Abstract
Fusarium palustre is an endophyte/pathogen of Spartina alterniflora, a saltmarsh grass native to North America that has been associated in the USA with a saltmarsh decline known as Sudden Vegetation Dieback (SVD). Since the intentional introduction of S. alterniflora to stabilize mud flats on Chongming Island, Shanghai, China, S. alterniflora has become invasive, but shows no symptoms of dieback even though F. palustre can be isolated from the plant. When declining S. alterniflora from SVD sites in the northeastern USA were assayed for Fusarium species, an average of 8 % of tissues sampled gave rise to a species of Fusarium of these, 64 % were F. palustre and 16 % were F. incarnatum, a nonpathogenic species. To determine if low densities of F. palustre could explain the lack of dieback symptoms on S. alterniflora from Chongming Island, we assessed the incidence and distribution of Fusarium spp. on S. alterniflora from 12 sites on Chongming Island. On average, 26 % of the stem and root tissues sampled were colonized by a Fusarium species. Of 196 isolates recovered from S. alterniflora, 44 % were F. incarnatum and 41 % were F. palustre. Species determinations were confirmed for a subset of these isolates using a phylogenetic analysis of partial sequences of the translation elongation factor (tef) gene. The observation that Fusarium incidence on S. alterniflora was much greater on Chongming Island than in the USA survey raises the question as to why S. alterniflora on Chongming Island is showing no dieback. Other factors, such as predator release, enhanced nutritional, edaphic and/or other unidentified environmental constraints on Chongming Island may afford S. alterniflora protection from dieback.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The introduction of non-native plants into new habitats can result in successful invasion as well as many other unintended consequences, including loss or decline of native species (Wilcove et al. 1998; Mack et al. 2000). In other instances, pests, saprophytes, or pathogens that accompany the non-native, invading plants may undergo changes in their relationship with the host facilitated by the new environment or may impact the native flora, possibly resulting in expanded host ranges, especially under conducive environmental conditions (Flory and Clay 2013; Strauss et al. 2012). For example, two plant pathogens, Cryphonectria parasitica, associated with chestnut blight, and Ophiostoma ulmi, associated with Dutch elm disease, were inadvertently introduced into the USA on plants, which had substantial impacts on the native host species (Anagnostakis 1987; Potter et al. 2011).
Spartina alterniflora Loisel was intentionally introduced to Chongming Island near Shanghai, China, in 2001 to stabilize mud flats. The source of the S. alterniflora germplasm was vegetative ramets from Yancheng in Jiangsu Province that had originated from an intentional introduction in 1979 via seeds and ramets obtained from coastal marshes from the southeastern USA (Morehead City, NC, Altamaha Estuary and Sapelo, GA and Tampa Bay, FL) (An et al. 2007; Li et al. 2009). Coincident with the invasion of S. alterniflora on Chongming Island was the decline of native sedges (Scirpus mariqueter Wang et Tang and Carex spp.) (Chen et al. 2004) and dieback of common reed grass (Phragmites australis Cav. Trin. ex Steud. (Chen et al. 2004; Xu and Zhao 2005).
While S. alterniflora continues to flourish on Chongming Island, it has undergone a major decline along the east and Gulf coasts of the USA where it is native. Known as Sudden Vegetation Dieback (SVD), this saltmarsh decline was first reported about 15 years ago (Alber et al. 2008; Elmer et al. 2013). Many hypotheses have been proposed, but no explanation adequately explains the cause of the occurrence. In 2011, a new fungal pathogen, Fusarium palustre Elmer and Marra, was isolated and described from declining S. alterniflora (Elmer and Marra 2011). The fungus was subsequently found in every USA marsh where SVD occurred, but pathogenicity tests never demonstrated that F. palustre could alone cause dieback without a contribution from other environmental stressors such as drought (Elmer et al. 2011, 2013). In a survey of salt marshes along the eastern and Gulf seaboards of the USA, more than half (55 %) of all Fusarium isolates recovered from SVD-affected S. alterniflora (n = 504) were identified as F. palustre (Elmer et al. 2013). Also recovered in high numbers (20 %) in the USA were isolates belonging to a group of species known as the F. incarnatum-equiseti species complex (FEISC) (O’Donnell et al. 2009); these isolates are collectively identified in this report as F. incarnatum. Species in this group were rarely found to be pathogenic on S. alterniflora (Elmer and Marra 2011; Elmer et al. 2011) or other plants (Castellá and Cabañes 2014), but given their ubiquity in salt marshes and other niches, their ecological contribution may be significant as secondary decomposers or modulators of infection.
