartmoor stream. J Anim Ecol 30:311–338
Johnson L (1989) The anadromous Arctic charr, Salvelinus alpinus of Nauyuk Lake, N.W.T., Canada. Physiol Ecol Japan 1(sp 1):201–228
Jonsson N, Jonsson B (1999) Trade-off between egg mass and egg number in brown trout. J Fish Biol 55:767–783
Kamler E (1992) Early life history of fish. An energetics approach. Chapman and Hall, London
Klemetsen A, Amundsen PA, Dempson JB, Jonsson B, Jonsson N, O’Connell MF, Mortensen E (2003) Atlantic salmon Salmo salar L., brown trout Salmo trutta L. and Arctic charr Salvelinus alpinus (L.): a review of aspects of their life histories. Ecol Freshw Fish 12:1–59
L’Abée-Lund JH, Hindar K (1990) Interpopulation variation in reproductive traits of anadromous female brown trout, Salmo trutta L. J Fish Biol 37:755–763
L’Abée-Lund JH, Jensen AJ, Johnsen BO (1990) Interpopulation variation in male parr maturation of anadromous brown trout (Salmo trutta) in Norway. Can J Zool 68:1983–1987
Lobon-Cervia J, Montanes C, De Sostoa A (1986) Reproductive ecology and growth of a population of brown trout (Salmo trutta L.) in an aquifer-fed stream of Old Castile (Spain). Hydrobiologia 135(1–2):81–94
MacCrimmon HR, Marshall TL, Gotos BL (1970) World distribution of brown trout, Salmo trutta: further observations. J Fish Res Board Can 27:811–818
Needham PR, Moffert JW, Slater AW (1945) Fluctuations in wild brown trout populations in Convict Creek, California. J Wildl Manage 9:9–15
Nicola GG, Almodovar A (2002) Reproductive traits of stream-dwelling brown trout Salmo trutta in contrasting neighbouring rivers of central Spain. Freshwat Biol 47:1353–1365
Nikolsky GW (1963) The ecology of fishes. Academic, London
Olsen EM, Vollestad LA (2003) Microgeographical variation in brown trout reproductive traits: possible effects of biotic interactions. Oikos 100:483–492
Olsen EM, Vollestad LA (2005) Small-scale spatial variation in age and size at maturity of stream-dwelling brown trout, Salmo trutta. Ecol Freshw Fish 142:202–208
Pender DR, Kwak TJ (2002) Factors influencing brown trout reproductive success in Ozark tailwater rivers. Trans Am Fish Soc 131:698–717
Rifflart R, Marchand F, Rivot E, Bagliniere JL (2006) Scale reading validation for estimating age from tagged fish recapture in brown trout (Salmo trutta) population. Fish Res 78:380–384
Rubin JF, Glimsater C, Jarvi T (2005) Spawning characteristics of the anadromous brown trout in a small Swedish stream. J Fish Biol 66:107–121
Sorensen PW, Cardwell JR, Essington T, Weigel DE (1995) Reproductive interactions between sympatric brook and brown trout in a small Minnesota stream. Can J Fish Aqua Sci 52:1958–1965
Taube CM (1976) Sexual maturation and fecundity in brown trout of the Platte river, Michigan. Trans Am Fish Soc 4:529–533
Thomas JD (1964) Study on the growth of trout, Salmo trutta from four contrasting habitats. Proc Zool Soc London 142:459–510
Yin MC (1995) Fish ecology. China Agriculture Press, Beijing, pp 105–131
Zhang CL, Wang WB (1962) A preliminary report on the fishes from Tibet. Acta Zoologica Sinica 14:529–536
Acknowledgements
This study was supported financially by the National Natural Science Foundation of China (Grant No. 30123004), the Knowledge Innovation Program of the Chinese Academy of Sciences (Grant No. KSCX2-SW-125) and Tibet Autonomous Region Program. Huiru Tang and Fuqiang Xu of Wuhan Institute of Physics and Mathematics are acknowledged for his help in critical reading of the manuscript. We also acknowledge helpful comments and suggestions from referees and editors which enable the manuscript to be improved substantially.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hao, F., Chen, Y. The reproductive traits of brown trout (Salmo trutta fario L.) from the Yadong River, Tibet. Environ Biol Fish 86, 89–96 (2009). https://doi.org/10.1007/s10641-008-9363-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-008-9363-5