Abstract
Stimulated contraction of the diaphragm by non-invasive electrical stimulation of the phrenic nerve is demonstrated. A specialized device including a programmable current source and an electrode system has been developed to generate the stimulating signal. We report here a study on the effectiveness of stimulation on the contractile activity of the diaphragm by assessment of transdiaphragmatic pressure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Artificial pulmonary ventilation (APV) is a key technique used in resuscitation practice to maintain respiratory function in a wide range of diseases [1]. Despite the significant advantages of this approach, long-term use of mechanical ventilation is associated with a number of complications [2]. One of the most common of these is the development of mechanical ventilation-induced diaphragmatic dysfunction, which has many underlying mechanisms, such as oxidative stress, decreased protein synthesis, and accelerated proteolysis, as well as decreased contractile function of muscle fibers [3, 4]. All these processes lead to progressive atrophy of the diaphragm [5, 6].
Prevention of progressive atrophy requires independent contractions of the diaphragmatic muscle to be induced by stimulation [7]. A variety of methods are used for this purpose: invasive methods, i.e., stimulation of the phrenic nerve or the diaphragmatic muscle itself, and non-invasive methods, i.e., transcutaneous nerve stimulation. Invasive methods involve placing electrodes—able to pass an electric current—directly around the phrenic nerve in the neck or in the thoracic cavity near the pericardium, or attaching them to the diaphragmatic muscle through incisions in the anterior abdominal wall [8,9,10]. However, invasive techniques have drawbacks: creation of new entry points for infection, risk of hemorrhage, and the inappropriateness of its use with short-term APV.
Non-invasive stimulation methods are based on the percutaneous action of electrodes on the phrenic nerve [11]. Despite the obvious advantages of significant simplification and shorter time to initiation of stimulation, this approach has not yet become widespread because of its more complex technical base and more stringent requirements for selection of the stimulating signal. The creation and testing of new software and hardware systems, along with detailed studies of the optimal parameters for transcutaneous stimulation, will expand and simplify the use of this approach in clinical practice.
Materials and methods
The present study used three male Soviet chinchilla rabbits weighing 3.7–3.9 kg. All experiments were performed in compliance with the rules adopted by the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Purposes and Russian state standard GOST 33044–2014 “Principles of Good Laboratory Practice.” Animal procedures were reviewed and approved by the Bioethics Committee of the St. Petersburg State Chemical and Pharmaceutical University, Russian Ministry of Health (Bioethics Committee No. Rabbit-2023/1). Animals were kept in compliance with the standards specified in The Guide for the Care and Use of Laboratory Animals. Animals were obtained from the Rappolovo laboratory animals supplier (Leningrad Region) and were clinically healthy.
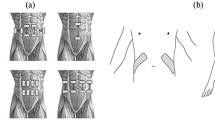
Anesthesia was induced by i.m. injection of a mixture of 10% Zoletil solution (Virbac, France) at a dose of 15 mg/kg and 2% Xylazine solution (Interchemie, Netherlands) at a dose of 5 mg/kg. Sedation was then maintained with i.v. 1% propofol at a dose of 1.3 mg/kg/min via infusion pump. Rabbits were intubated with an endotracheal tube (Covidien LLC, USA) with an internal diameter of 4 mm. Local anesthesia of the larynx was provided with 10% lidocaine solution. The fur of the neck and the area from the level of the sixth rib to the lower edge of the liver on the right was removed and the skin was treated with antiseptic AHD 2000 (Lysoform, Russia).
Diaphragmatic contractility was assessed in terms of changes in transdiaphragmatic pressure (∆Pdi), i.e., the difference in pressures between the abdominal and pleural cavities, during stimulated inhalation [12, 13]. Transdiaphragmatic pressure was recorded using two sensors (PhysExp, Russia) recording pressure in the abdominal and pleural cavities. The sensors were connected to balloons inflated with saline solution and values were recorded in the PhysExp Mini recorder program (Cardioprotect LLC, Russia). The pleural probe balloon was placed in the pleural cavity through a 1.5-cm incision between the eighth and ninth ribs on the right. The abdominal sensor balloon was positioned between the liver and the right half of the diaphragm through a 1.5-cm incision parallel to the tenth rib at a distance of 1–1.5 cm from the edge of the rib. After balloon emplacement, incisions were hermetically sutured (Fig. 1).
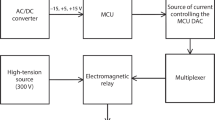
Phrenic nerve stimulation was applied using a TESD (transcutaneous electrical stimulation of the diaphragm) device (St. Petersburg State Electrotechnical University “LETI,” Russia). Electrodes in the form of clamps were positioned in the projection of the phrenic nerve behind the cleidomastoid muscles on both sides. Before use of the device, the animal was hyperventilated using a neonatal Ambu bag (Medplant LLC, Russia) to provide temporarily suppression of the animal’s spontaneous inhalation reflex.
