Abstract
The tomato potato psyllid Bactericera cockerelli (Sulcer) (Hemiptera: Triozidae) is a serious pest of several solanaceous crops in different parts of the world. We examined the biological control potential of the zoophytophagous bug Engytatus varians (Distant) (Hemiptera: Miridae), in combination with the ectoparasitoid Tamarixia triozae (Burks) (Hymenoptera: Eulophidae) on B. cockerelli-infested pepper (Capsicum annuum L.) plants in cages under greenhouse conditions. A single release rate (one adult per plant) of either E. varians or T. triozae was used and the timing of predator releases varied (before or after pest establishment). The number of nymphs and adults of B. cockerelli or the number of mirids (nymphs plus adults) and parasitoids (pupae plus adults) was determined three or four weeks after release. Both E. varians and T. triozae successfully established on pepper plants and significantly reduced the pest population density (by 91 to 96% and by 84 to 100% of nymphs and adults, respectively) when they were released separately or in combination, even when the predator was released before the establishment of B. cockerelli. At the end of the experiment, the density of E. varians was between one and two individuals per leaf, whereas that of the parasitoid was between one and six individuals per leaf in the treatments in which each natural enemy was released. These results could contribute to the integrated pest management of B. cockerelli. However, further studies are needed to validate the impact of both natural enemies on the control of this pest at a larger scale.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The pepper (Capsicum annuum L.) has its center of origin, diversity, and domestication in Mexico (Luna-Ruiz et al. 2018). It is the seventh most consumed vegetable worldwide (Omondi 2018). The global production of pepper is 41 million tons on 4.17 million hectares of cultivated land (FAOSTAT 2021). Currently, Mexico is the world's second main pepper producer with the majority of the production under field conditions all year round (3.09 million tons on ~ 148,000 hectares; SIAP 2021, 2022). The tomato potato psyllid Bactericera cockerelli (Sulcer) (Hemiptera: Triozidae), a severe invasive pest, has been one of the main limiting factors in pepper production and that of other solanaceous crops such as potato (Solanum tuberosum L.), tomato (Solanum lycopesicum L.), eggplant (Solanum melongena L.), and tamarillo (Solanum betaceum Cav.) (Liefting et al. 2008; Munyaneza et al. 2009). This pest is native to Central and North America but in the last 20 years it has spread through the temperate areas and become established in the USA, Mexico, Ecuador, New Zealand, the Norfolk Island, and West Australia (Gill 2006; Teulon et al. 2009; Butler and Trumble 2012a; Castillo et al. 2019; DPIRD 2021).
In addition to direct damage caused by phloem feeding, B. cockerelli is an efficient vector of different plant pathogens in several crops. It transmits permanent yellowing in tomato (Munyaneza et al. 2009), purple tip in potato (Rubio-Covarrubias et al. 2006), and the phloem-limited bacterium Candidatus Liberibacter solanacearum (CLso), which causes zebra chip disease in potatoes, tomato, and pepper (Hansen et al. 2008; Liefting et al. 2008; Camacho-Tapia et al. 2011). Chemical control is mainly used against B. cockerelli, with up to 15–30 foliar applications of broad-spectrum synthetic insecticides on potatoes or tomatoes (Rubio-Covarrubias et al. 2006; Guenthner et al. 2012; Anderson et al. 2013). However, this control tactic has limited impact because of the insect's capacity to develop resistance (Cerna et al. 2013; Chávez et al. 2015), the decline of natural enemy populations in frequently treated crops, and outbreaks of secondary pests, among others (Cloyd and Bethke 2011; Pimentel and Burgess 2014). Therefore, it is necessary to identify alternatives for the control of B. cockerelli that are environmentally benign and that can be included in integrated pest management (IPM) programs. In this regard, biological pest control, through the use of predators and parasitoids, has attracted particular interest.
Currently, there are 41 species of arthropod natural enemies that have been reported to prey on B. cockerelli, of which ten are arachnids (Acari and Araneae) and 31 are insects from the orders Coleoptera, Dermaptera, Diptera, Hemiptera, Hymenoptera, Neuroptera, and Thysanoptera (Sarkar et al. 2023a). However, only some of these have been evaluated for biological control purposes. In New Zealand and Australia, the use of coccinellids (O’Connell et al. 2012; Sarkar et al. 2022) and phytoseiid mites (Xu and Zhang 2015) have been identified as promising alternatives for the control of this pest.
The zoophytophagous mirids Dicyphus hesperus Knight (Calvo et al. 2016, 2018a), Engytatus nicotianae (Koningsberger) (Veronesi et al. 2021, 2022a, b), and Engytatus varians (Distant) (Pineda et al. 2020, Mena-Muciño et al. 2021) have also shown high potential against B. cockerelli. These species are generalist as they prey on several pests including whiteflies, thrips, pseudococcids or lepidopterans (Shipp and Wang 2006; Silva et al. 2016; Calvo et al. 2018a; Pineda et al. 2020; Veronesi et al. 2023). Engytatus varians was detected for the first time in Mexico in 2014 feeding on nymphs of B. cockerelli on tomato plants (Martínez et al. 2015). The rather wide prey spectrum of this mirid makes it an interesting candidate for use in IPM.
