Abstract
The abundance and size-structure of a formerly allopatric brown trout (Salmo trutta L.) population in an inlet stream to a small lake were monitored for 9 years (1987–1995) after the first observation of introduced pike (Esox lucius L.), and revisited 18 years later (2013). All age groups of brown trout were reduced after the pike introduction, especially older fish of age-≥2+, but less so for age-0+ and 1+ fish. We suggest that the decline of older brown trout is mainly due to the high predation pressure from pike when migrating into the adjacent lake to feed. Young stream-dwelling pike of age-0+ and 1+ which ranged between 6 and 27 cm in length may also exert a predation pressure on juvenile brown trout that remains in the stream.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Introduction of non-native species is considered a serious threat to native biodiversity (Davis, 2009), and the establishment of a new species may cause serious disruption in the recipient ecosystem (e.g. Moyle, 1999). In some cases, it may be an event tipping the system into a new stable state (cf. Roy et al., 2010, Sandlund et al., 2013a, b). The invading species may affect the native species populations in various ways: constitute a serious competitor for food and habitat (space), be a new potential prey, or be a predator.
Several studies have shown that pike (Esox lucius L.) is a selective and effective predator with regard to prey species and fish size (Shafi & Maitland, 1971; Eklöv & Hamrin, 1989; Robinson, 1989; Adams, 1991; Sharma & Borgstrøm, 2008). Pike might include invertebrates in their diet, but when suitable fish prey is available, the species is mainly piscivorous (Chapman et al., 1989; Chapman & Mackay, 1990). It may become piscivorous from a very early stage (Raat, 1988), as showed in Windermere where fish prey was detected in pike from 35 mm in length (Frost, 1954). Through predation, pike may influence the abundance and population structure of fish prey populations in lakes (Kipling, 1984; Rahel, 1984; Mills & Hurley, 1991; Hein et al., 2014). The impact of pike may, however, be context dependent and have been shown to vary with both lake size and morphology, climate (Hein et al., 2014), and species composition (Mann et al., 1989). Due to temperature dependent predation efficiency and the reliance on shallow littoral areas for effective hunting, brown trout (Salmo trutta L.) will generally only coexist with pike in large cold-water lakes (Hein et al., 2014). However, it has been shown that sea trout may survive in an inlet river to a shallow Danish reservoir with pike as well as pikeperch (Stizostedion L.), although their mortality during smolt migration was high (Jepsen et al., 2000). Thus, tributary streams may serve as refuge for brown trout in systems containing pike.
There is a general lack of detailed case studies documenting the impact of fish introductions on other fish species in communities with few species (but see, e.g., Museth et al., 2006; Winfield et al., 2011; Sandlund et al., 2013a, b). Moreover, few studies have documented the impact of introduction of pike over time, from the moment of first occurrence to a new stable state. In national strategies to prevent human-mediated spreading of invasive species, it is important to document case studies covering different species and fish communities, in order to provide concrete and convincing arguments.
In several parts of Norway, many illegal introductions of pike have occurred in recent decades (Hesthagen & Sandlund, 2012). Most lakes subjected to these introductions contain few species of fish, in many cases only brown trout. Brown trout utilizes nursery streams while young, and as they grow, most fish leave for the better feeding habitat in their home lake (Jonsson, 1985; Jonsson & Jonsson, 1993). However, a certain component of the population usually remains in the nursery stream and becomes small sized, stationary, sexually mature fish (Jonsson & Jonsson, 2011).
In this study, we describe the impact of introduced pike on an allopatric population of brown trout in an inlet spawning and nursery stream to a small lowland Norwegian lake. We hypothesized that the brown trout would be seriously affected through predation from pike. We also hypothesized that the larger fish, that leave the stream to feed in the lake, will suffer higher mortality rates than younger fish that may remain in the stream.