A dieback of the native reed Phragmites australis has been well documented on Chongming Island (Chen et al. 2004; Li et al. 2013; Xu and Zhao 2005). Li et al. (2014) isolated F. palustre from both S. alterniflora and P. australis sampled from Chongming Island and demonstrated in greenhouse tests that it was pathogenic to both plants by satisfying Koch’s postulates. Despite proof of pathogenicity in the greenhouse, no symptoms of dieback have ever been observed on S. alterniflora on Chongming Island. Even though the presence of F. palustre was confirmed by Li et al. (2014), we questioned whether the relative incidence of F. palustre on Chongming Island on S. alterniflora was comparable to S. alterniflora in the USA. A survey conducted along the coast of Connecticut and Massachusetts, USA, found the mean incidence of Fusarium spp. isolated from S. alterniflora tissue to be 8 % in sites with SVD, but <1 % from tissue from marshes where no SVD was evident (Elmer et al. 2011). Of 240 isolates recovered in that survey, 65 % were F. palustre.
The relationship between inoculum density and disease expression can often dictate whether or not symptoms appear (Seem 1984), therefore, the presence of a lower incidence of F. palustre on S. alterniflora on Chongming Island may explain the lack of disease symptoms. Furthermore, it is not clear if Fusarium species composition could influence S. alterniflora dieback. Therefore, a detailed examination of the Fusarium community colonizing S. alterniflora may provide information on why the invasive S. alterniflora exhibits no symptoms of dieback. If S. alterniflora supports different incidences and distributions of Fusarium species in China than the USA, it may suggest some competitive interaction with resident species of Fusarium in China. Our objective in this study was to assess Fusarium species composition on S. alterniflora on Chongming Island and compare results with a survey done in the USA (Elmer et al. 2011).
Materials and methods
Field sampling
The Dongtan wetland on Chongming Island (31°250–31°380 N 121°500–122°050E) is a 32,600 ha salt marsh once dominated by native sedges (e.g., Scirpus spp. and Carex spp.) and P. australis. Over the past decade, S. alterniflora has invaded large areas. Twelve sites were sampled where S. alterniflora had invaded a marsh area (Table 1). From each site, three to five plants were sampled within a 5 m radius. Plants were uprooted so that crown roots were still attached along with about 60 cm of the above ground tissue. Tissue was thoroughly washed in tap water to remove all soil, pressed between absorbent paper towels, and allowed to dry at room temperature until examination. Plants were processed within 72 h of being sampled.
Isolation and Identification
Eighty pieces of stem and 20 pieces of root tissue (0.5 cm long) were cut from the three to five bulked plants from each site. Tissue was surface-disinfested in 10 % household bleach (0.053 % Na hypochlorite), and placed on Peptone PCNB agar (PPA) (10 pieces per plate). Plates were incubated at 22–25 °C for 5–7 days whereupon colonies were counted, then sub-cultured to carnation leaf agar (CLA), and grown for 7–10 days under cool white fluorescent lights set at 12-h photoperiods. Colonies were examined under 100× magnification and all Fusarium colonies were sub-cultured again by placing a single conidium onto CLA as described above. Colonies were identified to species based on spore morphology (Leslie and Summerell 2006; Elmer and Marra 2011).