In Mexico, small-scale inundative release programs, or small caged experiments, have demonstrated that E. varians can suppress populations of nymphs and adults of B. cockerelli under greenhouse conditions on tomato plants (Pérez-Aguilar et al. 2019). The successful establishment of predatory mirids, including E. varians, on greenhouse tomato crops have been, in part, due to the hirsute nature of these plants, which seem to offer more suitable conditions for mirid development than glabrous plants (Perdikis and Lykouressis 2000; Sánchez et al. 2004; Orozco et al. 2012).
Two species of parasitoid of B. cockerelli have been identified: the ectoparasitoid Tamarixia triozae (Burks) (Hymenoptera: Eulophidae) (Martínez et al. 2015) and the endoparasitoid Metaphycus psyllidus Compere (Hymenoptera: Encyrtidae) (Jensen 1957). The former species appears to be more effective than the latter. Tamarixia triozae is a synovigenic species that causes pest mortality by both parasitism and host feeding (Morales et al. 2013; Martínez et al. 2015). Under laboratory conditions, T. triozae caused between 40 and 100% of parasitism on different instars of B. cockerelli (Morales et al. 2013; Yang et al. 2015; Veronesi et al. 2021). In the field, in Mexico, USA, and New Zealand, the parasitism of this psyllid by this parasitoid varied widely (between 5 and 85%) on several solanaceous plants, with higher rates on pesticide-free crops (Bravo and López 2007; Butler and Trumble 2012b; Liu et al. 2012; Davidson et al. 2023).
To better understand the integration of these two types of natural enemies, we compared two different E. varians release timings (before or after B. cockerelli establishment) in combination with parasitism by T. triozae for control of this pest on a glabrous plant (pepper). We hypothesized that the combination of parasitism and an omnivorous predator would improve the biological control of this pest on pepper crop compared to each natural enemy alone.
Materials and methods
Insects and rearing
Bactericera cockerelli and E. varians were obtained from colonies maintained at the Instituto de Investigaciones Agropecuarias y Forestales (IIAF), Universidad Michoacana de San Nicolás de Hidalgo (UMSNH), Mexico. Adults and nymphs of both species were reared on tomato plants (variety Río Grande) in frame boxes (50 × 50 × 50 cm) covered by a mesh screen. Eggs of the grain moth, Sitotroga cerealella (Olivier) (Lepidoptera: Gelechiidae) (Bio-bich, Uruapan, Michoacán, Mexico), were deposited on tomato leaves as food for adults and nymphs of E. varians. A 5% (w/v) solution of sugar was also supplied to this predator in microcentrifuge tubes (1.5 ml) with a piece of cotton and replaced at 3-day intervals to prevent fungal growth. The entire rearing process of B. cockerelli and E. varians was performed at ∼ 25 °C and 56% RH, with a photoperiod of ∼ L:D 12:12. New tomato plants were supplied as needed. Adults of the parasitoid T. triozae were obtained from the suppliers of the commercial product TETRAPAR (Koppert Biological Systems, El Marqués, Querétaro, Mexico).
Plant material and greenhouse
Pepper seeds (serrano type, variety Platino) were allowed to germinate in peat moss mixture 3 (Sunshine®; Sun Gro Horticulture Distribution Inc., Agawam, MA, USA) in individual pots of 2.5 cm diameter. One month later, the plants were transplanted individually to polyethylene black plastic bags (20 cm diameter × 25 cm high) containing a 1:1 mixture of a porous volcanic gravel known locally as tezontle (size of 2–5 mm) and humus-rich soil.
The experiment was carried out in a greenhouse (25 m long × 8 m wide) covered with polyethylene and anti-aphid screening located at the IIAF, UMSNH, Tarímbaro, Michoacán, Mexico. Fifteen experimental cages (2 m long × 1.6 m wide × 2.5 m high), made of plastic tubes and covered by a mesh screen to prevent the insects from escaping or entering, were used. After transplanting, conventional practices for greenhouse pepper cultivation were followed and, in each experimental cage, pepper plants were tied-up by the main stem using polyethylene string. Through weekly pruning, secondary shoots and insect-free old leaves were removed. During the experiment, each pepper plant was fertilized every three or four days with 500 ml of a nutritive solution (Kelatex-Multi®, San Nicolás de Los Garza, Nuevo León, Mexico). No pesticides were applied during the experiment.
Experimental design
To determine the impact of E. varians and T. triozae in reducing B. cockerelli densities on pepper plants, we varied the timing of predator releases (before or after pest establishment). The following five treatments were compared: (1) control, B. cockerelli only, (2) B. cockerelli + E. varians, (3) B. cockerelli + T. triozae, (4) E. varians + B. cockerelli, and (5) B. cockerelli + E. varians + T. triozae. Twenty days after transplanting (on May 08, 2019), five pepper plants were placed in two rows, each with three or two plants separated by 30 cm, inside each of the experimental cages (each cage was a replicate). Each treatment was replicated three times in a completely randomized design. Each cage could be accessed through an opening secured using Velcro strips (Velcro®; Grupo Parisina, Mexico City, Mexico). The release of E. varians before B. cockerelli establishment was based on studies with another mirid species: Nesidiocoris tenuis Reuter (Mirhosseini et al. 2019). The establishment and colonization of this predator on tomato plants, prior to pest establishment, was improved by providing it with alternative and complementary foods (i.e., eggs of Ephestia kuehniella Zeller [Lepidoptera: Pyralidae] and honey solution). As a supplementary food, we used a sugar solution at 8.5% to increase the probability of E. varians establishment (Pérez-Aguilar et al. 2019).
