Abstract
Objective
IL-8-251A>T polymorphisms have been reported to influence the risk for breast cancer in many studies; however, the results still remain controversial and ambiguous. The aim of this study was to determine more precise estimations for the relationship between IL-8-251A>T polymorphisms and the risk for breast cancer.
Methods
Electronic searches for all publications were conducted on association between this variant and breast cancer in several databases through November 2010. Crude odds ratios (ORs) with 95% confidence intervals (CIs) were calculated to estimate the strength of the association. Six studies were identified, including 1,880 breast cancer patients and 2,013 controls.
Results
Overall, no significant associations between IL-8-251A>T polymorphism and breast cancer (codominant model: TA vs. AA OR = 1.075, 95%CI = 0.864–1.337; TT vs. AA, OR = 0.900, 95%CI = 0.598–1.354; dominant model: OR = 1.011, 95%CI = 0.783–1.304; and recessive model: OR = 0.854, 95%CI = 0.623–1.171). In the subgroup analysis by ethnicity, significantly decreased risk was found for Africans (TT vs. AA OR = 0.541; 95%CI = 0.396–0.741; dominant model: OR = 0.737, 95%CI = 0.570–0.953; recessive model: OR = 0.594; 95%CI = 0.459–0.768). In the stratified analysis by control sources, significant association was observed in population-based studies (recessive model: OR = 0.692; 95%CI = 0.566–0.861).
Conclusions
This meta-analysis suggests the IL-8-251A/T polymorphism is associated with breast cancer risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most prevalent common cancer among women around the world (Parkin et al. 2005). The mechanism of breast cancer is still unclear. In recent years, genetic factors are increasingly recognized as major contributors to breast cancer risk (Weber and Nathanson 2000; Ponder 2001). Single nucleotide polymorphisms in cytokine genes are thought to influence expression of the encoding proteins and/or activity of encoding proteins thereby predisposing to breast cancer (De Jong et al. 2002). An important one is the interleukin-8 (IL-8) gene, located on chromosome 4q13–q21 in humans, comprises of four exons, three introns, and a proximal promoter region (Mukaida et al. 1989). A common single nucleotide polymorphism at position -251 of the IL-8 promoter region was described in 2000, and consequent evidences demonstrated the IL-8-251A/T polymorphism was associated with IL-8 production or protein expression both in vivo and in vitro (Hull et al. 2000; Taguchi et al. 2005; Ohyauchi et al. 2005). To date, many epidemiological studies have been done to evaluate the association between IL-8-251A>T polymorphism and breast cancer (Liu et al. 2007; Kamali–Sarvestani et al. 2007; Smith et al. 2004; Snoussi et al. 2006, 2010; Vogel et al. 2007). However, the results were rather inconsistent, partially due to the relative small sample size of individual studies. Therefore, we performed this meta-analysis of 6 eligible studies to get a more precise estimation of the association.
Materials and methods
Publication search
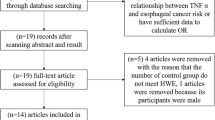
Articles were identified by an electronic search on Medline, Embase, and the Cochrane Library using the following keywords: interleukin-8 or IL-8 or IL8 and breast (last search: November 30, 2010). Eligible studies were retrieved and examined carefully. Review articles and bibliographies of other relevant studies identified were hand-searched to find additional eligible studies. The studies included must meet the following criteria: (1) evaluation of the IL-8-251A>T polymorphism and breast cancer risk; (2) case–control study; (3) at least two comparison groups (breast cancer group vs. control group).
Data extraction
Two authors (FS and JHY) independently extracted data and entered them in a customized database. Reviews, non-original articles, and studies on breast cancer cell lines and animal models were excluded from our meta-analysis. Discrepancies about inclusion of studies and interpretation of data were resolved by discussion, consensus, and arbitration by (QLJ or QH). The following data were collected from each study: first author’s name, year of publication, ethnicity, country of origin, sources of controls, genotyping method, Hardy–Weinberg equilibrium (HWE), and number of different genotypes in cases and controls.