The following basic characteristics of the pulse were specified: signal amplitude 3 mA, signal period 2000 msec, signal duty cycle 50%, number of pulses per signal 40, pulse duty factor 80%, pulse shape sinusoidal.
In addition, the experiment tested the influences of the following electrical stimulation characteristics on diaphragm contraction parameters:
-
signal amplitude (1, 3, 5, and 7 mA);
-
signal duration (500 and 2000 msec);
-
signal duty cycle (15, 50, and 80%).
The reactions of the muscles adjacent to the phrenic nerve stimulation zone were assessed visually in terms of the intensity of muscle contractions in the rabbit’s shoulder girdle and forelimbs. Results were expressed semi-quantitatively in points (0—absent, 1—low, 2—moderate, 3—marked reactions by surrounding muscles).
All animals were euthanized by anesthetic overdose when experiments were complete.
Results
After sensor emplacement in the pleural and abdominal cavities, accurate recordings of the dynamics of changes in pressure in the cavities depending on respiratory phases were captured in all animals. ∆Pdi during inhalation with the animal breathing freely was 9.2 ± 1.1 mmHg.
Application of stimulatory impulses to the phrenic nerves produced stable stimulation of diaphragmatic contractions in all rabbits studied. ∆Pdi on the background of exposure to an impulse with the characteristics specified above was 12.2 ± 0.3 mmHg.
The effect of signal amplitude in the range 1–7 mA on ∆Pdi was not strictly linear, such that there were no significant changes in ∆Pdi on exposure to impulses with amplitudes of 1, 3, and 5 mA, (12.1 ± 0.4, 12.2 ± 0.3, and 12.4 ± 0.6 mmHg respectively); conversely, stimulation with a 7-mA impulse led to a significant increase in ∆Pdi (15.6 ± 0.5 mmHg; p < 0.05) (Fig. 2).
Influence of changes in signal amplitude on the characteristics of non-invasive stimulation of diaphragm contractions: a plots showing impulse parameters; b relationship between changes in transdiaphragmatic pressure (∆Pdi) and the amplitude of the applied stimulus during stimulation; c relationship between the reaction of surrounding muscles and the amplitude of the applied stimulus; d combined plot of the relationship between ∆Pdi and the reaction of surrounding muscles and applied stimulus amplitude (autonomous respiration of the animal without artificial stimulation)
The duration of the signal period correlated directly with the stimulated respiratory rate (RR) (Fig. 3). With a signal period of 2000 msec, RR was 30 per min. With a signal period of 500 msec, RR was 120 per min. Baseline RR in anesthetized rabbits was 21.9 ± 6.3 per min. There was no significant change in ∆Pdi during the signal period durations studied (12.2 ± 1.1 mmHg at 2000 msec; 12.6 ± 1.1 mmHg at 500 msec).
Influence of changes in the signal period on the characteristics of non-invasive stimulation of diaphragm contractions: a plots of impulse parameters; b relationship between the frequency of stimulated respiratory movements and the signal period (autonomous respiration of the animal without artificial stimulation)
Changes in the duty cycle of the signal led to a linear change in the duration of inhalation (Fig. 4). At a signal period duration of 2000 msec (stimulated respiratory rate 30 per min), inspiratory durations were 15% (0.18 sec), 50% (0.75 sec), and 80% (1.3 sec). The duration of inspiration during quiet breathing in anesthetized animals at a respiratory rate of 30 per minute was 1.23 ± 0.14 sec. It should be noted that ∆Pdi was significantly lower at a signal duty cycle of 80% (7.6 ± 2.2 mmHg) than 15% (11.8 ± 1.2 mmHg) and 50% (12.2 ± 2.3 mmHg) (p < 0.05).
Effects of changing the signal duty cycle on the characteristics of non-invasive stimulation of diaphragm contractions; a plots of impulse parameters; b relationship between duration of inhalation and duty cycle of signal; c relationship between changes in transdiaphragmatic pressure (∆Pdi) during stimulation on the stimulus duty cycle (autonomous respiration of the animal without artificial stimulation)
Discussion
This study showed that the TESD device effectively stimulated diaphragmatic contractions by means of non-invasive transmission of electrical signals to the phrenic nerve. Inhalation was successfully carried out with each repetition of the stimulus.
The study addressed the influences of three parameters (signal amplitude, period, and duty cycle); the relationships between changes in transdiaphragmatic pressure and the intensity of skeletal muscle contraction and changes in the characteristics of impulses were established.