The test initiated on May 15, 2019. On this date, all experimental cages were infested with eight B. cockerelli adults (four females + four males; ≤ five days old) per plant, with exception of the treatment (4) E. varians + B. cockerelli in which five adults of E. varians (three females + two males) were released in each cage in the absence of the pest. On May 29, 2019, five adults of either E. varians or T. triozae (three females + two males) were released in the experimental cages of the treatments (2) B. cockerelli + E. varians and (3) B. cockerelli + T. triozae. In treatment (5) B. cockerelli + E. varians + T. triozae, five adults of both E. varians and T. triozae (three females + two males of each species) were released. Finally, eight B. cockerelli adults per plant were released in the cages of treatment (4) E. varians + B. cockerelli.
In cages in which E. varians or T. triozae adults were released, two plastic cups (50 ml) containing 40 ml of sugar solution (8.5% w/v) with a cotton wick were placed one day after the release as a food supplement. The cups were placed at a height of 20 cm between the rows of pepper plants. The sugar solution was replaced every four days during the experiment. The release of E. varians and T. triozae adults was performed on one occasion and never repeated. The number of E. varians individuals released was selected based on the study of Pérez-Aguilar et al. (2019), who reported a good pest suppression of B. cockerelli. The release of T. triozae was established based on the study of Calvo et al. (2018b) and on the supplier's recommendations for augmentative biocontrol of this pest with T. triozae (https://www.koppert.mx/tetrapar/).
Sampling and evaluations
After B. cockerelli and E. varians release on week zero of the experiment, adults of these insects were left undisturbed for three or four weeks thereby allowing their population to establish, as in Veronesi et al. (2022b). Therefore, the numbers of nymphs and adults of B. cockerelli were counted separately at three and four weeks after starting the experiment, respectively, and continued weekly until the 10th week. The numbers of mirids (nymphs and adults) and parasitoids (pupae [= mummified nymphs of B. cockerelli] and adults) were also counted at the same times, respectively. One mummified nymph of the host, with a coppery color, was considered as one pupa of T. triozae. The pest and natural enemies were counted on three leaves; each one from the upper, middle, and bottom third of each pepper plant in each treatment.
Climatic conditions
Temperature and RH in the experimental greenhouse were registered hourly during all tests using a datalogger (Hobo®, model U10, Bourne, MA, USA), which was hung at a height of 1.5 m in the centre of the greenhouse. Daily temperature averaged at 26.10 ± 0.24 °C, with the daily absolute maxima and minima during the experiment being 9.70 and 47.19 °C, respectively. RH averaged 44.20 ± 1.67%, with absolute minimum and maximum of 9.42 and 89.50%, respectively.
Data analysis
The count of individuals of B. cockerelli, E. varians, and T. triozae was modeled using Generalized Linear Mixed Models (GLMM) assuming a negative binomial distribution and a log link function (Gbur et al. 2012). The numbers of nymphs and adults of B. cockerelli per leaf were analyzed independently with a 5 × 8 and 5 × 7 factorial design, respectively, with the five treatments as a common factor and different evaluation weeks (time effect) (8 and 7 for nymphs and adults, respectively). For the analysis of the number of mirids (nymphs plus adults) and the parasitoids (pupae plus adults), experiments comprised a 3 × 7 and 2 × 7 factorial design, respectively. In all cases, treatment and time were considered as fixed effect and plants within cage as a random effect. Because zero values were present in the data on the number of mirids, the analysis was conducted following x + 1 transformation. An analysis by repeated-measures using the PROC GLIMMIX, with the LSMEANS test (P < 0.05) to separate means, was used for all analyses. All statistical tests were performed using SAS/STAT (version 9.4; SAS Institute, Cary, NC, USA) and all data are expressed as the mean ± SE.
Results
Bactericera cockerelli population density
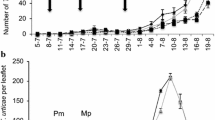
The mean numbers of B. cockerelli nymphs per leaf, from weeks 3–10, oscillated between 0.72 ± 0.38 and 13.20 ± 3.28, 1.81 ± 0.64 and 11.20 ± 3.32, 1.46 ± 0.48 and 8.20 ± 2.12, and 0.92 ± 0.35 and 11.66 ± 2.26 in the treatments of B. cockerelli + E. varians, B. cockerelli + T. triozae, E. varians + B. cockerelli, and B. cockerelli + E. varians + T. triozae, respectively (Fig. 1a). The treatment (F4,516 = 61.38; P < 0.0001), the time (F7,516 = 4.27; P = 0.0001), and the interaction between these two factors (F28,516 = 2.51; P < 0.0001), significantly affected the number of B. cockerelli nymphs per leaf. In all evaluation weeks, the mean number of B. cockerelli nymphs per leaf was significantly higher in the control compared with the remaining treatments, with exception of the 3rd week where there were no significant differences between the control and B. cockerelli + E. varians (P = 0.362; Fig. 1a). The latter treatment was significantly different to B. cockerelli + T. triozae treatment (P = 0.003). In week 4, significant differences were only observed between E. varians + B. cockerelli and B. cockerelli + E. varians + T. triozae treatments (P = 0.048).