Study area
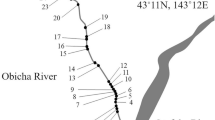
The study was carried out in Skjeltjønna Beck, the inlet stream to Lake Skjeltjønna, situated in Sør-Trøndelag county about 20 km east of Trondheim in central Norway, in the Sagelva river system, at 63°22′43.45″–10°40′34.39″. The drainage area consists mainly of pine and spruce forest, with some bog areas. The anthropogenic impact in the catchment area is very limited as there is no cultivated land, only an unpaved road and some logging, but not in close proximity to Skjeltjønna Beck. The stream runs for 670 m from Lake Mørkdalstjønna to Lake Skjeltjønna at altitudes of 221 and 209 m a.s.l., respectively. Thus, the gradient is moderate (2.8%). The stream has an average width of 3–4 m, with a water-covered area of approximately 2,200 m2 at normal summer flow. The stream habitat is dominated by riffles with depths mainly between 10 and 30 cm, and a coarse gravel substratum of 5–15 cm in diameter. The stream contains only three deeper pools of 50–70 cm in depth, each covering an area of 4–6 m2. The stream is low in nutrients with a conductivity and calcium concentration of 15.0 µS cm−l and 1.2 mg l–l, respectively. Lake Skjeltjønna has a surface area of 3.65 ha with dominating depths of 2–5 m, and with the deepest area at 9.5 m. The water has a high humic content with a secchi disk transparency of 3.0 m only. Approximately 20% of the lake surface area is covered with aquatic vegetation along the shoreline during summer and early fall, mainly water lilies, horsetails, and sedges. There were no detectable temporal trends in air temperatures or precipitation at Lake Skjeltjønna during the study period (1987–2013), by analyzing linear effect of monthly average temperature and precipitation adjusted for seasonal effects (P ≥ 0.280, based on interpolated data, ftp://ftpvm.met.no/projects/klimagrid/).
The lakes and streams in the Sagelva river system previously supported only brown trout, in addition to Arctic charr (Salvelinus alpinus L.) in two lakes. However, during the late 1970s, pike was illegally introduced into a couple of lakes in the lower part of the water system (Hesthagen & Sandlund, 2012). During the 1980s and 1990s, pike was further introduced into several lakes in the upper part of the water system.
The introduction of pike into Lake Skjeltjønna probably occurred in 1986. The species was first observed during beach seining in the spring of 1987, when one larger specimen weighing 3.0 kg was caught. Then, only 0+ pike was caught in Skjeltjønna Beck in August that same year. Lake Skjeltjønna supported a dense population of small-sized brown trout in 1987, with a catch of 11.4 specimens per 100 m2 net area (n = 74) during a 6-h period at day time with one standard gill net series of 10–45 mm mesh size knot to knot. Test-fishing employing the same gill netting effort and series during one night in the following 4 years showed that this lentic brown trout population was virtually extinct by 1991 as no brown trout was caught that year (Hesthagen et al., unpublished data). Skjeltjønna Beck is the only spawning and nursery stream for brown trout in Lake Skjeltjønna.
Materials and methods
We sampled fish in Skjeltjønna Beck by means of a portable backpack electrofishing apparatus (1,600 V, DC unloaded) in late August or early September, 1987–1995. The stream was revisited in September 2013 to check for any long-term changes. However, we did not include the data from 2013 in our analysis, but showed it in the figures. The sampling was carried out at normal water flow, and at water temperatures ranging between 12.4 and 15.1°C. Population estimates with 95% confidence limits were carried out by a mark-recapture experiment, using the adjusted Petersen method (Ricker, 1975): N = (M + l) × (C + l)/(R + 1), where N is the number of fish in the population, M is the number captured and marked in the first sampling day, C is the number caught during the second sampling day, and R is the number of marked fish recaptured. Estimates were performed for brown trout in age-classes 0+, 1+ and ≥2+, which were separated by means of length-frequency distribution and some length at age data. Estimates for pike were performed for age-classes 0+ and 1+ combined. The stream was divided into six sections of 100 m in length, and one uppermost section of 70 m length. Electrofishing was done separately for each section by a single electrofishing run. Each fish was measured for total length to the nearest mm and marked by cutting off a small part of the caudal fin. The fish were held in a cage in the stream for about 30 min to 1 h to check for short term mortality. This affected fewer than 15 specimens each year, which were excluded from the number of marked fish (cf. Table 1). All other fish were released back into their original stream section. The same sampling procedure was carried out one day later to check for marked specimens. A small sample of fish was then removed from each section for age analysis.
