Abstract
Pancreatic cancer remains the common cancer with the worst prognosis because of its late diagnosis and extensive metastasis. This study aimed to investigate the effects of GABRP on pancreatic cancer metastasis and the molecular mechanism. The expression of GABRP was measured using the quantitative real-time PCR and western blot. The biological behaviors of cancer cells were assessed using the cell counting kit-8, Transwell assay, and western blot. The regulation of GABRP on the MEK/ERK pathway was detected by western blot. The results indicated that GABRP was overexpressed in pancreatic cancer tissues and cells. Knockdown of GABRP suppressed cell viability, invasion, migration, and epithelial–mesenchymal transition (EMT), whereas GABRP overexpression facilitated these biological behaviors. Inactivation of the MEK/ERK pathway reversed the effects on cellular processes induced by GABRP. Moreover, silencing of GABRP inhibited tumor growth. In conclusion, GABRP promoted the progression of pancreatic cancer by facilitating cell metastasis and tumor growth via activating the MEK/ERK pathway. The findings suggest that GABRP has the potential to be a therapeutic target for the metastatic pancreatic cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic cancer, originating from the ductal epithelium and acinar cells of the pancreas, is an important cause of cancer-associated death and healthcare burden globally (Klein 2021). Recently, the incidence of pancreatic cancer is increasing, and over 90% of them are aggressive pancreatic ductal adenocarcinoma (Hu et al. 2021; Wang et al. 2021). The onset of pancreatic cancer is insidious and asymptomatic in its early stage, so it is usually diagnosed at the advanced stage with extensive metastasis (Du et al. 2016). With the development of chemoresistance and metastasis, pancreatic cancer leads to poor clinical outcomes (Zeng et al. 2019; Bhattacharya et al. 2021). Additionally, the prognosis of pancreatic cancer is worst among all main cancers (Loveday et al. 2019). Thus, focusing on the metastasis of pancreatic cancer and searching for appropriate treatment strategies will contribute to improving the survival rate of patients with pancreatic cancer.
Gamma-aminobutyric acid (GABA) is a neurotransmitter in the central nervous system (Xu et al. 2021). GABRP is the type A receptor subunit pi of GABA that consists of five subunits, which are encoded by 19 genes. Except for neurons, GABRP is expressed in multiple human tissues, such as the prostate, ovarian, stomach, esophagus, and pancreas. Yang et al. have identified that GABRP may be a prognostic marker and therapeutic target (Yang et al. 2022). Moreover, overexpression of GABRP promotes the growth of pancreatic cancer cells (Takehara et al. 2007). However, the effects of GABRP on pancreatic cancer metastasis remain unclear.
The mitogen-activated protein kinase (MAPK) pathways are the signal transduction pathways that are commonly abnormal activation or inactivation in diseases (Lee et al. 2020). Extracellular signal-regulated kinase (ERK), JNK, and p38 are the main factors in MAPKs. Among them, The ERK signaling plays a key role in cancer cell proliferation, migration, and invasion (Kim and Choi 2010). It has been revealed that the MEK/ERK pathway is activated in pancreatic cancer (Wang et al. 2017). Nevertheless, whether GABRP could mediate the MEK/ERK pathway to affect pancreatic cancer metastasis is largely unknown.
In the current study, the effects of GABRP on metastasis in pancreatic cancer were explored. We speculated that GABRP promoted the metastasis of pancreatic cancer by modulating the MEK/ERK pathway. The data may provide a new therapeutic target for metastatic pancreatic cancer.
Materials and Methods
Bioinformatic Analysis
The expression of GABRP in tumor and normal tissues and the overall survival of patients with high or low GABRP were predicted using the GEPIA database (http://gepia.cancer-pku.cn/).
Tissue Sample Collection
Tumor tissues and adjacent non-tumor tissues were both collected from patients with pancreatic cancer (n = 35) who were diagnosed and underwent surgery in Xi’an No.3 Hospital. Written informed consent was obtained from all patients. The protocol was approved by the Ethics Committee of Xi’an No.3 Hospital.
Cell Culture
Pancreatic cancer cell lines (CFAPC-1, Panc-1, Aspc-1) and normal cells (HPDE6-C7) were cultured at Dulbecco’s modified eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (Gibco) under the condition of 37 °C with 5% CO2.
