Abstract
Protein phosphatase, Mg2+/Mn2+ dependent, 1D (PPM1D) is emerging as an oncogene by virtue of its negative control on several tumor suppressor pathways. However, the clinical significance of PPM1D in pancreatic cancer (PC) has not been defined. In this study, we determined PPM1D expression in human PC tissues and cell lines and their irrespective noncancerous controls. We subsequently investigated the functional role of PPM1D in the migration, invasion, and apoptosis of MIA PaCa-2 and PANC-1 PC cells in vitro and explored the signaling pathways involved. Furthermore, we examined the role of PPM1D in PC tumorigenesis in vivo. Our results showed that PPM1D is overexpressed in human PC tissues and cell lines and significantly correlated with tumor growth and metastasis. PPM1D promotes PC cell migration and invasion via potentiation of the Wnt/β-catenin pathway through downregulation of apoptosis-stimulating of p53 protein 2 (ASPP2). In contrast to PPM1D, our results showed that ASPP2 is downregulated in PC tissues. Additionally, PPM1D suppresses PC cell apoptosis via inhibition of the p38 MAPK/p53 pathway through both dephosphorylation of p38 MAPK and downregulation of ASPP2. Furthermore, PPM1D promotes PC tumor growth in vivo. Our results demonstrated that PPM1D is an oncogene in PC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Protein phosphatase, Mg2+/Mn2+ dependent, 1D (PPM1D), also referred to as wild-type p53 inducible protein 1 (Wip1) phosphatase, is a member of the protein phosphatase 2C (PP2C) family of Ser/Thr protein phosphatases. The expression of this gene is induced in a p53-dependent manner in response to various environmental stresses such as radiation, H2O2, and anisomycin [1, 2]. In addition to p53, PPM1D is also a target of other transcription factors such as estrogen receptor-α and NF-κB [3, 4]. Once induced, PPM1D dephosphorylates and inactivates p38 MAPK and p53, suppressing p53-mediated transcription and apoptosis in response to stress [5]. In addition to its role in stress response, PPM1D is emerging as an important oncogene by virtue of its negative control on several key tumor suppressor pathways including ATM, CHK2, p38 MAPK, and p53 [6]. PPM1D is overexpressed and/or mutated in various human primary cancers [6–8]. Moreover, the high PPM1D expression has been associated with tumor progression and poor prognosis of a number of cancers such as non-small cell lung cancer and nasopharyngeal carcinoma [9–11]. The oncogenic properties of PPM1D have been demonstrated in various cellular and animal studies. PPM1D deletion in mice limits tumorigenesis in various mouse cancer models [12–14]. PPM1D knockdown inhibits whilst its overexpression promotes cancer cell growth in vitro and in vivo [11, 15]. However, the clinical and functional significance of PPM1D in pancreatic cancer (PC) is undefined.
Apoptosis-stimulating of p53 protein 2 (ASPP2, also known as 53BP2L) is a tumor suppressor that binds to p53 and enhances p53-mediated transcription of proapoptotic genes [16]. All the amino acids of p53 that are important for binding ASPP2 are mutated in human cancers, highlighting the importance of ASPP2 in human malignancies [17]. ASPP2 is frequently downregulated in tumors and cancer cells expressing wild type p53, and to a lesser extent mutant p53 [18, 19]. The proapoptotic activity of ASPP2 is increased by the RAS/Raf/MAPK signaling cascade as phosphorylation of ASPP2 by MAPK leads to RAS-induced translocation of ASPP2, which results in the increased binding to p53 [20]. Independent of p53 binding, ASPP2 inhibits cancer metastasis through β-catenin-dependent regulation of ZEB1 [21].
In the present study, we examined PPM1D expression in human PC tissues and paired adjacent noncancerous tissues. We also examined the functional role of PPM1D in PC cell migration, invasion, and apoptosis in vitro and PC tumor growth in vivo. Finally, we showed that the oncogenic activities of PPM1D in PC are mediated by regulation of the Wnt/β-catenin and p38 MAPK/p53 pathways through ASPP2 downregulation.
Materials and methods
Animals
Nude mice (6–7 weeks old) were obtained from the Shanghai SLAC Laboratory Animal Co., Ltd. All animal studies were performed in accordance with the Guide for the Care and Use of Laboratory Animals. All study protocols were approved by the Ethics Committee of Shanghai Sixth People’s Hospital of China.
Cell lines and cultures
The human PC cell lines MIA PaCa-2 and PANC-1 were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured following manufacturer’s instructions. Human pancreas ductal epithelial HPDE6-C7 cells were obtained from the lab (Department of General Surgery, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, Shanghai, China) and maintained as previously described [22].
Tissue samples
The human pancreatic tumor tissues and paired adjacent noncancerous tissues were collected from PC patients who underwent surgical resection in the Department of Hepatobiliary Surgery, Shanghai Sixth People’s Hospital, China, from January 2013 to February 2015. Tissues were immediately embedded in OCT compound. All patients gave informed consent. All procedures were reviewed and approved by the Ethics Committees of Shanghai Sixth People’s Hospital of China.