Results
Abundance of brown trout
The abundance of age-0+ and 1+ brown trout in Skjeltjønna Beck varied considerably during the study period, between 30 (1993) to 3130 specimens (1989) and 25 (1993) to 636 specimens (1987), respectively (Table 1). However, no significant change in their density over the investigated period was found, based on data from 1987 to 1995 ( Fig. 1a, b). However, among specimens of age-≥2+, there has been a significant reduction in abundance during the same period (Fig. 1c). However, the reduction in abundance continued onward to 2013 for all three age groups. Furthermore, the abundance of age 1+ brown trout was related to the density of the same cohort (age-0+) in the previous year (Fig. 2).
Densities of brown trout for age groups 0+ (a), 1+ (b) and ≥2+ (c) during the period 1987–1995 in Skjeltjønna Beck. Data from 2013 are included for visual comparison only. Solid lines are based on estimated least square regression between brown trout density (log transformed) and year (ln ≥2+ = 507.97 −0.25 year, F 1,7 = 19.69, P < 0.023, R 2 = 0.50). No significant time trend was detected for 0+ (F 1,7 = 0.56, P = 0.475, R 2 = 0.01) and 1+ (F 1,7 = 0.32, P = 0.107, R 2 = 0.23)
Density of brown trout in age-class 1+ versus density of the same age-class as 0+ in the previous year from Skjeltjønna Beck, 1987–1995. Gray line is least square fitted Beverton–Holt stock-recruitment function; 1+ = α0+ /(β + 0+), with the fitted parameter α = 430.0 (P = 0.010) and β = 594.9 (P = 0.189)
Population structure and size of brown trout
As indicated from the abundance data, the number of the largest specimens has been strongly reduced during the study period (Fig. 3). The length distribution and age at length data allow us to define a cutoff point between age-0+ and older fish, which facilitates estimation of the annual mean length of age-0+ fish (cf. Fig. 3). Their lengths ranged between 52 and 66 mm, and have increased significantly over the sampling period (Fig. 4a). Furthermore, their lengths correlated negatively with cohort densities (Fig. 4b).
Length frequency distribution (%) of brown trout caught in Skjeltjønna Beck, 1987–1995, and 2013. All fish caught during the first sampling day and unmarked specimens from the second day are included (N). Vertical grey lines and associated number indicate visually determined cutoff lengths (in mm) between age 0+ and older specimens in the catches
a Mean lengths of age 0+ brown trout in Skjeltjønna Beck from 1987 to 1995, and in 2013, and b mean lengths of age 0+ brown trout versus cohort abundance (N 0+) in Skjeltjønna Beck. Error bars indicate 95% confidence intervals of means. Grey lines are least square regression between a 0+ length (L0+) and sampling year (ln L0+ = −115.58 + 15.75 lnYear, F 1,8 = 6.41, P = 0.035, R 2 = 0.36), and b 0+ length and estimated 0+ density (N0+) (L0+ = 4.27 − 0.05 ln N0+, F 1,8 = 4.96, P = 0.056, R 2 = 0.31)
Abundance and size of pike
Pike of age-0+ and 1+ was caught in Skjeltjønna Beck, with a variation in numbers between 2 and 53 individuals during the study period (Table 1). Specimens in these two age groups ranged in length of 6–12 and 14–27 cm, respectively (Fig. 5). In 1987, seven fry (age-0+) were caught, ranging between 82 and 116 mm in length (mean ± standard deviation: 110 ± 10). In 1988, 17 specimens were caught, ranging between 137 and 200 mm in length (mean ± standard deviation: 168 ± 17), all belonging to age group 1+. In 2013, when pike densities were higher, specimens in age-0+ cohort ranged between 72 and 118 mm in length (mean ± standard deviation: 93 ± 11).