Cell Transfection
siRNA (si)-GABRP 1#, si-GABRP 2#, si-negative control (NC), GABRP overexpression vector (oe-GABRP), and empty vector (oe-NC) were acquired from Genepharma (Shanghai). CFAPC-1 cells were transfected with the vectors mentioned above using the Lipofectamine 3000 (Invitrogen). Forty-eight hours post-transfection, cells were harvested.
AZD6244 Treatment
AZD6244 (MEK inhibitor; MCE) was dissolved in DMSO (Sigma-Aldrich). The CFAPC-1 cells were treated with 20-nM AZD6244 solution for 48 h (14).
Real-Time Quantitative PCR (qPCR)
Total RNA was isolated using the TRIzol reagent (Invitrogen). After measuring the concentration and purity, 1-μg RNA was reverse transcribed to complementary DNA using a miScript II RT kit (Qiagen). qPCR was assessed using the miScript SYBR Green PCR kit (Qiagen) on a LightCycle real-time PCR system (Roche). Relative gene expression was calculated using the 2−ΔΔCt formula as normalized to the expression of GAPDH.
Cell Counting Kit-8 (CCK-8) Assay
A CCK-8 kit (Dojindo) was used to analyze cell viability. The cells were seeded into 96-well plates and incubated for 24 h. Subsequently, the CCK-8 reagent was incubated with the cells for 4 h. The absorbance was measured using a microplate reader (Bio-Rad) at 450 nm.
Transwell Assay
Transwell chambers (24-well) were precoated with 50-μL Matrigel to assess cell invasion. The cells were suspended in DMEM without FBS and added to the top chambers. The DMEM supplemented with 10% FBS was added to the bottom chambers. Following 24 h, cells in the upper chambers were removed and the others that invaded the lower chamber were stained with crystal violet. Images were taken by a microscope in 5 random fields.
Transwell chambers without Matrigel were used to assess cell migration. The other operations were the same as mentioned above.
Western Blot
Total protein was extracted using the RIPA lysis buffer (Beyotime) and the concentration was measured using the BCA kit (Beyotime). About 30-μg protein was separated using 10% SDS-PAGE. The separated proteins were electro-transferred to PVDF membranes (Millipore). The membrane was blocked in 5% nonfat milk for 1 h at 25 °C and incubated with primary antibodies overnight at 4 °C. On a secondary day, the membrane was incubated with HRP-conjugated secondary antibodies for 2 h at 25 °C. Protein bands were visualized using the ECL reagent under a Gel Doc EZ Gel imaging system (Bio-Rad).
In Vivo Study
The CFAPC-1 cells were stably transfected with sh-NC and sh-GABRP. BALB/c nude mice (male, 4–6 weeks old; SLAC, Shanghai) were housed in SPF conditions. The mice were randomly divided into two groups: sh-NC and sh-GABRP groups (8 mice per group). Transfected cells (2 × 105 cells) were inoculated subcutaneously in nude mice to establish the xenograft tumor model. After inoculation, the mice were grown under the standard conditions. Tumor volume was detected every week using the formula: length × width2 × 1/2. Five weeks later, the mice were euthanized and tumors were collected and weighed. The animal study was approved by the Ethics Committee of Xi’an No.3 Hospital.
Statistical Analysis
Data were acquired from three repeated experiments and analyzed using the GraphPad Prism 7.0 software. Data were expressed as mean ± standard deviation. Comparisons between the two groups were carried out using the student’s t test, and comparisons among multiple groups were performed using one-way ANOVA. P < 0.05 was identified as statistical significance.
Results
GABRP Levels are Upregulated in Pancreatic Cancer
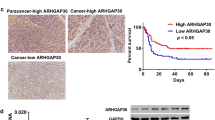
We first predicted the levels of GABRP in pancreatic cancer. The data from the GEPIA database showed that GABRP was upregulated in tumor tissues compared with normal tissues (Fig. 1A). The overall survival of high GABRP was lower than that of low GABRP (Fig. 1B). We detected GABRP expression in tissues and confirmed that GABRP expression was increased in pancreatic cancer (Fig. 1C and D). Compared to the HPDE6-C7 cells, the levels of GABRP were increased in the CFAPC-1, Panc-1, and Aspc-1 cells, especially in the CFAPC-1 cell line (Fig. 1E and F).