Wound-healing migration and transwell migration and invasion assays
The wound-healing migration and transwell migration and invasion assays were performed using MIA PaCa-2 and PANC-1 cells transfected with PPM1D, PPM1D siRNA, or their respective controls. In wound-healing migration assay, confluent monolayers of cells transfected for 48 h were wounded with a p20 pipette tip (time 0). Cell migration was monitored by time-lapse imaging in four separate fields (one of the four fields was shown in the Figs. 1, 7). The transwell migration assay was performed using transwell membrane (8 μm pore size, 6.5 mm diameter) from Corning Costar (USA). The bottom chambers of the Transwell were filled with migration-inducing medium containing 10 % fetal bovine serum. The top chambers were seeded with 105 MIA PaCa-2 or PANC-1 cells transfected for 48 h. After 16 h, cells migrated though pores to the bottom surface of the transwell were fixed with 10 % formaldehyde, stained with 0.5 % crystal violet, and counted under an Olympus inverted microscope. Six randomly selected microscopic fields were counted for each group. The invasion assays were performed using similar transwell membranes coated with Matrigel (Chemicon International, USA). Data were analyzed using ImageJ 1.42q software (National Institutes of Health).
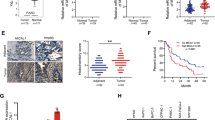
PPM1D is overexpressed in human PC tissues and cell lines. a Immunohistochemical (IHC) staining of human PC (right) and paired adjacent noncancerous tissues (left). Original magnification, ×200. b and c The PPM1D mRNA (c) and protein expression (b) in human PC and paired adjacent noncancerous tissues determined by qRT-PCR and western blotting, respectively. d and e The PPM1D mRNA (d) and protein (e) expression in MIA PaCa-2 and PANC-1 human PC cells and HPDE6-C7 human normal pancreatic epithelial cells determined by qRT-PCR and western blotting, respectively. GAPDH was used as a loading control. n = 3, *P < 0.05. NS no significantly difference. e and f The ASPP2 mRNA (f) and protein expression (e) in human PC and paired adjacent noncancerous tissues determined by qrt-PCR and western blotting, respectively. g and h The Aspp2 mrna (h) and protein (f) expression in MIA PaCa-2 and PANC-1 human PC cells and HPDE6-C7 human normal pancreatic epithelial cells determined by qRT-PCR and western blotting, respectively. GAPDH was used as a loading control. n = 3, **P < 0.01. NS no significantly difference. T cancer tissues. N normal tissues
Western blot analysis
Western blot analysis was carried out using cell lysates or tissue homogenates in urea buffer (8 M urea, 1 M thiourea, 0.5 % CHAPS, 50 mM dithiothreitol, 24 mM spermine). Cytoplasmic and nuclear protein fractions were prepared using NE-PER nuclear and cytoplasmic extraction reagents (Pierce, USA), respectively, following manufacturer’s protocols. B-tubulin was used as a loading control for the cytoplasmic fraction, whereas PARP was used as a loading control for the nuclear fraction. Samples (40 μg total protein) were separated on SDS-PAGE and transferred to nitrocellulose membranes (Millipore, USA). After blocking in 5 % nonfat milk for 1 h, the membranes were incubated with primary antibodies against ZEB1 (1:1000), PPM1D (1:200), β-catenin (1:200), p-β-catenin (1:200), ASPP2 (1:400), E-cadherin (1:300), N-cadherin (1:400), GSK3β (1:200), p-GSK3β (1:200), Vimentin (1:500), p38 MAPK (1:400), p-p38 MAPK (1:400), p53 (1:200), p-P53 (1:200), Bax (1:100), cleaved caspase-3 (1:1000), β-tubulin (1:1000), PARP (1:1000), and GAPDH (1:2000), respectively, at 4 °C overnight. All primary antibodies were from Santa Cruz Biotechnology (USA). After washing, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature. Signals were detected using an ECL detection system (GE Healthcare, USA) and analyzed by ImageJ 1.42q software (National Institutes of Health).
Construction and transfection
Full-length human PPM1D cDNA was generated by PCR using specific primers (forward, 5′-GGCCTGCAGTCACTTTCTTGAATCGAGTTCA-3′; reverse, 5′-CAGGAATTCCTACCCACTTGAGAAGCGGCTGATG-3′). The amplified fragment was subcloned into pEGFP-C1 plasmid (GeneChem, Shanghai, China) after Bgl II/Pst I restriction enzyme digestion. MIA PACA-2 and PANC-1 cells were transfected with pEGFP-C1 plasmid or control plasmid using Lipofectamine Plus (Invitrogen, USA).
Small interfering RNAs (siRNA) specific for PPM1D (siPPM1D) and β-catenin (siβ-catenin) and a scrambled siRNA used as control (SCR) were synthesized by GenScript (siPPM1D, 5′-GTGGGAGTGTAATGAACAATT-3′; siβ-catenin, 5′-CGGAGGAGATGTACATTCATT-3′; SCR, 5′-CGCTCGAGAACAAGATTCATT-3′). MIA PaCa-2 and PANC-1 cells were transfected with siRNA using oligofectamine following manufacturer’s instructions (Invitrogen, USA).
Immunoprecipitation
Cells were lysed in 20 mM Tris–HCl (pH 7.5) containing 1 mM EDTA, 1 M KCl, 5 mM MgCl2, 10 % glycerol (v/v), 1 % Triton X-100 (v/v), 0.05 % 2-mercaptoethanol (v/v), and protease and phosphatase inhibitors. The cell lysates (1 − 4 mg) were pre-cleared with protein G beads at 4 °C for 30 min and subsequently incubated with protein G beads pre-bound with antibody at 4 °C for 2 − 16 h. The beads were washed three times with 1 % NP40, mixed with 6 × sample buffer, and subjected to SDS-PAGE and immunoblotting.