Statistical methods
Logistic regression analysis was used to examine the association between the IL-8-251A>T polymorphism and breast cancer risk (Thakkinstian et al. 2005). Crude ORs with 95%CIs were used to assess the strength of association between the L-8-251A>T polymorphism and breast cancer risk. We evaluated the risk of the codominant model (TA vs. AA; TT vs. AA), the dominant model (TT/TA vs. AA), and the recessive model (TT vs. TA/AA), respectively. Heterogeneity assumption was checked by the chi-square based Q test and was regarded to indicate significance for P < 0.1 (Cochran 1954). The fixed model would be used if the test of heterogeneity was not significant, otherwise the random-effect model would be used (Mantel and Haenszel 1959; DerSimonian and Laird 1986). Heterogeneity among studies was assessed by I 2 statistic interpreted as the proportion of total variation contributed by variation between studies. Sensitivity analysis was carried out by including and excluding studies not in HWE (Thakkinstian et al. 2005). An estimate of potential publication bias was evaluated by the funnel plot, in which the standard error of log (OR) of each study was plotted against its log (OR). An asymmetric plot suggests a possible publication bias. Funnel plot asymmetry was assessed by the method of Egger’s linear regression test, if P < 0.05, the publication bias was statistically significant. Subgroup analyses were performed by ethnicities and sources of controls. All statistical analyses were carried out with STATA software, version 10.0 (STATA Corp., College Station, TX).
Results
Study characteristics
A total of 6 studies fulfilling the inclusion criteria were identified. Table 1 listed the detailed characteristics of these studies. Totally 1,880 breast cancer patients and 2,013 controls were included in our meta-analysis. Among these studies, two were hospital-based and four were population-based. There were two studies of Africans, two studies of Caucasians, and two studies of Asian population. To assess the main effect of IL-8-251 genotypes, the logistic regression test was used to compare the two models with and without the IL-8-251 genotype. These two models were significantly different (P < 0.0001), indicating that the IL-8-251A>T genotypes were associated with breast cancer risk.
Meta-analysis
The results of the association between IL-8-251A>T polymorphism and breast cancer, and the heterogeneity test were shown in Table 2. Overall, no significant associations between IL-8-251A>T polymorphism and breast cancer risk were found for TA versus AA (OR = 1.075; 95%CI = 0.864–1.337; P = 0.12 for heterogeneity), TT versus AA (OR = 0.900; 95%CI = 0.598–1.354; P = 0.000 for heterogeneity), TT + TA versus AA (OR = 1.011; 95%CI = 0.783–1.304; P = 0.018 for heterogeneity), and TT versus AA + TA (OR = 0.854; 95%CI = 0.623–1.171; P = 0.001 for heterogeneity). However, in the subgroup analysis by ethnicity, significant associations were found in Asians for TA versus AA (OR = 1.444; 95%CI = 1.092–1.908; P = 0.606 for heterogeneity) and TT + TA versus AA (OR = 1.435; 95%CI = 1.107–1.861; P = 0.62 for heterogeneity). Furthermore, significant associations were observed in Africans for TT versus AA (OR = 0.541; 95%CI = 0.396–0.741; P = 0.820 for heterogeneity), TT + TA versus AA (OR = 0.737; 95%CI = 0.570–0.953; P = 0.803 for heterogeneity), and TT versus AA + TA (OR = 0.594; 95%CI = 0.459–0.768; P = 0.862 for heterogeneity). Moreover, in the stratified analysis by control sources, significant association was observed in population-based studies for TT versus AA + TA (OR = 0.692; 95%CI = 0.566–0.861; P = 0.229 for heterogeneity).
Publication bias and sensitivity analysis
Begg’s funnel plot and Egger’s test were performed to access the publication bias of literatures. The shape of the funnel plot did not reveal obvious asymmetry (figures not shown) and the Egger’s test suggested the absence of publication bias (P = 0.663 for AT vs. AA; P = 0.928 for TT vs. AA; P = 0.732 for dominant model; P = 0.87 for recessive model). Sensitivity analysis was carried out by including and excluding one study not in HWE. The pooled ORs were not materially altered (data not shown), implying that our results were robust.
Discussion
In this study, we performed a systematic review of association between the IL-8-251A/T polymorphism and risk for breast cancer based on 6 case–control studies for which information was available. Our meta-analysis provided evidence that TT/TA genotypes of IL-8-251A/T were associated with a significantly increased risk in Asian population, whereas TT/TA genotypes of IL-8-251A/T were associated with a significantly decreased risk in African population. These results suggested that it might not be uncommon for the same polymorphism play different roles in cancer susceptibility among different ethnic populations. In Asians and Africans, the differences in genetic backgrounds and the environment they lived in may influence the association between the IL-8-251A/T polymorphism and risk for breast cancer. In addition, the limited number of studies also makes the results from subgroup analysis by ethnicity less reliable. Thus, our results should be interpreted with caution.
Our results indicated that TT versus AA/TA genotypes decreased cancer risk among population-based studies but not among hospital-based studies. This reason may be that hospital-based studies have a high risk of producing unreliable results because hospital-based controls may not always be truly representative of the general population. Therefore, a methodologically preferable design, such as using a proper and representative population-based study, is crucial to avoid selection bias.