GABRP levels are upregulated in pancreatic cancer. A The levels of GABRP in tumor and normal tissues were predicted using the GEPIA online database. B The overall survival of patients with high and low GABRP expression was predicted using the GEPIA database. C qPCR and D western blot were used to detect GABRP expression in tumor tissues and adjacent non-tumor tissues from patients with pancreatic cancer. E qPCR and F western blot measured GABRP expression in normal cells (HPDE6-C7) and tumor cells (CFAPC-1, Panc-1, Aspc-1). **P < 0.01. ***P < 0.001
Knockdown of GABRP Suppresses the Metastasis of Pancreatic Cancer Cells
To investigate the role of GABRP knockdown in pancreatic cancer, si-GABRP 1# and si-GABRP 2# were transfected into the CFAPC-1 cells. The results of efficiency illustrated that GABRP mRNA and protein levels were decreased after transfection (Fig. 2A and B). Cell viability was inhibited by GABRP knockdown (Fig. 2C). Additionally, silencing of GABRP inhibited cell migration and invasion (Fig. 2D). The protein levels of E-cadherin were elevated, whereas N-cadherin and Vimentin levels were reduced by GABRP downregulation (Fig. 2F).
Knockdown of GABRP suppresses the metastasis of pancreatic cancer cells. A GABRP expression was examined using qPCR after transfection. B GABRP levels were examined using western blot after transfection. C Cell viability was analyzed using CCK-8 analysis. D Cellular migration and E invasion were assessed using Transwell assay. F The protein levels of E-cadherin, N-cadherin, and Vimentin were measured by western blot. **P < 0.01. ***P < 0.001
Overexpression of GABRP Promotes Pancreatic Cancer Cell Metastasis
Then, we explored the role of GABRP overexpression in pancreatic cancer. The GABRP overexpression vectors were transfected into the CFAPC-1 cells, and the levels of GABRP were elevated (Fig. 3A and B). Overexpressing GABRP promoted cell viability, compared with empty vector (Fig. 3C). Cell migration and invasion were both facilitated by GABRP overexpression (Fig. 3D and E). In addition, GABRP reduced the levels of E-cadherin and increased the levels of N-cadherin and Vimentin (Fig. 3F).
Overexpression of GABRP promotes pancreatic cancer cell metastasis. A qPCR and B western blot were carried out to assess the efficiency after transfection. C CCK-8 assay measured the cell viability. D Cellular migration and E invasion were assessed using Transwell assay. F Western blot detected the protein levels of E-cadherin, N-cadherin, and Vimentin. ***P < 0.001
GABRP Activates the MEK/ERK Pathway
To identify the underlying mechanism, we analyzed the effects of GABRP on the MEK/ERK pathway. Knockdown of GABRP reduced the protein levels of p-MEK1 and p-ERK1/2, and did not affect MEK1 and ERK1/2 levels (Fig. 4A). On the other hand, overexpression of GABRP increased the protein levels of p-MEK1 and p-ERK1/2 and also did not affect MEK1 and ERK1/2 levels (Fig. 4B).
GABRP Promotes the Metastasis of Pancreatic Cancer via the MEK/ERK Pathway
We used AZD6244 to suppress the activation of the MEK/ERK pathway. The results of western blot indicated that the elevation of the p-MEK1 and p-ERK1/2 induced by GABRP was reversed by the AZD6244 treatment (Fig. 5A). GABRP promoted cell viability, migration, and invasion, whereas AZD6244 treatment counteracted the promotion induced by GABRP (Fig. 5B–D). The downregulation of E-cadherin, and the upregulation of N-cadherin and Vimentin induced by GABRP overexpression were partly abolished by AZD6244 (Fig. 5E).