Quantitative real-time PCR
Total RNA was extracted and purified using the Trizol reagent (Invitrogen, Carlsbad, CA, USA) following manufacturer’s instructions. cDNA was prepared as previously described [23]. Quantitative real-time polymerase chain reaction (PCR) was performed using the SYBR Green Real-time PCR Master Mix (Applied Biosystems, Foster City, CA, USA) on an ABI StepOnePlus real-time PCR system. The specific primers used in the PCR were as follows: GAPDH, 5′-ATGGGGAAGGTGAAGGTCG-3′ (sense) and 5′-GGGGTCATTGATGGCAACAATA-3′ (antisense); PPM1D, 5′- GAAGGATGACTTTGTCAG-3′ (sense) and 5′-CCCAGACTTGTTCATTAC-3′ (antisense); β-catenin, 5′-AGTCCGGAGGAGATGTACA-3′ (sense) and 5′-CGGTCTTCCGTCTCCGACC-3′ (antisense). The cycle threshold (Ct) values were standardized to Ct values of GAPDH. Fold difference in occupancy was calculated as follows: Fold difference = \(2^{{ - \varDelta \varDelta c_{\text{t}} }}\).
Immunohistochemical analysis
The immunohistochemical staining of PPM1D in tissue and cell samples was performed as described in our previous reports [24, 25] and scored by two investigators blinded to the treatment conditions. Staining intensity was denoted as 0 (negative), 1 (weak), 2 (moderate), or 3 (strong). Staining density was scored based on the percentage of positively stained cells as follows: 0, less than 5 %; 1, 5–25 %; 2, 25–50 %; or 3, more than 50 %. The final score was calculated as the sum of the intensity and density scores. A final score of 0–2 were considered low expression whereas a final score of 3–6 were considered high expression.
Flow cytometric analysis
Cells were harvested and fixed in precooled 70 % ethanol for 1 h. The cells were sequentially centrifuged at 100 g for 5 min and resuspended in phosphate-buffered saline (PBS). The cells were double stained with Annexin V-FITC and propidium iodide (Sigma-Aldrich) at 4 °C for 30 min in the dark and subjected to flow cytometric analysis. Each experiment was conducted in triplicate.
Animal experiments
Five-week-old male nude mice purchased from Shanghai Slac Laboratory Animal Co. Ltd. (Shanghai, China) were divided into three groups of six mice each. A total of 5 × 106 cells transfected with PPM1D, siPPM1D, or their irrespective controls were suspended in Eagle’s minimal essential medium (EMEM) and injected subcutaneously into the right flank of each mouse. The tumor diameter was measured 4, 8, 12, 16, 20, 24, and 28 days after the injection. The tumor volume was calculated using the formula V = 0.4 × ab2 (V, volume; a, the largest diameter; b, the smallest diameter). The mice were photographed and sacrificed on day 28, and the tumors were dissected and weighed.
Statistical analysis
All data are presented as mean ± SD (standard deviation). Differences between two groups were analyzed using the Student two-tailed unpaired t test. Differences with a P value < 0.05 were considered statistically significant.
Results
PPM1D is overexpressed in human PC tissues and cell lines
The PPM1D protein expression in 65 human PC and paired adjacent noncancerous tissues was assessed by both immunohistochemical staining and western blotting. The mRNA expression was determined by qRT-PCR. Our immunohistochemical staining assay showed that, in 36 out of the 65 tissue pairs, significantly increased PPM1D levels were detected in the PC tissues compared with the adjacent noncancerous tissues (Fig. 1a; Table 1). The PPM1D overexpression in PC tissues was confirmed by western blotting (Fig. 1b) and qRT-PCR analysis (Fig. 1b, c). Moreover, the high PPM1D expression in PC tissues was significantly correlated with tumor grade (P = 0.015), tumor size (P = 0.023), vascular invasion (P = 0.039), and lymph node metastases (P = 0.021) (Table 1). These results highlighted the prognostic significance of PPM1D in PC. Similarly, we found significantly higher mRNA and protein levels of PPM1D in the two human PC cell lines MIA PaCa-2 and PANC-1 than the human normal pancreatic epithelial cell line HPDE6-C7 (Fig. 1d, e). Therefore, PPM1D is overexpressed in both human PC tissues and cell lines.
In contrast to PPM1D, we detected lower Aspp2 levels in cancerous tissues in comparison with para-cancerous tissues in PC patients. Similarly, lower levels of Aspp2 were detected in MIA PaCa-2 and PANC-1 cells in comparison with HPDE6-C7 cells (data not shown). These results were consistent with previous findings by Song et al.
PPM1D overexpression promotes PC cell migration and invasion in vitro
The significant correlation between high PPM1D expression in human PC tissues and vascular invasion and lymph node metastases suggested that PPM1D might regulate PC cell migration and invasion. To test this hypothesis, we investigated the effects of PPM1D overexpression on PC cell migration and invasion in vitro. MIA PaCa-2 and PANC-1 cells transfected with PPM1D for 48 h showed significantly increased PPM1D mRNA expression than cells transfected with vector alone (Fig. 2a, b). In our wound-healing migration assay, PPM1D-transfected MIA PaCa-2 and PANC-1 cells migrated faster than vector-transfected cells (Fig. 2c). Furthermore, these PPM1D-transfected PC cells showed significantly higher migratory potential and invasiveness than vector-transfected cells in the Boyden transwell assays (Fig. 2d–g). Collectively, these data demonstrated that PPM1D promotes PC cell migration and invasion in vitro.