Discussion
We demonstrated that the abundance of brown trout in Skjeltjønna Beck became strongly reduced during a period of 9 years from 1987 to 1995, after the introduction of pike in the adjacent lake, Lake Skjeltjønna. A revisit 18 years later confirmed the development in population abundance. Test-fishing in Lake Skjeltjønna with gill nets has shown that the native brown trout population have been wiped out. A component of brown trout survived in Skjeltjønna Beck although all age groups have been strongly reduced, especially specimens of age ≥2+. Immigration of brown trout to our study stream does not occur, as test-fishing in the two upstream lakes with tributary streams have shown that their populations of brown trout have been wiped out (Hesthagen et al., unpublished data), probably also due to the introduction of pike.
At the start of this study, in 1987, both Lake Skjeltjønna and Skjeltjønna Beck sustained a relatively dense population of brown trout. However, over the next 4 years, until 1991, brown trout in the lake was virtually extirpated. This was verified based on a test-fishing in 2013 (Hesthagen et al., unpublished data). Pike was already in the system in 1987, as demonstrated by the 3 kg specimen caught in the beach seine in Lake Skjeltjønna that year, and the presence of age-0+ pike in Skjeltjønna Beck the same year. Thus, it seems reasonable to assume that the age-0+ fish represented the first pike cohort hatched in the system. Thus, the introduction of pike probably involved a few larger specimens in 1986, with first spawning in the spring of 1987.
We argue that the decline in the brown trout population is due to the impact of the development of the invasive pike population, representing the major environmental change in the system. Because Lake Skjeltjønna is a shallow and small lowland lake (3.65 ha), brown trout can hardly avoid predation from pike. However, separation of the effects of different environmental changes may be particularly difficult in long-term data series (Smith et al., 1993), as several environmental factors may vary over time and confound our attempted cause–effect analysis. However, there were no detectable temporal trends in air temperatures or precipitation at Lake Skjeltjønna during the study period (1987–2013). Our study site has also very low nutrient contents. Thus, no effects of eutrophication or any changes in the trophic state are likely to have occurred. Further, no encroachments have taken place during the study period, either in the catchment area nor in the stream, that might have caused any changes in water quality or in the stream bed.
However, to conclusively demonstrate the impact of some environmental perturbation on an ecosystem or on species populations, a proper BACI-design (before–after-control-impact) of the investigation would normally be required (Smith, 2013). This is often not possible, as the perturbation happens outside the control of the investigator (Stewart-Oaten et al., 1992). Data on relevant environmental parameters may also be lacking, due to budgetary and logistic constraints, as well as to a deficient understanding of the system under scrutiny when the monitoring programme was initiated (cf. Mayer-Pinto et al., 2012). From reviewing other environmental factors, we argue by causal inference (cf. Beyers, 1998) that no other environmental perturbation has been likely to produce the observed decline in brown trout population in this water system. As it is not possible to apply a proper BACI-design in this case, we will refer to data on the temporal development of brown trout in areas where no invasion of pike has occurred. First, an assessment of brown trout populations in lakes in Sør-Trøndelag county, in the project Nature index of Norway, which included statistically selected lakes only (n = 30), no change in their abundance was recorded during recent decades (Hesthagen et al., 2010; Schartau et al., 2010). Second, test-fishing in Lake Atnsjøen, which is located some 300 km south of our study area, indicated no changes in the abundance of either brown trout or Arctic charr during the period between 1985 and 2013 (Saksgård & Hesthagen, 2004, 2014).