PCR and Sequencing
Sixteen monosporic Fusarium cultures—thirteen morphologically identified as F. palustre and three as F. incarnatum—were chosen for molecular phylogenetic species confirmation using partial sequences of the translation elongation factor (tef) gene (Table 2). Mycelium was grown for 1 week at room temperature in potato dextrose broth (PDB). Following centrifugation and rinsing in sterile distilled water, mycelium was then lyophilized, and genomic DNA extracted using the Omniprep-for-Fungi kit (G-Biosciences, St. Louis, MO). Conditions and primers for PCR were as previously published (Elmer and Marra 2011). Briefly, concentrations of approximately 0.1 ng DNA/µL were combined in 30 µL volumes with 0.5 units of Invitrogen (Carlsbad, CA) High-Fidelity Platinum Taq, 2.5 mM MgSO4, 0.2 mM each of the four nucleotides and 0.25 mM each primer (EF1 and EF2; O’Donnell et al. 1998) in the accompanying PCR Buffer. Cycling parameters, amplicon sequencing, and alignment of the raw sequence data were as previously published (Elmer and Marra 2011), and as follows.
Partial tef sequences from the 16 Chongming isolates were aligned using MEGA 6.06 (Tamura et al. 2013) along with partial tef sequences from eight USA F. palustre isolates. Phylogenetic analysis was performed in MEGA using the Maximum Likelihood method (Guindon and Gascuel 2003; Tamura et al. 2013) with 500 bootstrap replicates based on the Kimura 2-parameter evolutionary model, which was chosen because it had the lowest Bayesian Inference Criterion (Schwarz 1978) score in a test of six evolutionary models (General Time Reversible; Kasegawa-Kishino-Yano; Tamura-Nei; Tamura 3-parameter; Kimura 2-parameter; Jukes-Cantor). From an initial alignment of 528 nucleotides, all positions with less than 95 % site coverage were eliminated, resulting in a total of 498 informative positions in the final dataset. Initial trees for the heuristic search were obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the Maximum Composite Likelihood (MCL) approach (Tamura et al. 2004), then selecting the topologies with superior log likelihood values.
Results
The relative incidence of Fusarium species recovered from S. alterniflora averaged 23 % and 29 % for the roots and stems pieces, respectively (Table 1). Of the 196 Fusarium isolates recovered from Chongming Island, 44 % were F. incarnatum, 41 % were F. palustre, 12 % were F. chlamydosporum-like, and 3 % were F. proliferatum. The distribution between the root and stems differed in that F. palustre was found in greater incidence on stems where F. incarnatum composed a greater percentage in the roots (Fig. 1).
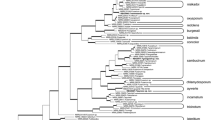
Species determinations were confirmed for a subset of these isolates using a molecular phylogenetic analysis of partial sequences of the tef gene using the Maximum Likelihood method (Table 2). This confirmed morphology-based species determinations, showing that those Chinese isolates identified as F. palustre based on morphology clustered as expected with isolates of F. palustre form the USA (Fig. 2). Partial tef sequences from the US isolates were deposited in Genbank database. Similarly, isolates from Chongming Island identified morphologically as F. incarnatum clustered with F. incarnatum from the USA.
Phylogenetic relationships among taxa of Fusarium associated with Spartina alterniflora on Chongming Island, China, and marshes of the eastern USA, inferred using the Maximum Likelihood method. Isolates ChF34, ChF35 were isolated from Phragmites. australis. Based on an analysis of partial gene sequences from translation-elongation factor 1-α, the tree shown had the highest log-likelihood value among the 500 bootstrap trees generated in the analysis. The percentage of trees in which the associated taxa clustered together is shown at the branches. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. See text for further details. Red triangles represent Chinese isolates
Discussion
These findings show that the incidence of Fusarium species on the invasive S. alterniflora samples from Chongming Island is much higher than that found on native S. alterniflora in the USA where plants exhibit SVD. What was not clear is why S. alterniflora exhibit no symptoms of dieback on Chongming Island, despite the presence of very high densities of the F. palustre. An earlier report by Li et al. (2014) showed that F. palustre isolated from S. alterniflora was capable of inciting disease on S. alterniflora using artificial inoculations under greenhouse conditions (Li et al. 2014). Although Li et al. (2014) implicated F. palustre as a contributing factor in the Phragmites dieback, there was no indication that S. alterniflora was adversely affected by the presence of F. palustre or any other biotic pests (Li et al. 2014). In the USA, the opposite has occurred, where F. palustre has been associated with SVD of S. alterniflora (Elmer et al. 2013). However, despite the appreciable differences in the relative incidences of colonization of S. alterniflora by Fusarium spp. between Chongming Island (26 %) and the USA survey (8 %), the overall distribution of Fusarium spp. on S. alterniflora was similar in that F. palustre totaled 41 and 65 % of the isolates recovered from Chongming Island and the USA, respectively.