PPM1D promotes PC cell migration and invasion. MIA PaCa-2 and PANC-1 cells were transfected with PPM1D or vector alone for 48 h. Untransfected cells were included as control. a and b The PPM1D mRNA and protein expression in transfected MIA PaCa-2 and PANC-1 cells. n = 3, *P < 0.05. c Migration of transfected MIA PaCa-2 and PANC-1 cells in the wound healing migration assay. Images shown are representative of three experiments. d–g Invasion and migration of transfected MIA PaCa-2 and PANC-1 cells assessed using Boyden transwell chambers. n = 3, *P < 0.05 versus vector alone. NS no significant difference
PPM1D downregulates ASPP2 expression, reduces the ASPP2-β-catenin-E-cadherin ternary complex formation, and enhances β-catenin nuclear translocation in PC cells
Epithelial to mesenchymal transition (EMT) is associated with enhanced cell migration and invasion, and cancer metastasis [26]. EMT requires a disruption of apical–basal polarity and loss of E-cadherin expression. The β-catenin–TCF (T-cell factor) complex activates the transcription of the zinc finger E-box binding homeobox 1 protein (ZEB1), a transcription factor that represses E-cadherin expression [27]. It has been shown that ASPP2 negatively regulates metastasis through inhibition of the Wnt/β-catenin signaling pathway and subsequent β-catenin-dependent ZEB1 expression [21]. ASPP2 forms a ternary complex with β-catenin–E-cadherin and thereby inhibits β-catenin nuclear translocation. Moreover, ASPP2 potentiates the RAS-AKT signaling, which inhibits glycogen synthase kinase 3β (GSK3β). GSK3β phosphorylates β-catenin at Ser-33, Ser-37, and Thr-41, leading to impaired β-catenin binding to E-cadherin [28, 29]. Thus, ASPP2 may stabilize the β-catenin–E-cadherin complex via inhibition of GSK3β-mediated β-catenin N-terminal phosphorylation through its ability to enhance RAS signaling [30]. To find out whether ASPP2 plays a role in increased migratory potential and invasiveness of PPM1D-transfected MIA PaCa-2 and PANC-1 cells, we assessed the expression of ASPP2, β-catenin, and E-cadherin, the formation of the ASPP2-β-catenin–E-cadherin ternary complex, and β-catenin nuclear translocation in these cells. Our immunofluorescence staining data showed that PPM1D-transfected PANC-1 or MIA PaCa-2 cells had decreased ASPP2 and E-cadherin expression but increased β-catenin expression compared with cells transfected with vector alone (Fig. 3a). Western blot analysis of the cytoplasmic and nuclear fractions revealed increased β-catenin nuclear translocation in PPM1D-transfected MIA PaCa-2 and PANC-1 cells (Fig. 3b, c). In addition, our western blot analysis of the whole cell lysates revealed enhanced GSK3β activity (decreased phosphorylation at Ser-9) and increased β-catenin phosphorylation at Ser-33, Ser-37, and Thr-41 in these PPM1D-transfected PC cells (Fig. 3d, e). Consistent with these results, our coimmunoprecipitation data suggested reduced formation of the ASPP2-β-catenin–E-cadherin ternary complex in these PC cells (Fig. 3d, e). Taken together, these data suggested that PPM1D potentiates the Wnt/β-catenin signaling pathway in PC cells by promoting β-catenin nuclear translocation, which is likely mediated by downregulation of ASPP2.
PPM1D downregulates ASPP2 expression, inhibits the ASPP2-β-catenin-E-cadherin ternary complex formation, and enhances β-catenin nuclear translocation in PC cells. a Immunofluorescence staining of ASPP2 (green), E-cadherin (green), and β-catenin (green) in PPM1D or vector-transfected PANC-1 cells. DAPI (blue) was used to stain nuclei. b and c The cytoplasmic and nuclear levels of β-catenin and AAPP2 of MIA PaCa-2 (b) and PANC-1 (c) cells transfected with PPM1D or vector alone assessed by western blotting. Beta-tubulin and PARP were used as loading controls for the cytoplasmic and nuclear fractions, respectively. d and e The protein levels of p-β-catenin, p-GSK3, GSK3, and ASPP2 in MIA PaCa-2 (d) and PANC-1 (e) cells transfected with PPM1D or vector alone assessed by western blotting. In addition, cell lysates were immunoprecipitated with an anti-ASPP2 antibody or control IgG and subjected to western blot analysis. GAPDH was used as a loading control (Color figure online)
PPM1D overexpression promotes EMT of PC cells through potentiation of the Wnt/β-catenin signaling pathway
To directly investigate the role of the Wnt/β-catenin signaling pathway in the regulatory effects of PPM1D on PC cell migration and invasion, we used small interfering RNA (siRNA) to inhibit β-catenin expression in MIA PaCa-2 and PANC-1 cells. Transfection with siβ-catenin resulted in over 70 % decrease in β-catenin mRNA expression whilst transfection with a scrambled siRNA had no significant effects (Fig. 4c, f). We subsequently assessed the protein levels of ZEB1, ASPP2, E-cadherin, N-cadherin, Vimentin (a EMT marker), p-β-catenin, and β-catenin in untransfected (control), PPM1D-transfected, or PPM1D and siβ-catenin-cotransfected MIA PaCa-2 and PANC-1 cells by western blotting. Our data showed that PPM1D-transfected cells had significantly increased ZEB1 expression and decreased E-cadherin expression (Fig. 4a, b, d, e), consistent with PPM1D-enhanced β-catenin nuclear translocation. Interestingly, PPM1D-transfected cells also had increased levels of N-cadherin, a cadherin family member that promotes metastasis. Moreover, PPM1D-transfected cells had significantly elevated levels of Vimentin, an EMT protein marker. These results indicated that PPM1D overexpression promotes EMT in PC cells. Importantly, these PPM1D overexpression-induced changes in EMT markers (E-cadherin, N-cadherin, and Vimentin) were reversed by β-catenin silencing with siβ-catenin transfection (Fig. 4a, b, d, e), providing direct evidence that PPM1D promotes EMT in PC cells through potentiation of the Wnt/β-catenin signaling pathway.