GABRP promotes the metastasis of pancreatic cancer via the MEK/ERK pathway. A Western blot assessed the protein levels of p-MEK1 and p-ERK1/2 in GABRP overexpression cells treated with AZD6244. B CCK-8 assessed the cell viability. Transwell assay was carried out to evaluate the C cell migration and D invasion. E The protein levels of E-cadherin, N-cadherin, and Vimentin were measured using the western blot. *P < 0.05. **P < 0.01. ***P < 0.001
Silencing of GABRP Inhibits Tumor Growth In Vivo
To explore the role of GABRP in vivo, a xenograft tumor model was established. Knockdown of GABRP inhibited tumor size, volume, and weight, compared with sh-GABRP (Fig. 6A–C).
Discussion
With the development of cancer treatment technology, pancreatic cancer is still associated with a poor prognosis. The new strategies are urgently needed to investigate. Epithelial–mesenchymal transition (EMT) plays a central role in cancer invasion and metastasis, which induces early dissemination of tumor cells (Zheng et al. 2015). Tumor invasion stimulates cell migration, promoting cell proliferation and survival (Keleg et al. 2003). In addition, the interaction of epithelial or tumor cells and stromal cells induces the tumor microenvironment, leading to tumor metastasis (Ren et al. 2018). Thus, controlling tumor metastasis can help decelerate tumor progression, which may improve patient survival.
Accumulating evidence has shown that GABRP is pivotal in cancer progression by regulating biological behaviors. For example, GABRP knockdown impedes ovarian cancer cell migration and invasion (Sung et al. 2017). GABRP is related to a worse prognosis of breast cancer, and knockdown of which suppresses the carcinogenicity and cytoskeletal changes (Sizemore et al. 2014). GABRP is a target of miR-320c and rescues the suppression of cervical cancer cell migration induced by miR-320c (Li et al. 2020). Moreover, GABRP promotes cell proliferation and inhibits apoptosis of oral squamous cell carcinoma and is linked to tumor differentiation (Ma et al. 2016). GABRP is a prognostic biomarker of pancreatic cancer, and inhibiting the expression of endogenous GABRP hinders the growth of pancreatic cancer cells (Yang et al. 2022; Takehara et al. 2007). Additionally, GABRP is contributed to tumor metastasis and infiltration of macrophages. Overexpression of GABRP has immunomodulatory effects in pancreatic cancer (Jiang et al. 2019). However, the effects of GABRP on the metastasis of pancreatic cancer remain unclear. Herein, we focused on whether GABRP affected tumor cell metastasis. The results showed that GABRP was upregulated in pancreatic cancer. Silencing of GABRP inhibited the migration, invasion, and EMT of pancreatic cancer cells, whereas overexpression of GABRP promoted these cellular processes. The data suggested that GABRP is an oncogene in pancreatic cancer and promoted cancer progression, consistent with previous studies mentioned above.
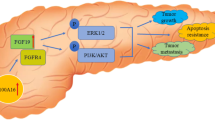
The MEK/ERK signaling is important in the regulation of cell survival, migration, apoptosis, and EMT (Chen et al. 2020; Sun et al. 2015). It is associated with tumorigenesis and functions as an oncogene in cancers, including pancreatic cancer (Griesmann et al. 2021). However, MEK inhibitor alone has no significant effects on pancreatic cancer (Yan et al. 2021). Therefore, exploring novel targets related to the MEK pathway is required. It has been proved that GABRP promotes tumor progression by regulating the MEK/ERK pathway (Takehara et al. 2007; Sung et al. 2017; Sizemore et al. 2014). In this study, we found that GABRP depletion inactivated the MEK/ERK pathway, whereas GABRP overexpression activated this pathway. Moreover, the inactivation of the pathway reversed the effects of GABRP on cell migration, invasion, and EMT. Thus, we believed that GABRP promoted the progression of pancreatic cancer via the MEK/ERK pathway.
The major limitation of this study is that we only explore the effects of GABRP on tumor growth in vivo. Whether GABRP regulate tumor metastasis to liver and lung has not been analyzed. We will further study in our future work.
In conclusion, GABRP was highly expressed in pancreatic cancer, which promoted cell metastasis in vitro and tumor growth in vivo by activating the MEK/ERK pathway. The data suggested that GABRP might be a novel target for the treatment of pancreatic cancer.
Data Availability
Data are available upon reasonable request from corresponding author.