This study does not provide any convincing explanation for why no dieback occurs on S. alterniflora on Chongming Island. Release from unknown environmental stressors may have allowed S. alterniflora to proliferate. Nutritional and edaphic factors may also govern whether or not symptoms appear on plants (Datnoff et al. 2007). Li et al. (2014) demonstrated that F. palustre was recovered from P. australis in areas where S. alterniflora had invaded, and they associated F. palustre with the dieback on Phragmites. These observations do support a hypothesis that S. alterniflora may be mediating the spread of F. palustre on Chongming Island.
Species determinations of putative F. palustre and F. incarnatum isolates from Chongming Island were confirmed through genetic analysis of partial tef gene sequences; all isolates identified morphologically as F. palustre or F. incarnatum clustered with known F. palustre and F. incarnatum isolates from eastern marshes of the USA. We note that there is less variation in F. palustre compared to F. incarnatum. The dominance of these two fungal species in two geographically distinct marshes is intriguing. Both species may have evolved in salt marsh habitats. Elmer and LaMondia (2014) demonstrated that both species have increased tolerance to NaCl. Hyphae of F. palustre and F. incarnatum could grow unimpeded on agar amended with NaCl at levels (0.27 M) equivalent to marsh water, whereas genetically similar terrestrial species of Fusarium showed substantial inhibition.
It is tempting to speculate that F. palustre may have been introduced to Chongming Island on the invasive S. alterniflora. However, data presented here do not provide sufficient evidence to establish the origin of Chinese strains of F. palustre; i.e., whether F. palustre was introduced from the USA with S. alterniflora, or was already present at the time that S. alterniflora’s was introduced. Testing these hypotheses would require population genetic analyses using molecular markers and a large set of isolates from each continent.
References
Alber M, Swenson EM, Adamowicz SC, Mendelssohn IA (2008) Salt marsh dieback: an overview of recent events in the US. Estuar Coast Shelf Sci 80:1–11
An SQ, Gu BH, Zhou CF, Wang ZS, Deng ZF, Zhi YB, Li HL, Chen L, Yu DH, Liu YH (2007) Spartina invasion in China: implications for invasive species management and future research. Weed Res 47:183–191
Anagnostakis SL (1987) Chestnut blight: the classical problem of an introduced pathogen. Mycologia 79:23–37
Castellá G, Cabañes FJ (2014) Phylogenetic diversity of Fusarium incarnatum-equiseti species complex isolated from Spanish wheat. Antonie Van Leeuwenhoek. doi:10.1007/s10482-014-0200-x
Chen ZY, Li B, Zhong Y, Chen JK (2004) Local competitive effects of introduced Spartina alterniflora on Scirpus mariqueter at Dongtan of Chongming Island, the Yangtze River estuary and their potential ecological consequences. Hydrobiologia 528:99–106
Datnoff LE, Elmer WH, Huber DN (2007) Mineral nutrition and plant disease. APS Press, St. Paul
Elmer WH, LaMondia JA (2014) Comparison of saline tolerance of genetically similar species of Fusarium and Meloidogyne recovered from marine and terrestrial habitats. Estuar Coast Shelf Sci 149:320–324
Elmer WH, Marra RE (2011) New species of Fusarium associated with dieback of Spartina alterniflora in Atlantic salt marshes. Mycologia 103:806–819