PPM1D promotes PC cell migration and invasion through potentiation of the Wnt/β-catenin signaling pathway. a, b and d, e The protein levels of ZEB1, ASPP2, E-cadherin, N-cadherin, Vimentin, p-β-catenin, and β-catenin in untransfected (control), PPM1D-transfected, or PPM1D and siβ-catenin-cotransfected MIA PaCa-2 (a, b) and PANC-1 (d, e) cells by western blotting. GAPDH was used as a loading control. c and f The β-catenin mRNA expression in untransfected (control) and SCR or siβ-catenin-transfected MIA PaCa-2 (c) and PANC-1 (f) cells by qRT-PCR. n = 3, *P < 0.05, **P < 0.01, ***P < 0.001 versus Control. NS no significant difference
PPM1D silencing inhibits EMT, migration, and invasion of PC cells
To further investigate the regulatory role of PPM1D in PC cell EMT, migration, and invasion, we used siRNA to inhibit PPM1D expression in MIA PaCa-2 and PANC-1 cells. Transfection with siPPM1D led to significantly reduced PPM1D mRNA and protein expression in these two PC cell lines (Fig. 5a–d). Our western blot analysis revealed significantly decreased p-β-catenin, ZEB1, N-cadherin, and Vimentin expression and increased E-cadherin expression (Fig. 6a–d) in siPPM1D-transfected cells compared with cells transfected with a scrambled siRNA. These data indicated that PPM1D silencing inhibits EMT in PC cells, likely via suppression of the Wnt/β-catenin pathway. In contrast to enhanced migratory activity and invasiveness of PPM1D-transfected MIA PaCa-2 and PANC-1 cells (Fig. 2d–g), siPPM1D-transfected cells exhibited significantly reduced migratory activity and invasiveness in the Boyden transwell assays (Fig. 6e). Therefore, opposite to the effects of PPM1D overexpression, PPM1D silencing inhibits EMT, migration, and invasion of PC cells.
PPM1D silencing inhibits EMT, migration, and invasion of PC cells through suppression of the Wnt/β-catenin pathway. PANC-1 and MIA PaCa-2 cells were transfected with siPPM1D or scrambled siRNA (SCR) for 48 h. a–d The protein levels of p-β-catenin, E-cadherin, N-cadherin, Vimentin, and ZEB1 in transfected PANC-1 (a and c) and MIA PaCa-2 (b and d) cells by western blotting. e Cell invasion and migration determined by Boyden transwell assays. n = 3, *P < 0.05, **P < 0.01, ***P < 0.001 versus control. NS no significant difference
PPM1D inhibits the p38 MAPK/p53 signaling pathway in PC cells through downregulation of ASPP2
PPM1D has been reported to negatively regulate the p38 MAPK/p53 signaling pathway through dephosphorylation of p38 MAPK, suppressing p53-mediated transcription and apoptosis in response to stress [5]. To investigate this possible functional relationship in PC cells, we determined the levels of signaling proteins involved in the p38 MAPK/p53 pathway in MIA PaCa-2 and PANC-1 cells transfected with PPM1D or siPPM1D. Our data showed that PPM1D overexpression in these two PC cell lines reduced the p-p38 MAPK and p-p53 levels, resulting in attenuated caspase-3 cleavage and Bax expression (Fig. 7a, b). In contrast, PPM1D silencing led to increased p38 MAPK and p53 phosphorylation and enhanced caspase-3 cleavage and Bax expression (Fig. 8a–d). Therefore, PPM1D negatively regulates the p38 MAPK/p53 pathway in PC cells. Moreover, PPM1D silencing in MIA PaCa-2 and PANC-1 cells led to increased ASPP2 expression (Fig. 8a–d). Treatment with the p38 MAPK inhibitor SB203580 reversed PPM1D silencing-induced p53 phosphorylation and ASPP2 phosphorylation and nuclear translocation, but not ASPP2 expression (Fig. 9a, b), suggesting that PPM1D regulates ASPP2 expression independent of the p38 MAPK/p53 pathway. ASPP2 has been reported to bind to p53 and enhances its transcriptional activity [16]. Our coimmunoprecipitation data revealed enhanced p53 binding to ASPP2 in siPPM1D-transfected MIA PaCa-2 and PANC-1 cells (Fig. 9c, d), presumably attributed to increased ASPP2 expression. These data suggested that increased ASPP2 expression contributed at least partially to PPM1D silencing-enhanced p38 MAPK/p53 signaling in PC cells.