Density (mean number of individuals per leaf ± SE) of B. cockerelli nymphs (a) and adults (b). The black solid arrow indicates the infestation of B. cockerelli in all treatments, with the exception to E. varians + B. cockerelli treatment, where the predator was first released. The black broken arrow indicates the release of E. varians and T. triozae in B. cockerelli + T. triozae, B. cockerelli + E. varians, and B. cockerelli + E. varians + T. triozae treatments, as well as the infestation of B. cockerelli in the E. varians + B. cockerelli treatment. Bars within each evaluation week followed by the same letter do not differ significantly (LSMEANS test, P < 0.05)
In week 5, the treatment B. cockerelli + T. triozae was significantly different compared with the following three treatments (P ≤ 0.053 in all cases): B. cockerelli + E. varians, E. varians + B. cockerelli, and B. cockerelli + E. varians + T. triozae. No significant differences were observed in the number of B. cockerelli nymphs per leaf from weeks 6 to 10 among treatments where E. varians and T. triozae were released (P ≥ 0.158 in all cases).
From weeks 4–10, the mean numbers of B. cockerelli adults per leaf oscillated between 0.60 ± 0.32 and 1.66 ± 0.42, < 0.1 ± < 0.1 and 1.26 ± 0.33, < 0.1 ± < 0.1 and 0.60 ± 0.25, and 0.33 ± 0.15 and 1.33 ± 0.59 in the treatments B. cockerelli + E. varians, B. cockerelli + T. triozae, E. varians + B. cockerelli, and B. cockerelli + E. varians + T. triozae, respectively (Fig. 1b). The treatment (F4,446 = 27.00; P < 0.0001) and the interaction treatment × time (F24,446 = 2.00; P = 0.003), significantly affected the number of B. cockerelli adults per leaf, but not the time factor (F6,446 = 1.80; P = 0.097). During all evaluation weeks, the number of B. cockerelli adults per leaf in the control (range from 1.0 ± 0.48 to 6.0 ± 2.44) was significantly higher than that recorded in the rest of the treatments (P ≤ 0.020 in all cases). The following two exceptions were observed: (1) between the control and B. cockerelli + E. varians treatments (P = 0.324) in the 5th week; and (2) among any of the treatments, including the control (P ≥ 0.360 in all cases) in the 7th week.
In week 4, significant differences were only observed between B. cockerelli + E. varians and E. varians + B. cockerelli treatments (P = 0.022). No significant differences were observed in the number of B. cockerelli adults per leaf in the weeks 6, 8, 9, and 10 in the treatments where E. varians or T. triozae where released (P ≥ 0.083 in all cases, Fig. 1b).
Engytatus varians and T. triozae populations density
From weeks 3–10 of the experiment, the mean numbers of E. varians per leaf oscillated between 0.20 ± 0.14 and 0.90 ± 0.30, < 0.1 ± < 0.1 and 1.30 ± 0.47, and < 0.1 ± < 0.1 and 0.71 ± 0.26 in the treatments of B. cockerelli + E. varians, E. varians + B. cockerelli, and B. cockerelli + E. varians + T. triozae, respectively (Fig. 2a). The number of E. varians per leaf was significantly affected by the time (F6,273 = 3.15; P = 0.005), but not by the treatment factor (F2,273 = 2.33; P = 0.099) or by the interaction between these two factors (F12,273 = 1.44; P = 0.148).
Density (mean number of individuals per leaf ± SE) of E. varians (nymphs and adults) (a) and T. triozae (pupae and adults) (b). The black solid arrow indicates the infestation of B. cockerelli in B. cockerelli + E. varians, B. cockerelli + T. triozae, and B. cockerelli + E. varians + T. triozae treatments, as well as the release of E. varians adults in the E. varians + B. cockerelli treatment. The black broken arrow indicates the release of E. varians and T. triozae adults, alone or in combination, in the B. cockerelli + E. varians, B. cockerelli + T. triozae, and B. cockerelli + E. varians + T. triozae treatments, as well as the infestation of B. cockerelli in the E. varians + B. cockerelli treatment. Bars within each evaluation week followed by the same letter do not differ significantly (LSMEANS test, P < 0.05)
In week 7 there was a significant difference between E. varians + B. cockerelli and the other two treatments involving the predator (B. cockerelli + E.varians [P = 0.001] and B. cockerelli + E. varians + T. triozae [P = 0.006]). In week 9, a significant difference between B. cockerelli + E.varians and E. varians + B. cockerelli treatments (P = 0.054) was observed.