Elmer WH, LaMondia JA, Caruso F (2011) Association between Fusarium spp. on Spartina alterniflora and dieback sites in Connecticut and Massachusetts. Estuaries Coasts 35:436–444
Elmer WH, Useman S, Schneider RW, Marra RE, del Mar LaMondiaJA, Jimenez-Gasco M, Mendelssohn IA, Caruso FL (2013) Sudden Vegetation Dieback in Atlantic and Gulf coast salt marshes. Plant Dis 97:436–444
Flory SL, Clay K (2013) Pathogen accumulation and long-term dynamics of plant invasions. J Ecol 101:607–613
Guindon S, Gascuel O (2003) A simple, fast and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52:696–704
Leslie JF, Summerell BA (2006) The fusarium laboratory manual. Blackwell, Ames, p 385
Li B, Liao CZ, Zhang XD, Chen HL, Wang Q, Chen ZY (2009) Spartina alterniflora invasions in the Yangtze River estuary, China: an overview of current status and ecosystem effects. Ecol Eng 35:511–520
Li H, Shao JJ, Qiu SY, Li B (2013) Native Phragmites dieback reduced its dominance in the salt marshes invaded by exotic Spartina in the Yangtze River estuary, China. Ecol Eng 57:236–241
Li H, Zhang X, Zheng R, Li X, Elmer WH, Wolfe LM, Li B (2014) Li Indirect effects of non-native Spartina alterniflora and its fungal pathogen (Fusarium palustre) on native salt marsh plants in China. J Ecol 102:1112–1119
Mack RN, Simberloff D, Lonsdale WM, Evans H, Clout M, Bazzaz FA (2000) Biotic invasions: causes, epidemiology, global consequences, and control. Ecol Appl 10:689–710
O’Donnell K, Cigelnik E, Nirenberg HI (1998) Molecular systematics and phylogeography of the Gibberella fujikuroi species complex. Mycologia 90:465–493
O’Donnell K, Sutton DA, Rinaldi MG, Gueidan C, Crous PW, Geiser DM (2009) Novel multilocus sequence typing scheme reveals high genetic diversity of human pathogenic members of the Fusarium incarnatum–F. equseti and F. chlamydosporum species complexes within the United States. J Clin Microbiol 47:3851–3861
Potter C, Harwood T, Knight J, Tomlinson I (2011) Learning from history, predicting the future: the UK Dutch elm disease outbreak in relation to contemporary tree disease threats. Phil Trans R Soc Lond Ser B Biol Sci 366:1966–1974
Schwarz GE (1978) Estimating the dimension of a model. Ann Stat 6(2):461–464
Seem RC (1984) Disease incidence and severity relationships. Ann Rev Phytopathol 22:133–150
Strauss A, White A, Boots M (2012) Invading with biological weapons: the importance of disease-mediated invasions. Funct Ecol 26:1249–1261
Tamura K, Nei M, Kumar S (2004) Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc Natl Acad Sci USA 101:11030–11035
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Wilcove DS, Rothstein D, Dubow J, Phillips A, Losos E (1998) Quantifying threats to imperiled species in the United States. Bioscience 48:607–615
Xu HF, Zhao YL (2005) Comprehensive surveys in Chongming Dongtan Nature Reserve for Migratory Birds, Shanghai (in Chinese). Chinese Forestry Publishing House, Beijing
Acknowledgments
The authors thank Peter Thiel and Michael Ammirata for Technical assistance. This work was funded in part from USDA Hatch Grant H 647.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: Alan Gray and Malika Ainouche/Invasive Spartina.
Rights and permissions
About this article
Cite this article
Elmer, W.H., Marra, R.E., Li, H. et al. Incidence of Fusarium spp. on the invasive Spartina alterniflora on Chongming Island, Shanghai, China. Biol Invasions 18, 2221–2227 (2016). https://doi.org/10.1007/s10530-015-1012-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-015-1012-2