PPM1D silencing in PC cells potentiates the p38 MAPK/p53 pathway. a–d The protein levels of ASPP2, p38 MAPK, p-p38 MAPK, p53, p-p53, cleaved caspase-3, and Bax in MIA PaCa-2 (c and d) and PANC-1 (a and b) cells transfected with siPPM1D or scrambled siRNA (SCR) for 48 h by western blotting. n = 3, *P < 0.05 versus control
PPM1D inhibits the p38 MAPK/p53 signaling pathway through downregulation of ASPP2. MIA PaCa-2 and PANC-1 cells were transfected with siPPM1D or scrambled siRNA (SCR) for 48 h. a and b The protein levels of ASPP2, p-ASPP2, nuclear p-ASPP2, p53, and p-p53 in transfected MIA PaCa-2 (a) and PANC-1 (b) cells by western blotting. Cells were treated with the p38 MAPK inhibitor SB203580 as indicated for 48 h. c and d Total cell lysates from transfected MIA PaCa-2 (c) and PANC-1 (d) cells were immunoprecipitated with an anti-ASPP2 antibody or control IgG and subjected to western blot analysis. GAPDH was used as a loading control
PPM1D silencing increases PC cell apoptosis
Since we have demonstrated that PPM1D negatively regulates the proapoptotic p38 MAPK/p53 signaling pathway in PC cells, we speculated that PPM1D silencing would potentiate p53-mediated PC cell apoptosis. Indeed, siPPM1D-transfected MIA PaCa-2 and PANC-1 cells exhibited significantly increased apoptosis than SCR-transfected cells when analyzed by flow cytometry using V-FITC/PI double staining (Fig. 10). Considering that we have shown that PPM1D is overexpressed in human PC tissues and its high expression is correlated with tumor progression and metastasis, our in vitro data suggested that PPM1D functions as an oncogene in PC.
PPM1D silencing increases PC cell apoptosis. MIA PaCa-2 and PANC-1 cells were transfected with siPPM1D or scrambled siRNA (SCR) for 48 h. Untransfected cells were included as control. Cells were double-labeled with Annexin V-FITC and PI and subjected to analysis by flow cytometry. n = 3, *P < 0.05 versus Control. NS no significant difference
PPM1D promotes PC tumor growth in vivo
To investigate the in vivo relevance of our in vitro findings, we established a PC xenograft model by implanting untransfected and PPM1D or siPPM1D-transfected PANC-1 cells into male nude mice. The tumor volume was measured at least three times a week up to four weeks. The mice were sacrificed after 4 weeks and the tumor weight was determined. Compared with tumors derived from untransfected cells, tumors from PPM1D-transfected cells grew at a significantly faster rate while those from siPPM1D-transfected cells grew at a significantly slower rate (Fig. 11b), resulting in significantly higher tumor weight in the PPM1D-transfected group and lower tumor weight in the siPPM1D-transfected group 4 weeks after the implantation (Fig. 11a, c). Thus, our data showed that PPM1D promotes PC tumor growth in vivo.
PPM1D promotes PC tumor growth in vivo. Untransfected and PPM1D or siPPM1D-transfected PANC-1 cells were implanted into the right flank of nude mice. a Images of representative tumors harvested 4 weeks after the implantation. b Tumor size measured at least three times a week up to 4 weeks. c Tumor weight determined 4 weeks after the implantation. n = 6, *P < 0.05 versus control
Discussion
Despite the recent medical advances, pancreatic cancer (PC) represents the fourth leading cause of cancer-related death in Western countries [31]. Efforts towards identifying sensitive molecular markers for early diagnosis are currently leading the clinical study of this fatal disease [32]. PPM1D, an oncogenic protein phosphatase, has been shown to be overexpressed in a number of cancers and associated with clinical outcome in patients, however, its clinical significance in PC has never been defined. In the present study, we determined the PPM1D expression in human PC tissues and paired adjacent noncancerous tissues. We detected significantly increased PPM1D levels in the PC tissues in 36 out of the 65 tissue pairs using immunohistochemical staining (Fig. 1a; Table 1). Our western blot and qRT-PCR analysis confirmed the PPM1D overexpression in PC tissues (Fig. 1b, c). Importantly, we found that the high PPM1D expression in PC tissues was significantly correlated with tumor grade (P = 0.015), tumor size (P = 0.023), vascular invasion (P = 0.039), and lymph node metastases (P = 0.021) (Table 1). We also detected significantly higher PPM1D levels in two human PC cell lines MIA PaCa-2 and PANC-1 than the human normal pancreatic epithelial cell line HPDE6-C7 (Fig. 1d, e). These clinical and in vitro data provided evidence that PPM1D may be a potential molecular marker for PC diagnosis and prognosis.
To define the functional significance of PPM1D overexpression in PC vascular invasion and lymph node metastases, we studied the effects of PPM1D overexpression and silencing on PC cell migratory properties and invasiveness in vitro. The results showed that PPM1D overexpression in MIA PaCa-2 and PANC-1 cells significantly increased cell migration and invasion (Fig. 2c–g) whilst PPM1D silencing had the opposite effects (Fig. 7e). Although PPM1D has been shown to promote cancer cell migration in vitro [33], the underlying molecular mechanisms are not defined. The Wnt/β-catenin signaling pathway promotes cell migration by inducing ZEB1 expression. A recent article has reported that ASPP2 inhibits β-catenin nuclear translocation and thereby suppresses Wnt/β-catenin-mediated cancer metastasis [21]. ASPP2 inhibits β-catenin nuclear translocation by [1] directly forming a ternary complex with β-catenin–E-cadherin and [2] stabilizing the β-catenin–E-cadherin complex via inhibition of GSK3β-mediated β-catenin N-terminal phosphorylation through its ability to enhance RAS signaling [30]. We found that PPM1D overexpression in MIA PaCa-2 and PANC-1 cells resulted in reduced ASPP2 expression and increased β-catenin nuclear translocation, which were accompanied by enhanced GSK3β activity, increased β-catenin N-terminal phosphorylation, and reduced formation of the ASPP2-β-catenin–E-cadherin ternary complex (Fig. 3). These data suggested that PPM1D overexpression potentiates the Wnt/β-catenin signaling via downregulation of ASPP2 in PC cells. Further studies showed that PPM1D overexpression in MIA PaCa-2 and PANC-1 cells significantly increased the expression of ZEB1, N-cadherin, and the EMT marker Vimentin, but decreased E-cadherin expression (Fig. 4a, b, d, e) whilst PPM1D silencing had the opposite effects (Fig. 7a, b), indicating that PPM1D promotes EMT, a hallmark of cancer cell invasion and metastasis, in PC cells. Moreover, the PPM1D overexpression-induced changes in the expression of EMT-related proteins (ZEB1, E-cadherin, N-cadherin, and Vimentin) were reversed by β-catenin silencing (Fig. 4). Taken together, these data suggested that PPM1D promotes PC cell migration and invasion via potentiation of the Wnt/β-catenin signaling pathway through its ability to downregulate ASPP2.