The mean numbers of pupae plus adults of T. triozae per leaf varied between 0.80 ± 0.60 and 6.53 ± 2.16 and 1.13 ± 0.68 and 2.40 ± 0.71 from week 4 to the end of the experiment in the treatments comprising B. cockerelli + T. triozae and B. cockerelli + E. varians + T. triozae, respectively (Fig. 2b). The number of pupae plus adults of T. triozae per leaf was only significantly affected by the treatment factor in the week 6 (F1,174 = 9.88; P = 0.002). No significant differences were observed either in the time factor (F6,174 = 1.35; P = 0.238) or in the interaction treatment × time (F6,174 = 1.83; P = 0.095).
Discussion
Zoophytophagous mirids have had a successful history of use for almost the last 25 years in Europe, both in conservation and augmentative biological control programs against arthropod pests in greenhouses. Following the success of predatory mirids in the Mediterranean area (Calvo et al. 2012; Pérez-Hedo et al. 2021), expectations that mirids can contribute to the control of horticultural pests in the Americas have increased (Calvo et al. 2016, 2018b; van Lenteren et al. 2018; Pérez-Aguilar et al. 2019). However, studies have been performed on tomato crops. To our knowledge, there is no information available on the use of E. varians and T. triozae for the control of B. cockerelli on pepper plants. In the present study, both natural enemies established well under greenhouse conditions.
In general, E. varians reduced the population of nymphs and adults of B. cockerelli during all weeks of experiment and at the end of experiment this reduction was between 84 and 96% when the predator was released before or after pest establishment. Although this is the first study reporting the capacity of E. varians to predate B. cockerelli on pepper plants under greenhouse conditions, it was not the first of its kind. On tomato plants, this mirid reduced the population of B. cockerelli up to ∼ 80 and ∼ 90% with release rates of one and four individuals per plant, respectively (Pérez-Aguilar et al. 2019). Similarly, E. nicotianae reduced the population of eggs and nymphs of B. cockerelli by ~ 75% following the release of eight adults (four male and four female) per tomato plant (Veronesi et al. 2021, 2022a). Moreover, the mirid D. hesperus suppressed B. cockerelli adults and nymphs by ~ 90% when released at a rate of one individual per plant in a tomato greenhouse (Calvo et al. 2016). It is important to point out that the release rates of the natural enemy as well as the pest infestation method were different in the studies of Calvo et al. (2016), Veronesi et al. (2021, 2022a) and ours. We obtained pepper plants that were more heavily infested with a single release of eight B. cockerelli adults per plant that those in the previous studies. Veronesi et al. (2021) released weekly only two adults per plant during three consecutive weeks, whereas releases of between 0.1 and 0.2 adults per plant during the 14th weeks were done by Calvo et al. (2016). Therefore, our predator E. varians exhibited a higher predation rate than E. nicotianae and D. hesperus for the control of B. cockerelli because it was able to control its population following the release of just one adult per plant. However, it is important to consider that, although E. varians caused high predation at a low release rate, the producing and application costs of this predator must be estimated to determine if its mass release is affordable for growers.
In the present study, we observed a decline in the pepper plants of the controls (release of B. cockerelli alone), compared to those that received E. varians and T. triozae, in the last three weeks of the experiment. Plants with natural enemies had a more intense green color, were taller, and had more and larger leaves than those of the controls, in which the leaves were yellowing and covered with honeydew. Although not quantified, we assume that this was due to a reduced chlorophyll and dry matter of the pepper plants. Similarly, Sarkar et al. (2023b) reported that the quantity of chlorophyll and dry matter was higher in tomato plants on which the lady beetle Hippodamia variegata (Goeze) (Coleoptera: Coccinellidae) was released to control B. cockerelli compared to plants on which the pest was released without the predator. This is due, presumably, to the direct impact of feeding by this pest, which is consistent with the high number of adults recorded in our study (between 20 and 30 per leaf, in weeks 8–10 of the experiment).
The density of E. varians nymphs + adults ranged from one to two per leaf during the period of the experiment in the three treatments in which the predator was present (B. cockerelli + E. varians, E. varians + B. cockerelli, and B. cockerelli + E. varians + T. triozae). The successful of establishment of E. varians under the conditions of our experiment was likely due to food availability and the optimal conditions for development of both prey and predator (Pineda et al. 2016). When D. hesperus was released after establishment of B. cockerelli on greenhouse tomato plants, the number of nymphs plus adults was up to 1.7 individuals per leaf in spring–summer but it was only 0.4 individuals per leaf in autumn–winter (Calvo et al. 2016). The low number of individuals of D. hesperus recorded in the experiment in autumn–winter was presumably due the lower temperatures. However, this density was sufficient to provide a high degree of pest suppression.