Some limitations of this meta-analysis should be acknowledged. First, the total subject number of studies seems to be insufficient (approximately 1,880 cases and 2,013 controls) to reach a reliable conclusion. The limited number of studies also makes the results from subgroup analysis less reliable. Second, some controls were selected from hospital populations; such women might have benign breast disease and correspond to a potentially incremental risk of breast cancer. Third, the overall outcomes were based on unadjusted estimates, while a more precise evaluation should be adjusted by other covariants including age, body mass index, menopausal status, ethnicity, drinking status, and environment factors. Last, but not the least, Egger’s linear regression test is known to be unreliable when there are fewer than 10 studies in meta-analysis (Higgins and Green 2008).
In conclusion, the current meta-analysis suggests that the AA/TA genotypes are associated with a significantly decreased risk for breast cancer among population-based studies. Well designed, unbiased prospective studies with larger sample size should be conducted to confirm these results. Moreover, further studies estimating the effect of gene–gene and gene–environment interactions are needed to clarify the role of IL-8 in breast carcinogenesis.
References
Cochran WG (1954) The combination of estimates from different experiments. Biometrics 10:101–129
De Jong MM, Nolte IM, Meerman GJ, van der Graaf WT, Oosterwijk JC, Kleibeuker JH, Schaapveld M, de Vries EG (2002) Genes other than BRCA1 and BRCA2 involved in breast cancer susceptibility. J Med Genet 39:225–242
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Higgins JPT, Green S (2008) Cochrane handbook for systematic reviews of interventions. Cochrane Collaboration and John Wiley
Hull J, Thomson A, Kwiatkowski D (2000) Association of respiratory syncytial virus bronchiolitis with the interleukin 8 gene region in UK families. Thorax 55:1023–1027
Kamali-Sarvestani E, Aliparasti MR, Atefi S (2007) Association of interleukin-8 (IL-8 or CXCL8) −251T/A and CXCR2 +1208C/T gene polymorphisms with breast cancer. Neoplasma 54:484–489
Liu JY, Zhai XJ, Jin GF et al (2007) A study of relationship between polymorphisms of interleukin-8 and risk of breast cancer in Chinese population. China Cancer 16:8–10
Mantel N, Haenszel W (1959) Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 22:719–748
Mukaida N, Shiroo M, Matsushima K (1989) Genomic structure of the human monocyte-derived neutrophil chemotactic factor IL-8. J Immunol 143:1366–1371
Ohyauchi M, Imatani A, Yonechi M et al (2005) The polymorphism interleukin 8-251 A/T influences the susceptibility of Helicobacter pylori related gastric diseases in the Japanese population. Gut 54:330–335
Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics 2002. CA Cancer J Clin 55:74–108
Ponder BA (2001) Cancer genetics. Nature 411:336–341
Smith KC, Bateman AC, Fussell HM, Howell WM (2004) Cytokine gene polymorphisms and breast cancer susceptibility and prognosis. Eur J Immuno genet 31:167–173
Snoussi K, Mahfoudh W, Bouaouina N et al (2006) Genetic variation in IL-8 associated with increased risk and poor prognosis of breast carcinoma. Hum Immunol 67:13–21
Snoussi K, Mahfoudh W, Bouaouina N et al (2010) Combined effects of IL-8 and CXCR2 gene polymorphisms on breast cancer susceptibility and aggressiveness. BMC Cancer 10:283
Taguchi A, Ohmiya N, Shirai K, Mabuchi N, Itoh A, Hirooka Y (2005) Interleukin-8 promoter polymorphism increases the risk of atrophic gastritis and gastric cancer in Japan. Cancer Epidemiol Biomarkers Prev 14:2487–2493
Thakkinstian A, McElduff P, D’Este C, Duffy D, Attia J (2005) A method for meta-analysis of molecular association studies. Stat Med 24:1291–1306
Vogel U, Christensen J, Nexo BA et al (2007) Peroxisome proliferator-activated receptor-gamma2 Pro12Ala, interaction with alcohol intake and NSAID use, in relation to risk of breast cancer in a prospective study of Danes. Carcinogenesis 28:427–434
Weber BL, Nathanson KL (2000) Low penetrance genes associated with increased risk for breast cancer. Eur J Cancer 36:1193–1199
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, Q., Wang, C., Qiu, LJ. et al. IL-8-251A>T polymorphism is associated with breast cancer risk: a meta-analysis. J Cancer Res Clin Oncol 137, 1147–1150 (2011). https://doi.org/10.1007/s00432-011-0981-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-011-0981-5