It has previously been shown, that piscivorous fish may cause the extirpation of populations of predation-intolerant species (e.g. Harvey, 1981; Rahel, 1984; Englund et al., 2009). Some analyses seem to indicate that brown trout cannot coexist with pike (Spens & Ball, 2008). Similar, a Swedish study showed that when pike was introduced in small and warm lakes, brown trout populations were exterminated (Hein et al., 2014). However, the two species do coexist in relatively large natural lakes, for example in southeastern Norway (Hesthagen & Sandlund, 2012). This is also the case for sea trout to a Danish reservoir (Jepsen et al., 2000). However, due to its slender body shape, brown trout is a preferred prey species for pike in general (Hambright et al., 1991). The small-sized brown trout in Lake Skjeltjønna, with few individuals larger than 25 cm, was probably highly vulnerable to predation from pike. It has been shown that pike may take prey up to a size of more than 50% of their own body length. Thus, even adult brown trout in our study system would be vulnerable to predation from pike as small as 30–35 cm in length, or age-2+ and older (Diana, 1979). Moreover, the habitat use of brown trout in lakes do to a large extent overlap with that of pike, since both species to a large degree prefer littoral areas (Diana et al., 1977). Lake Skjeltjønna is shallow, with substantial areas covered by higher aquatic vegetation,
We expect that brown trout which remained stationary in Skjeltjønna Beck would be less vulnerable to pike predation, as the stream is a less suitable habitat for pike. However, some juvenile pike of age-0+ and 1+ also occupy Skjeltjønna Beck. Although these are small specimens, and also relatively few in number, they are probably able to exert a certain predation pressure on the youngest age groups of brown trout (cf. Frost, 1954). The size of age 0+ brown trout in the stream typically ranged between 4 and 6 cm in length, which increased significantly over the sampling period, probably due to reduced density of conspecifics. However, this increase in body length among young brown trout may only marginally reduce their predation risk from pike in the stream, as age-1+ pike ranged in size between 14 and 27 cm.
Stream-resident brown trout use different parts of a stream during ontogeny; fry and small juveniles dwell in shallow gravelly rapids, whereas larger individuals often migrate to deeper areas like pools and backwaters (Bohlin, 1977; Näslund et al., 1998). In Skjeltjønna Beck, the three small pools available would originally have been the preferred habitat for age-1+ and older brown trout. However, pike would prefer the pools as well, and as age-1+, and even older brown trout in Skjeltjønna Beck are smaller than 10 cm, they would in most years face a high risk of pike predation in the pools. It has been shown that the presence of pike may cause brown trout to decrease their use of pools, the habitat in which pike occurred, and increase their use of other habitats (Greenberg, 1992). The impact from pike has been less dramatic on age-0+ and 1+ brown trout than on older fish in Skjeltjønna Beck, probably because they to some extent avoid predation by staying in the rapids. In our study system, specimens that leave the stream will be subjected to an even higher predation pressure in Lake Skjeltjønna. Brown trout may temporarily be forced to leave such small and shallow streams because of droughts (cf. Elliott, 1994). Effects of droughts are considered to be of less importance as a limiting factor for young brown trout in our case. First, Skjeltjønna Beck is fed by two lakes located further upstream. Second, there was no significant temporal trends in precipitation at Lake Skjeltjønna during the study period (1987–2013), indicating that drought have not been of vital importance.
Our analysis of stomach content from young pike caught in Skjeltjønna Beck revealed only aquatic insects (unpublished data). Pike is not entirely piscivorous (Chapman et al., 1989), but rather an opportunistic predator, taking whatever prey is most easily available (Chapman et al., 1989; Sepulveda et al., 2013). Mills (1964) recorded a marked seasonal variation in the diet of pike. In River Avon, juveniles (age-0+) ate mainly invertebrates and minnow. However, Mann (1985) attributed the absence of brown trout in pike stomachs to limited natural recruitment and thereby low availability of age-0+ fish. Several investigations have shown that the presence of pike strongly affects the behavior and habitat use of brown trout juveniles in streams (Greenberg et al., 1997; Vehanen & Hamari, 2004).
We observed great changes in the population structure of brown trout in Skjeltjønna Beck after the introduction of pike. While the number of age-0+ and 1+ fish did not exhibit a significant decline over the years from 1987 to 1995, older fish did so. Further, densities of age-1+ brown trout were significantly correlated with that of the same cohort in the previous year. This is in accordance with the results obtained for migratory brown trout in Black Brows Beck in England, where the number of alevins at the start of each year-class was the chief factor affecting the subsequent number of survivors throughout the life cycle (Elliott, 1984, 1985).