References
Bhattacharya A, Santhoshkumar A, Kurahara H, Harihar S (2021) Metastasis suppressor genes in pancreatic cancer: an update. Pancreas 50(7):923–932. https://doi.org/10.1097/MPA.0000000000001853
Chen Y, Liu P, Shen D, Liu H, Xu L, Wang J, Shen D, Sun H, Wu H (2020) FAM172A inhibits EMT in pancreatic cancer via ERK-MAPK signaling. Biol Open. https://doi.org/10.1242/bio.048462
Diep CH, Munoz RM, Choudhary A, Von Hoff DD, Han H (2011) Synergistic effect between erlotinib and MEK inhibitors in KRAS wild-type human pancreatic cancer cells. Clin Cancer Res 17(9):2744–2756. https://doi.org/10.1158/1078-0432.CCR-10-2214
Du YX, Liu ZW, You L, Wu WM, Zhao YP (2016) Advances in understanding the molecular mechanism of pancreatic cancer metastasis. Hepatobiliary Pancreat Dis Int 15(4):361–370. https://doi.org/10.1016/s1499-3872(15)60033-9
Griesmann H, Mühl S, Riedel J, Theuerkorn K, Sipos B, Esposito I, Vanden Heuvel GB, Michl P (2021) CUX1 enhances pancreatic cancer formation by synergizing with KRAS and inducing MEK/ERK-dependent proliferation. Cancers (basel) 13(10):2462. https://doi.org/10.3390/cancers13102462
Hu JX, Zhao CF, Chen WB, Liu QC, Li QW, Lin YY, Gao F (2021) Pancreatic cancer: a review of epidemiology, trend, and risk factors. World J Gastroenterol 27(27):4298–4321. https://doi.org/10.3748/wjg.v27.i27.4298
Jiang SH, Zhu LL, Zhang M, Li RK, Yang Q, Yan JY, Zhang C, Yang JY, Dong FY, Dai M, Hu LP, Li J, Li Q, Wang YH, Yang XM, Zhang YL, Nie HZ, Zhu L, Zhang XL, Tian GA, Zhang XX, Cao XY, Tao LY, Huang S, Jiang YS, Hua R, Qian Luo K, Gu JR, Sun YW, Hou S, Zhang ZG (2019) GABRP regulates chemokine signalling, macrophage recruitment and tumour progression in pancreatic cancer through tuning KCNN4-mediated Ca2+ signalling in a GABA-independent manner. Gut 68(11):1994–2006. https://doi.org/10.1136/gutjnl-2018-317479
Keleg S, Büchler P, Ludwig R, Büchler MW, Friess H (2003) Invasion and metastasis in pancreatic cancer. Mol Cancer 2:14. https://doi.org/10.1186/1476-4598-2-14
Kim EK, Choi EJ (2010) Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta. https://doi.org/10.1016/j.bbadis.2009.12.009
Klein AP (2021) Pancreatic cancer epidemiology: understanding the role of lifestyle and inherited risk factors. Nat Rev Gastroenterol Hepatol 18(7):493–502. https://doi.org/10.1038/s41575-021-00457-x
Lee S, Rauch J, Kolch W (2020) Targeting MAPK signaling in cancer: mechanisms of drug resistance and sensitivity. Int J Mol Sci 21(3):1102. https://doi.org/10.3390/ijms21031102
Li Y, Huang Y, Zhou C, Jiang PC, Pan W (2020) MiR-320c prevents the malignant development of cervical cancer by regulating GABRP level. Eur Rev Med Pharmacol Sci 24(17):8731–8739. https://doi.org/10.26355/eurrev_202009_22810
Loveday BPT, Lipton L, Thomson BN (2019) Pancreatic cancer: an update on diagnosis and management. Aust J Gen Pract 48(12):826–831. https://doi.org/10.31128/AJGP-06-19-4957
Ma J, Zhang Y, Wang J, Zhao T, Ji P, Song J, Zhang H, Luo W (2016) Proliferative effects of gamma-amino butyric acid on oral squamous cell carcinoma cells are associated with mitogen-activated protein kinase signaling pathways. Int J Mol Med 38(1):305–311. https://doi.org/10.3892/ijmm.2016.2597
Ren B, Cui M, Yang G, Wang H, Feng M, You L, Zhao Y (2018) Tumor microenvironment participates in metastasis of pancreatic cancer. Mol Cancer 17(1):108. https://doi.org/10.1186/s12943-018-0858-1