Due the zoophytophagous habit of mirid predators, these insects can cause plant damage when prey are scarce (Silva et al. 2016). To avoid this, the use of supplementary foods such as E. kuehniella eggs (Calvo et al. 2016, 2018a, b) or Artemia sp. cysts (Alonso 2015) have been successfully used to increase the probability of establishment of the predator population. When a sugar solution at 8.5% was supplied, E. varians achieved rapid establishment on tomato plants under greenhouse conditions, reaching one and 1.4 nymphs per leaf and one and two adults per leaf following the release of one and four adults per tomato plant, respectively (Pérez-Aguilar et al. 2019). Sugar and moth/shrimp eggs provide different types of nutrients, as the former is a carbohydrates source, and the latter are protein sources. According to our results, this sugar solution could be used as supplementary food because it is cheaper (US$1.25 per kg) than E. kuehniella eggs (US$400 per kg; Urbaneja-Bernat et al. 2015) or Artemia sp. cysts (US$250 per kg; Alonso 2015). However, further research is needed to determine the cost–benefit and how this could be translated from caged conditions to commercial greenhouses.
When T. triozae was released alone, it reduced the density of nymphs and adults of B. cockerelli by 91 and 100%, respectively, by the end of the experiment. The only study in which the effect of the release of this parasitoid alone has been assessed under greenhouse conditions on this pest is that of Veronesi et al. (2021). These authors reported that this parasitoid did not reduce the population of B. cockerelli after three and six weeks after its release, although, after three weeks, the density of nymphs was reduced by 79%.
Intragremial interactions can be used in the context of IPM as they significantly impact on the reduction of insect pest populations. The present study represents the first report of the interaction between the predator E. varians and the parasitoid T. triozae that exploit the same food source. In this regard, the combined release of both natural enemies reduced the density of nymphs and adults of B. cockerelli by 95 and 92%, respectively, at the end of experiment. The release of one adult of D. hesperus per plant + one pupa of T. triozae per tomato plant reduced the density of nymphs and adults of B. cockerelli by ∼ 70 and ∼ 85%, respectively (Calvo et al. 2018b). Similarly, the configuration of E. nicotianae + T. triozae reduced the population of eggs, nymphs, and adults of B. cockerelli by ∼ 71, 65, and 78%, respectively, when the predator and parasitoid were released on a weekly basis and twice, respectively, across all the experiment (Veronesi et al. 2022b). We assume that the high reduction in the pest population recorded by Calvo et al. (2018b), Veronesi et al. (2022b), and in the present study was due to predation by E. varians, E. nicotianae, or D. hesperus, which did not interfere with the feeding habits of the parasitoid and vice versa. Supporting this, Ramírez-Ahuja et al. (2017) demonstrated that there was no intragremial competition between D. hesperus and the parasitoid T. triozae on B. cockerelli nymphs under laboratory conditions. In this case, the predator preferred to feed on second and third instars of the pest, whereas the parasitoid preferred to parasitize fourth and fifth instars. This was also demonstrated in choice and non-choice trials using E. varians and T. triozae against B. cockerelli (Mena-Muciño et al. 2021; Morales-Alonso, personal communication) or by Veronesi et al. (2021, 2022a) in the complex E. nicotianae-T. triozae-B. cockerelli.
It has been reported that the combination of E. varians + T. triozae could increase the effect of biological control of B. cockerelli (Mena-Muciño et al. 2021). However, in the present study, the combined release of both natural enemies did not show significant differences in the reduction of the pest population compared with the release of either the predator or parasitoid alone. For this reason, the combined release of E. varians and T. triozae would not be recommendable because it would increase production costs. On the other hand, E. varians prefers to feed on second and third nymphal instars of B. cockerelli while T. triozae kill first and second nymphal instars of the same host on which they feed (Martínez et al. 2015; Rojas et al. 2015). Therefore, further studies are needed to assess whether these behaviors represent a source of competition between these natural enemies. This information could be important for the development of strategies that optimize the augmentative biological control of B. cockerelli using these two natural enemies.
Similar to our results, the combined release of the mirid E. nicotianae together with T. triozae did not result in a significant increase in the biological control of eggs, nymphs, and adults of B. cockerelli compared to each natural enemy alone (Veronesi et al. 2021). These authors demonstrated that the pest was better controlled, at least in the nymphal stage, when the parasitoid was released alone. These authors concluded that this was probably because the pest population was already too high before the introduction of the natural enemies three weeks after pest infestation. In another study, the tomato leaf miner Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) was better controlled when N. tenuis was released alone, rather than in combination with the parasitoid Trichogramma brassicae Bezdenko (Hymenotera: Trichogrammatidae) (Mirhosseini et al. 2019). A possible explanation is that this predator can detect and avoid feeding on parasitized eggs of T. absoluta (Cabello et al. 2015) and this feeding behavior may change towards herbivory when released in combination with T. brassicae (Mirhosseini et al. 2019).
In conclusion, the hypothesis that the combination of E. varians and T. triozae would improve the biological control of B. cockerelli compared to each natural enemy alone is rejected. In this study, we demonstrated that E. varians caused a higher predation at a low release rate, which could be very important for mass rearing or commercial releases. In addition, we observed that both E. varians and T. triozae can survive on pepper on a B. cockerelli and sugar-based diet. However, to validate the impact of these natural enemies on the control of this pest, more studies are needed in a larger experimental area to determine the effects of repetitive releases, as employed for other mirid-parasitoid complexes (Cabello et al. 2015; Mirhosseini et al. 2019). The developmental rate of E. varians on pepper plants without prey and its climatic preferences also should be studied before this predator can be recommended as a biological control agent for B. cockerelli on this crop.