The reduction in density of larger brown trout in the stream is probably also related to the fact that almost no adult specimens would return from the lake to spawn in the stream, as the lake component of the stock was virtually wiped out. However, because there was no significant reduction in age-0+ densities over the years in the stream, it seems that the remaining stationary spawning stock produces a sufficient number of fry to prevent the population from going extinct. The significant reduction in the density of older stream-dwelling brown trout, however, may be a combined effect of predation by pike in the stream and in the lake. Thus, it may be expected that the future long-term impact on the brown trout population is a continuous decline. Data from 2013, compared with that from 1987 to 1995, strongly suggest such a development.
References
Adams, C. E., 1991. Shift in pike, Esox lucius L., predation pressure following the introduction of ruffe, Gymnocephalus cernuus (L.) to Loch Lomond. Journal of Fish Biology 38: 663–667.
Beyers, D. W., 1998. Causal inference in environmental impact studies. Journal of the North American Benthological Society 17: 367–373.
Bohlin, T., 1977. Habitat selection and intercohort competition of juvenile sea-trout, Salmo trutta. Oikos 29: 112–117.
Chapman, L. J. & W. C. Mackay, 1990. Ecological correlates of feeding flexibility in northern pike (Esox lucius). Journal of Freshwater Ecology 5: 313–322.
Chapman, L. J., W. C. Mackay & C. W. Wilkinson, 1989. Flexibility in northern pike (Esox lucius): Fish versus invertebrate prey. Canadian Journal of Fisheries and Aquatic Sciences 46: 666–669.
Davis, M. A., 2009. Invasion Biology. Oxford University Press, Oxford, UK: 244.
Diana, J. S., 1979. The feeding pattern and daily ration of a top carnivore, the northern pike (Esox lucius). Canadian Journal of Zoology 57: 2121–2127.
Diana, J. S., W. C. Mackay & M. Ehrman, 1977. Movement and habitat preference of northern pike (Esox lucius) in Lac Ste. Anne, Alberta. Transactions of the American Fisheries Society 106: 560–565.
Eklöv, P. & S. F. Hamrin, 1989. Predatory efficiency and prey selection: Interactions between pike Esox lucius, perch Perca fluviatilis and rudd Scardinus erythrophthalmus. Oikos 56: 149–156.
Elliott, J. M., 1984. Numerical changes and population regulation in young migratory trout Salmo trutta in a Lake District stream. Journal of Animal Ecology 53: 327–350.
Elliott, J. M., 1985. Population regulation for different life stages of migratory trout Salmo trutta in a Lake District stream. Journal of Animal Ecology 54: 617–638.
Elliott, J. M., 1994. Quantitative Ecology and the Brown Trout. Oxford University Press, Oxford.
Englund, G., F. Johansson, P. Olofsson, J. Salonsaari & J. Öhman, 2009. Predation leads to assembly rules in fragmented fish communities. Ecological Letters 12: 663–671.
Frost, W. E., 1954. The food of pike (Esox lucius L.) in Windermere. Journal of Animal Ecology 23: 339–360.
Greenberg, L. A., 1992. The effect of discharge and predation on habitat use by wild and hactchery brown trout (Salmo trutta). Regulated Rivers: Research & Management 7: 205–212.
Greenberg, L. A., E. Bergman & G. Eklöv, 1997. Effects of predation and intraspecific interactions on habitat use and foraging by brown trout in artificial streams. Ecology of Freshwater Fish 6: 16–26.
Hambright, K. D., R. W. Drenner, S. R. McComas & N. G. Hairston, 1991. Gape-limited piscivores: Planktivore size refuges, and the trophic cascade hypothesis. Archiv für Hydrobiologie 121: 389–404.
Harvey, H. H., 1981. Fish communities of the Manitoulin Island lakes. Verhandlungen Internationale Vereinigung für Theoretische und Angewandte Limnologie Verhandlungen 20: 2031–2038.
Hein, C. L., G. Öhlund & G. Englund, 2014. Fish introductions reveal the temperature dependence of species interactions. Proceedings of the Royal Society B 281: 20132641.