PPM1D, which is a negative regulator of the p38 MAPK/p53 pathway, has been reported to inhibit p53-mediated cancer cell apoptosis [10, 33]. To investigate the clinical significance of PPM1D overexpression in PC tumorigenesis, we studied the effects of PPM1D overexpression and silencing on the p38 MAPK/p53 pathway and apoptosis of PC cells in vitro. We found that PPM1D overexpression in MIA PaCa-2 and PANC-1 cells significantly reduced the p-p38 MAPK and p-p53 protein levels and attenuated caspase-3 cleavage and Bax expression (Fig. 5a, b) whilst PPM1D silencing had the opposite effects (Fig. 8a–d), indicating that PPM1D negatively regulates the p38 MAPK/p53 pathway and p53-dependent transcription of proapoptotic genes in PC cells, likely by directly dephosphorylating p38 MAPK as previously reported [5]. Indeed, we found that PPM1D silencing in MIA PaCa-2 and PANC-1 cells significantly increased cell apoptosis (Fig. 10). ASPP2 has been reported to bind to p53 and enhances its transcriptional activity [16]. Importantly, We found that PPM1D silencing in MIA PaCa-2 and PANC-1 cells led to increased ASPP2 expression (Fig. 8a–d) and enhanced p53 binding to ASPP2 (Fig. 9c, d). These data suggested that PPM1D inhibits p53-mediated PC cell apoptosis through both direct and indirect mechanisms. Directly, PPM1D inactivates p38 MAPK and p53 by dephosphorylation. Indirectly, PPM1D reduces p53 transcriptional activity by downregulating ASPP2. Although the p38 MAPK inhibitor SB203580 inhibited ASPP2 phosphorylation and nuclear translocation, it had no effects on ASPP2 expression in MIA PaCa-2 and PANC-1 cells (Fig. 9a, b), suggesting that PPM1D regulates ASPP2 expression independent of the p38 MAPK/p53 pathway in these cells.
Finally, the oncogenic properties of PPM1D in PC were confirmed in our in vivo study, in which PPM1D overexpression accelerated whilst PPM1D silencing slowed PC tumor growth in a mouse xenograft model (Fig. 11).
In summary, our data demonstrated that PPM1D is overexpressed in PC and correlated with disease progression and metastasis. PPM1D promotes PC cell migration and invasion through potentiation of the Wnt/β-catenin pathway and suppresses PC cell apoptosis through inhibition of the p38 MAPK/p53 pathway. These oncogenic effects of PPM1D in PC are mediated by downregulation of ASPP2 (Fig. 12). Our results suggest that PPM1D is a biomarker for PC diagnosis and prognosis and a potential target for therapeutic intervention of this fatal disease.