References
Alonso M (2015) Desarrollo biológico e instalación del mírido depredador Nesidiocoris tenuis en el cultivo del tomate con quistes desencapsulados de Artemia sp. como alimento alternativo. Dissertation, Universidad Politécnica de Valencia, Valencia, Spain
Anderson JAD, Walker GP, Alspach PA, Jeram M, Wright PJ (2013) Assessment of susceptibility to zebra chip and Bactericera cockerelli of selected potato cultivars under different insecticide regimes in New Zealand. Am J Potato Res 90:58–65
Bravo ME, López LP (2007) Principales plagas del chile de agua en los valles centrales de Oaxaca. Agroproduce. https://www.researchgate.net/publication/311509157_Principales_plagas_del_chile_de_agua_en_los_Valles_Centrales_de_Oaxaca. Accessed 25 April 2023
Butler CD, Trumble JT (2012a) The potato psyllid, Bactericera cockerelli (Sulc) (Hemiptera: Triozidae): life history, relationship to plant diseases, and management strategies. Terr Arthropod Rev 5(2):87–111
Butler CD, Trumble JT (2012b) Identification and impact of natural enemies of Bactericera cockerelli (Hemiptera: Triozidae) in Southern California. J Econ Entomol 105(5):1509–1519
Cabello T, Bonfil F, Gallego JR, Fernández FJ, Gamez M, Garay J (2015) Can interactions between an omnivorous hemipteran and an egg parasitoid limit the level of biological control for the tomato pinworm? Environ Entomol 44(1):12–26
Calvo FJ, Lorente MJ, Stansly PA, Belda JE (2012) Preplant release of Nesidiocoris tenuis and supplementary tactics for control of Tuta absoluta and Bemisa tabaci in greenhouse tomato. Entomol Exp Appl 143(2):111–119
Calvo FJ, Torres A, Velázquez-González JC, Rodríguez-Leyva E, Lomelí-Flores JR (2016) Evaluation of Dicyphus hesperus for biological control of sweet potato whitefly and potato psyllid on greenhouse tomato. BioControl 61(4):415–424
Calvo FJ, Torres A, González EJ, Velázquez MB (2018a) The potential of Dicyphus hesperus as a biological control agent of potato psyllid and sweetpotato whitefly in tomato. Bull Entomol Res 108(6):765–772
Calvo FJ, Torres-Ruiz A, Velázquez-González J, Rodríguez-Leyva E, Lomelí-Flores JR (2018b) Improved sweetpotato whitefly and potato psyllid control in tomato by combining the mirid Dicyphus hesperus (Heteroptera: Miridae) with specialist parasitic wasps. J Econ Entomol 111(2):549–555
Camacho-Tapia M, Rojas-Martínez RI, Zavaleta-Mejía E, Hernández-Deheza MG, Carrillo-Salazar JA, Rebollar-Alviter A, Ochoa-Martínez DL (2011) Aetiology of chili pepper variegation from Yurécuaro. México J Plant Pathol 93(2):331–335
Castillo CC, Fu Z, Burckhardt D (2019) First record of the tomato potato psyllid Bactericera cockerelli from South America. Bull Insectol 72(1):85–91
Cerna E, Ochoa Y, Aguirre LA, Flores M, Landeros J (2013) Determination of insecticide resistance in four populations of potato psyllid Bactericera cockerelli Sulc (Hemiptera: Triozidae). Phyton 82:63–68
Chávez EC, Bautista OH, Flores JL, Uribe LA, Fuentes YMO (2015) Insecticide resistance ratios of three populations of Bactericera cockerelli (Hemiptera: Psylloidea: Triozidae) in regions of northern México. Fla Entomol 98(3):950–953
Cloyd RA, Bethke JA (2011) Impact of neonicotinoid insecticides on natural enemies in greenhouse and interiorscape environments. Pest Manag Sci 67(1):3–9
Davidson M, Sachtleben T, MacDonald F, Watkins L, Barnes AM, Drayton G, Walker M (2023) The establishment and spread of Tamarixia triozae, a parasitoid of the potato psyllid, in New Zealand. BioControl 68:363–373
DPIRD (Department for Primary Industries and Regional Development) (2021) Tomato potato psyllid. https://www.agric.wa.gov.au/tomato-potato-psyllid-tpp. Accessed 19 June 2023
FAOSTAT (Food and Agriculture Organization of the United Nations) (2021) http://www.fao.org/faostat/es/#data/QCL/visualize. Accessed 22 April 2023
Gbur E, Stroup WW, McCarter KS et al (2012) Analysis of generalized linear mixed models in the agricultural and natural resources sciences. American Society of Agronomy, Madison
Gill G (2006) Tomato psyllid detected in New Zealand. Biosecurity 69:10–11
Guenthner J, Goolsby J, Greenway G (2012) Use and cost of insecticides to control potato psyllids and zebra chip on potatoes. Southwest Entomol 37(3):263–270