Hesthagen, T. & O. T. Sandlund. 2012. Gjedde, sørv og suter: status, vektorer og tiltak mot uønsket spredning. Norwegian Institute for Nature Research, Report 669. 45. (in Norwegian).
Hesthagen, T., P. Fiske, B. Barlaup & E. B. Thorstad. 2010. Fisk. In Nybø, S. (ed), Datagrunnlag for “Naturindeks for Norge”. DN-utredning 4-2010: 85–89 (in Norwegian).
Jepsen, N., S. Pedersen & E. Thorstad, 2000. Behavioural interactions between prey (trout smolts) and predators (pike and pikeperch) in an impounded river. Regulated Rivers: Research & Management 16: 189–198.
Jonsson, B., 1985. Life history patterns of freshwater resident and sea-run brown trout in Norway. Transactions of the American Fisheries Society 114: 182–194.
Jonsson, B. & N. Jonsson, 1993. Partial migration: Niche shift versus sexual maturation in fishes. Reviews in Fish Biology and Fisheries 3: 348–365.
Jonsson, B. & N. Jonsson, 2011. Ecology of Atlantic salmon and brown trout. Habitat as a template for life histories. Fish & Fisheries series, Vol. 33. Springer, Dordrecht: 708.
Kipling, C., 1984. A study of perch (Perca fluviatilis L.) and pike (Esox lucius L.) in Windermere from 1941 to 1982. Conceil International Pour L’Exploration de la Mer 41: 259–267.
Mann, R. H., 1985. A pike management strategy for a trout fishery. Journal of Fish Biology 27(Supplement A): 227–234.
Mann, R. M., J. H. Blackburn & R. C. Beaumont, 1989. The ecology of brown trout in English chalk streams. Freshwater Biology 21: 57–70.
Mayer-Pinto, M., B. L. Ignacia, M. T. M. Széchy, M. S. Viana, M. P. Curbelo-Fernandez, et al., 2012. How much is too little to detect impacts? A case study of a nuclear power plant. PloS ONE 7(10): e47871.
Mills, D. H. 1964. The ecology of the young stages of the Atlantic salmon in the River Bran, Roo-shire. Freshwater and Salmon Fisheries Research 32: 58.
Mills, C. A. & M. A. Hurley, 1991. Long-term studies on the Windermere populations of perch (Perca fluviatilis), pike (Esox lucius) and Arctic charr (Salvelinus alpinus). Freshwater Biology 23: 119–136.
Moyle, P. B., 1999. Effects of invading species on freshwater and estuarine ecosystems. In Sandlund, O. T., P. J. Schei & Å. Viken (eds), Invasive species and biodiversity management. Kluwer Academic Publishers, Dordrecht: 177–191.
Museth, J., O. T. Sandlund, T. E. Brandrud, S. W. Johansen, G. Kjellberg, J. E. Løvik, O. Reitan, O. T. Taugbøl & K. J. Aanes. 2006. The river reservoir Løpsjøen in River Søndre Rena—A survey of vegetation, zooplankton, fish and birds 35 years after establishment. Norwegian Institute for Nature Research, Report 168: 53. (in Norwegian).
Näslund, I., E. Degerman & F. Nordwall, 1998. Brown trout (Salmo trutta) habitat use and life history in Swedish streams: Possible effects of biotic interactions. Canadian Journal of Fisheries and Aquatic Sciences 55: 1034–1042.
Raat, A. J. P. 1988. Synopsis of biological data on the northern pike, Esox Lucius Linneaus, 1758. Food and Agriculture Organization of the United Nations. FAO Fisheries Synopsis No 30 Rev. 2: 178.
Rahel, F. J., 1984. Factors structuring fish assemblages along a bog lake successional gradient. Ecology 65: 1276–1289.
Ricker, W. E. 1975. Computation and interpretation of biological statistics of fish populations. Bulletine of Fisheries Research Board of Canada. Report No 191: 382.
Robinson, C. L. K., 1989. Laboratory survival of four prey in the presence of northern pike. Canadian Journal of Zoology 67: 418–420.