References
Rossi M, Demidov ON, Anderson CW, Appella E, Mazur SJ (2008) Induction of PPM1D following DNA-damaging treatments through a conserved p53 response element coincides with a shift in the use of transcription initiation sites. Nucleic Acids Res 36:7168–7180
Lowe J, Cha H, Lee MO, Mazur SJ, Appella E, Fornace AJ Jr (2012) Regulation of the Wip1 phosphatase and its effects on the stress response. Front Biosci (Landmark Ed) 17:1480–1498
Han HS, Yu E, Song JY, Park JY, Jang SJ, Choi J (2009) The estrogen receptor alpha pathway induces oncogenic Wip1 phosphatase gene expression. Mol Cancer Res 7:713–723
Lowe JM, Cha H, Yang Q, Fornace AJ Jr (2010) Nuclear factor-kappaB (NF-kappaB) is a novel positive transcriptional regulator of the oncogenic Wip1 phosphatase. J Biol Chem 285:5249–5257
Takekawa M, Adachi M, Nakahata A et al (2000) p53-Inducible wip1 phosphatase mediates a negative feedback regulation of p38 MAPK-p53 signaling in response to UV radiation. EMBO J 19:6517–6526
Le Guezennec X, Bulavin DV (2010) WIP1 phosphatase at the crossroads of cancer and aging. Trends Biochem Sci 35:109–114
Emelyanov A, Bulavin DV (2014) Wip1 phosphatase in breast cancer. Oncogene
Ruark E, Snape K, Humburg P et al (2013) Mosaic PPM1D mutations are associated with predisposition to breast and ovarian cancer. Nature 493:406–410
Yang H, Gao XY, Li P, Jiang TS (2015) PPM1D overexpression predicts poor prognosis in non-small cell lung cancer. Tumour Biol 36:2179–2184
Sun GG, Zhang J, Ma XB, Wang YD, Cheng YJ, Hu WN (2015) Overexpression of wild-type p53-induced phosphatase 1 confers poor prognosis of patients with nasopharyngeal carcinoma. Pathol Oncol Res 21:283–291
Liu S, Qi L, Han W et al (2014) Overexpression of wip1 is associated with biologic behavior in human clear cell renal cell carcinoma. PLoS ONE 9:e110218
Bulavin DV, Phillips C, Nannenga B et al (2004) Inactivation of the Wip1 phosphatase inhibits mammary tumorigenesis through p38 MAPK-mediated activation of the p16(Ink4a)-p19(Arf) pathway. Nat Genet 36:343–350
Nannenga B, Lu X, Dumble M et al (2006) Augmented cancer resistance and DNA damage response phenotypes in PPM1D null mice. Mol Carcinog 45:594–604
Demidov ON, Timofeev O, Lwin HN, Kek C, Appella E, Bulavin DV (2007) Wip1 phosphatase regulates p53-dependent apoptosis of stem cells and tumorigenesis in the mouse intestine. Cell Stem Cell 1:180–190
Wang W, Zhu H, Zhang H, Zhang L, Ding Q, Jiang H (2014) Targeting PPM1D by lentivirus-mediated RNA interference inhibits the tumorigenicity of bladder cancer cells. Braz J Med Biol Res 47(12):1044–1049
Samuels-Lev Y, O’Connor DJ, Bergamaschi D et al (2001) ASPP proteins specifically stimulate the apoptotic function of p53. Mol Cell 8:781–794
Trigiante G, Lu X (2006) ASPP [corrected] and cancer. Nat Rev Cancer 6:217–226
Sgroi DC, Teng S, Robinson G, LeVangie R, Hudson JR Jr, Elkahloun AG (1999) In vivo gene expression profile analysis of human breast cancer progression. Cancer Res 59:5656–5661
Liu ZJ, Lu X, Zhang Y et al (2005) Downregulated mRNA expression of ASPP and the hypermethylation of the 5′-untranslated region in cancer cell lines retaining wild-type p53. FEBS Lett 579:1587–1590
Godin-Heymann N, Wang Y, Slee E, Lu X (2013) Phosphorylation of ASPP2 by RAS/MAPK pathway is critical for its full pro-apoptotic function. PLoS ONE 8:e82022
Wang Y, Bu F, Royer C et al (2014) ASPP2 controls epithelial plasticity and inhibits metastasis through beta-catenin-dependent regulation of ZEB1. Nat Cell Biol 16:1092–1104
Radulovich N, Qian JY, Tsao MS (2008) Human pancreatic duct epithelial cell model for KRAS transformation. Methods Enzymol 439:1–13
Dudgeon C, Shreeram S, Tanoue K et al (2013) Genetic variants and mutations of PPM1D control the response to DNA damage. Cell Cycle 12:2656–2664
Yuan J, Liu S, Yu Q et al (2013) Down-regulation of human leukocyte antigen class I (HLA-I) is associated with poor prognosis in patients with clear cell renal cell carcinoma. Acta Histochem 115:470–474
Liu S, Qi L, Yu Q et al (2014) Survivin and HLA-I expression predicts survival of patients with clear cell renal cell carcinoma. Tumour Biol 35:8281–8288
Thiery JP, Acloque H, Huang RY, Nieto MA (2009) Epithelial-mesenchymal transitions in development and disease. Cell 139:871–890
Sanchez-Tillo E, de Barrios O, Siles L, Cuatrecasas M, Castells A, Postigo A (2011) Beta-catenin/TCF4 complex induces the epithelial-to-mesenchymal transition (EMT)-activator ZEB1 to regulate tumor invasiveness. Proc Natl Acad Sci USA 108:19204–19209
Sadot E, Conacci-Sorrell M, Zhurinsky J et al (2002) Regulation of S33/S37 phosphorylated beta-catenin in normal and transformed cells. J Cell Sci 115:2771–2780
Maher MT, Mo R, Flozak AS, Peled ON, Gottardi CJ (2010) Beta-catenin phosphorylated at serine 45 is spatially uncoupled from beta-catenin phosphorylated in the GSK3 domain: implications for signaling. PLoS ONE 5:e10184
Wang Z, Liu Y, Takahashi M et al (2013) N terminus of ASPP2 binds to Ras and enhances Ras/Raf/MEK/ERK activation to promote oncogene-induced senescence. Proc Natl Acad Sci USA 110:312–317
Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ (2003) Cancer statistics, 2003. CA Cancer J Clin 53:5–26
He XY, Yuan YZ (2014) Advances in pancreatic cancer research: moving towards early detection. World J Gastroenterol 20:11241–11248
Castellino RC, De Bortoli M, Lu X et al (2008) Medulloblastomas overexpress the p53-inactivating oncogene WIP1/PPM1D. J Neurooncol 86:245–256
Acknowledgments
This study was supported by Department of General Surgery, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, Shanghai, China.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Bo Wu and Bo-Min Guo are co-first authors and these authors have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wu, B., Guo, BM., Kang, J. et al. PPM1D exerts its oncogenic properties in human pancreatic cancer through multiple mechanisms. Apoptosis 21, 365–378 (2016). https://doi.org/10.1007/s10495-015-1211-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-015-1211-4