Sizemore GM, Sizemore ST, Seachrist DD, Keri RA (2014) GABA(A) receptor pi (GABRP) stimulates basal-like breast cancer cell migration through activation of extracellular-regulated kinase 1/2 (ERK1/2). J Biol Chem 289(35):24102–24113. https://doi.org/10.1074/jbc.M114.593582
Sun Y, Liu WZ, Liu T, Feng X, Yang N, Zhou HF (2015) Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J Recept Signal Transduct Res 35(6):600–604. https://doi.org/10.3109/10799893.2015.1030412
Sung HY, Yang SD, Ju W, Ahn JH (2017) Aberrant epigenetic regulation of GABRP associates with aggressive phenotype of ovarian cancer. Exp Mol Med. https://doi.org/10.1038/emm.2017.62
Takehara A, Hosokawa M, Eguchi H, Ohigashi H, Ishikawa O, Nakamura Y, Nakagawa H (2007) Gamma-aminobutyric acid (GABA) stimulates pancreatic cancer growth through overexpressing GABAA receptor pi subunit. Cancer Res 67(20):9704–9712. https://doi.org/10.1158/0008-5472.CAN-07-2099
Wang J, Guo X, Xie C, Jiang J (2017) KIF15 promotes pancreatic cancer proliferation via the MEK-ERK signalling pathway. Br J Cancer 117(2):245–255. https://doi.org/10.1038/bjc.2017.165
Wang SS, Xu J, Ji KY, Hwang CI (2021) Epigenetic alterations in pancreatic cancer metastasis. Biomolecules 11(8):1082. https://doi.org/10.3390/biom11081082
Xu B, Sai N, Gilliham M (2021) The emerging role of GABA as a transport regulator and physiological signal. Plant Physiol 187(4):2005–2016. https://doi.org/10.1093/plphys/kiab347
Yan L, Tu B, Yao J, Gong J, Carugo A, Bristow CA, Wang Q, Zhu C, Dai B, Kang Y, Han L, Feng N, Jin Y, Fleming J, Heffernan TP, Yao W, Ying H (2021) Targeting glucose metabolism sensitizes pancreatic cancer to MEK inhibition. Cancer Res 81(15):4054–4065. https://doi.org/10.1158/0008-5472.CAN-20-3792
Yang Y, Ren L, Li S, Zheng X, Liu J, Li W, Fu W, Wang J, Du G (2022) GABRP is a potential prognostic biomarker and correlated with immune infiltration and tumor microenvironment in pancreatic cancer. Transl Cancer Res 11(4):649–668. https://doi.org/10.21037/tcr-21-2021
Zeng S, Pöttler M, Lan B, Grützmann R, Pilarsky C, Yang H (2019) Chemoresistance in Pancreatic Cancer. Int J Mol Sci 20(18):4504. https://doi.org/10.3390/ijms20184504
Zheng X, Carstens JL, Kim J, Scheible M, Kaye J, Sugimoto H, Wu CC, LeBleu VS, Kalluri R (2015) Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 527(7579):525–530. https://doi.org/10.1038/nature16064
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by YM, SG, WC, WJ, ZL, JH, and ZX. The first draft of the manuscript was written by YM and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflicts of Interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics Approval
This study was performed in line with the principles of the Declaration of Helsinki. The animal study was performed in line with the Guide for the care and use of laboratory animals. The human and animal approvals were granted by the Ethics Committee of Xi’an No.3 Hospital.
Consent to Participate
Written informed consent was obtained from all patients.
Consent to Publish
The authors affirm that human research participants provided informed consent for publication of the images in Fig. 1C.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Meng, Y., Li, R., Geng, S. et al. GABRP Promotes the Metastasis of Pancreatic Cancer by Activation of the MEK/ERK Signaling Pathway. Biochem Genet 62, 242–253 (2024). https://doi.org/10.1007/s10528-023-10410-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10528-023-10410-z