Roy, E. D, J. F. Martin, E. G. Irwin, J. D. Conroy & D. A. Culver. 2010. Transient social–ecological stability: The effects of invasive species and ecosystem restoration on nutrient management compromise in Lake Erie. Ecology and Society 15 (1): 20 [online]. http://www.ecologyandsociety.org/vol15/iss1/art20/.
Saksgård, R. & T. Hesthagen, 2004. A 14-year study of habitat use and diet of brown trout (Salmo trutta) and Arctic charr (Salvelinus alpinus) in Lake Atnsjøen, a subalpine Norwegian lake. Hydrobiologia 521: 187–199.
Saksgård, R. & T. Hesthagen. 2014. Fisk i Atnsjøen. In Jensen, T. C. (ed), Nettverk for biologisk mangfold i ferskvann—resultater 2012. Norwegian Institute for Nature Research, Minirapport 502: 18–22 (in Norwegian).
Sandlund, O. T., T. Hesthagen & Å. Brabrand, 2013a. Coregonid introductions in Norway: Well-intended and successful, but destructive. Advances in Limnology 64: 341–358.
Sandlund, O. T., K. Ø. Gjelland, T. Bøhn, R. Knudsen & P.-A. Amundsen, 2013b. Contrasting population and life history responses of a young morph-pair of European whitefish to the invasion of a specialised coregonid competitor, vendace. PLOS One 8(7): e68156.
Schartau, A. K., T. Hesthagen, B. M. Larsen & M. Lindholm. 2010. Ferskvann. In Nybø, S. (ed), Naturindeks for Norge. DN-utredning 3-2010: 60–69 (in Norwegian).
Sepulveda, A. J., D. S. Rutz, S. S. Ivey, K. J. Dunker & J. A. Gross, 2013. Introduced northern pike predation on salmonids in southcentral Alaska. Ecology of Freshwater Fish 22: 268–279. doi:10.1111/eff.12024.
Shafi, M. & M. S. Maitland, 1971. Comparative aspects of the biology of pike Esox lucius in two Scottish lochs. Proceedings of the Royal Society of Edinburgh 71B: 41–60.
Sharma, C. M. & R. Borgstrøm, 2008. Shift in density, habitat use, and diet of perch and roach: An effect of changed predation pressure after manipulation of pike. Fisheries Research 91: 98–106.
Smith, E. P. 2013. BACI design. In El-Shaarawi, A. H. & W. W. Piegorsch (eds), Encyclopedia of Environmetrics, Vol. 1. Wiley, New York: 141–148.
Smith, E. P., D. R. Orvos & J. Jr Cairns, 1993. Impact assessment using the Before-After-Control-Impact (BACI) model: Concerns and comments. Canadian Journal of Fisheries and Aquatic Sciences 50: 627–637.
Spens, J. & J. P. Ball, 2008. Salmonid or nonsalmonid lakes: Predicting the fate of northern boreal fish communities with hierarchial filters relating to a keystone piscivore. Canadian Journal of Fisheries and Aquatic Sciences 65: 1945–1955.
Stewart-Oaten, A., J. R. Bence & C. W. Osenberg, 1992. Assessing effects of unreplicated perturbations: No simple solutions. Ecology 73: 1396–1404.
Vehanen, T. & S. Hamari, 2004. Predation threat affects behaviour and habitat use by hatchery brown trout (Salmo trutta L.) juveniles. Hydrobiologia 525: 229–237.
Winfield, I. J., J. M. Fletcher & J. B. James, 2011. Invasive fish species in the largest lakes of Scotland, Northern Ireland, Wales and England: The collective UK experience. Hydrobiologia 660: 93–103.
Acknowledgments
This study was financed by Norwegian Institute for Nature Research. We thank our colleague Ola Ugedal and two anonymous referees for valuable comments on this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Zhengwen Liu
Rights and permissions
About this article
Cite this article
Hesthagen, T., Sandlund, O.T., Finstad, A.G. et al. The impact of introduced pike (Esox lucius L.) on allopatric brown trout (Salmo trutta L.) in a small stream. Hydrobiologia 744, 223–233 (2015). https://doi.org/10.1007/s10750-014-2078-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-014-2078-z