Stimuli with signal amplitudes of 1, 3, 5, and 7 mA were tested. However, even at a current of 1 mA, ∆Pdi was greater than during spontaneous breathing. A further increase in ∆Pdi was observed only at the level of 7 mA. Changes in signal amplitude revealed a direct relationship between current strength and contractions of the surrounding muscles. In this regard, the optimal amplitude range among those tested was the range 1–3 mA. Future studies should also test lower current values, to reduce undesirable muscle effects and help reduce ∆Pdi to optimal values. Tidal volume was recorded on rabbits at stimulation amplitudes of 10, 16, 20, 26, and 32 mA [14]. As in the present study, a direct relationship between changes in signal amplitude and the force of diaphragm contraction, measured by determining tidal volume, was found.
The signal period was adjusted with the aim of controlling the frequency of stimulated respiratory movements. This parameter is integral for devices of this type. Use of a period of 2000 msec corresponds to a respiratory rate of 30 per minute, which was optimal for the experimental conditions.
The duty cycle of the signal reflects the duration of the stimulation pulse within a given cycle. The higher the coefficient, the longer the inhalation period, while the duration of the respiratory cycle remains the same. However, it was noted that ∆Pdi decreased as the signal duty cycle increased to 80%.
Conclusions
Future development of the method discussed here will support effective prevention of atrophy of the diaphragm. Thus, use of non-invasive stimulation of the phrenic nerve in clinical practice will provide opportunities for rapid disconnection of the patient from mechanical ventilation, thereby reducing the durations of intensive care unit stays and accelerating the recovery process as a whole.
References
Walter JM, Corbridge TC, Singer BD (2018) Invasive mechanical ventilation. South Med J 111(12):746–753
Jaber S, Petrof BJ, Jung B et al (2011) Rapidly progressive diaphragmatic weakness and injury during mechanical ventilation in humans. Am J Respir Crit Care Med 183(3):364–371
Davis RT 3rd, Bruells CS, Stabley JN et al (2012) Mechanical ventilation reduces rat diaphragm blood flow and impairs oxygen delivery and uptake. Crit Care Med 40(10):2858–2866
Zergeroglu MA, McKenzie MJ, Shanely RA et al (2003) Mechanical ventilation-induced oxidative stress in the diaphragm. J Appl Physiol 95(3):1116–1124
Powers SK, Wiggs MP, Sollanek KJ et al (2013) Ventilator-induced diaphragm dysfunction: cause and effect. Am J Physiol Regul Integr Comp Physiol 305(5):R464–R477
Schepens T, Verbrugghe W, Dams K et al (2015) The course of diaphragm atrophy in ventilated patients assessed with ultrasound: a longitudinal cohort study. Crit Care 19:422
DiMarco AF (2018) Diaphragm pacing. Clin Chest Med 39(2):459–471
Headley DB, Martins AG, McShane KJ et al (2023) Diaphragm pacing using the minimally invasive cervical approach. J Spinal Cord Med 46(1):26–34
Le Pimpec-Barthes F, Gonzalez-Bermejo J, Hubsch JP et al (2011) Intrathoracic phrenic pacing: A 10-year experience in France. J Thorac Cardiovasc Surg 142(2):378–383
DiMarco AF, Onders RP, Ignagni A et al (2005) Phrenic nerve pacing via intramuscular diaphragm electrodes in tetraplegic subjects. Chest 127(2):671–678
Keogh C, Saavedra F, Dubo S et al (2022) Non-invasive phrenic nerve stimulation to avoid ventilator-induced diaphragm dysfunction in critical care. Artif Organs 46(10):1988–1997
Rugg C, Schmid S, Kreutziger J et al (2020) Assessing transpulmonary pressure via direct pleural manometry. Ann Intens Care 10(1):100
Smith SE, Sande AA (2012) Measurement of intra-abdominal pressure in dogs and cats. J Vet Emerg Crit Care 22(5):530–544
Ghedini RG, de Espinel Felix JOEA et al (2013) Effectiveness of diaphragmatic stimulation with single-channel electrodes in rabbits. Braz J Pulmonol 39(4):490–494
Funding
This research was supported by a grant from the Russian Science Foundation (project No. 22-25-20214) and a grant from the St. Petersburg Science Foundation in accordance with agreement No. 15/2022 dated April 14, 2022.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Meditsinskaya Tekhnika, Vol. 58, No. 1, pp. 21–25, January–February, 2024.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Akhmetova, A.A., Shapovalov, S.V., Li, R.V. et al. A method for noninvasive electrical stimulation of the diaphragm. Biomed Eng 58, 30–35 (2024). https://doi.org/10.1007/s10527-024-10360-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10527-024-10360-9