Hansen AK, Trumble JT, Stouthamer R, Paine TD (2008) A new Huanglongbing species, “Candidatus Liberibacter psyllaurous” found to infect tomato and potato, is vectored by psyllid Bactericera cockerelli (Sulc). Appl Environ Microbiol 74(18):5862–5865
Jensen DD (1957) Parasites of the Psyllidae. Hilgardia 27(2):71–99
Liefting LW, Perez-Egusquiza ZC, Clover GRG, Anderson JAD (2008) A new ‘Candidatus Liberibacter’ species in Solanum tuberosum in New Zealand. Plant Dis 92(10):1474–1474
Liu TX, Zhang YM, Peng LN, Rojas P, Trumble JT (2012) Risk assessment of selected insecticides on Tamarixia triozae (Hymenoptera: Eulophidae), a parasitoid of Bactericera cockerelli (Hemiptera: Triozidae). J Econ Entomol 105(2):490–496
Luna-Ruiz JJ, Pérez-Chávez MS, Martínez-de Anda JA, Sosa-Ramírez J (2018) Distribución ecogeográfica del chile silvestre en México y su conservación ex situ. In: Aguilar-Meléndez A, Vásquez-Dávila MA, Katz E, Hernández-Colorado MR (eds) Los chiles que le dan sabor al mundo. IRD Éditions, Marseille, pp 93–107
Martínez AM, Chavarrieta JM, Morales SI, Caudillo KB, Figueroa JI, Díaz O, Bujanos R, Gómez B, Viñuela E, Pineda S (2015) Behavior of Tamarixia triozae females (Hymenoptera: Eulophidae) attacking Bactericera cockerelli (Hemiptera: Triozidae) and effects of three pesticides on this parasitoid. Environ Entomol 44(1):3–11
Mena-Mociño LV, Pineda S, Martínez AM, Palma-Castillo LJ, Gómez-Ramos B, Viñuela E, Figueroa JI (2021) Effects of sex ratio on different biological parameters of Engytatus varians (Distant) (Hemiptera: Miridae) adults and their offspring: prey preference for Bactericera cockerelli (Sulcer) (Hemiptera: Triozidae). Bull Entomol Res 111(6):733–740
Mirhosseini MA, Fathipour Y, Holst N, Soufbaf M, Michaud JP (2019) An egg parasitoid interferes with biological control of tomato leafminer by augmentation of Nesidiocorus tenuis (Hemiptera: Miridae). Biol Control 133:34–40
Morales ASI, Martínez CAM, Figueroa JI, Espino AMH, Chavarrieta JM, Ortiz R, Rodríguez EChL, Pineda S (2013) Parámetros de vida del parasitoide sinovigénico Tamarixia triozae (Hymenoptera: Eulophidae). Rev Colomb Entomol 39(2):243–249
Munyaneza JE, Sengoda VG, Crosslin JM, Garzón-Tiznado JA, Cardenas-Valenzuela OG (2009) First report of “Candidatus Liberibacter solanacearum” in pepper plants in Mexico. Plant Dis 93(10):1076–1076
O’Connell DM, Wratten SD, Pugh AR, Barnes AM (2012) “New species association” biological control? Two coccinellids species and an invasive psyllid pest in New Zealand. Biol Control 62(2):86–92
Omondi S (2018) The most popular vegetables in the world. World facts, WorldAtlas https://www.worldatlas.com/articles/the-most-popular-vegetables-in-the-world.html. Accessed 22 May 2023
Orozco MA, Villalba VV, López SN (2012) Desarrollo de Tupiocoris cucurbitaceus (Hemiptera: Miridae) sobre Bemisia tabaci (Hemiptera: Aleyrodidae) en diversas hortalizas. Fitosanidad 16(3):147–153
Perdikis D, Lykouressis DP (2000) Effects of various items, host plants, and temperatures on the development and survival of Macrolophus pygmaeus Rambur (Hemiptera: Miridae). Biol Control 17(1):55–60
Pérez-Aguilar DA, Martínez AM, Viñuela E, Figueroa JI, Gómez B, Morales SI, Pineda S (2019) Impact of the zoophytophagous predator Engytatus varians (Hemiptera: Miridae) on Bactericera cockerelli (Hemiptera: Triozidae) control. Biol Control 132:29–35
Pérez-Hedo M, Riahi Ch, Urbaneja A (2021) Use of zoophytophagous mirid bugs in horticultural crops: current challenges and future perspectives. Pest Manag Sci 77(1):33–42
Pimentel D, Burgess M (2014) Environmental and economic costs of the application of pesticides primarily in the United States. In: Pimentel D, Peshin R (eds) Integrated pest management. Springer, Dordrecht, pp 47–71
Pineda S, Medina M, Figueroa JI, Henry TJ, Mena LV, Chavarrieta JM, Martínez AM (2016) Life history, diagnosis, and biological aspects of Engytatus varians (Hemiptera: Miridae), a predator of Bactericera cockerelli (Hemiptera: Triozidae). Biocontrol Sci Technol 26(8):1073–1086
Pineda S, Hernández-Quintero O, Velázquez-Rodríguez YB, Viñuela E, Figueroa JI, Morales SI, Martínez-Castillo AM (2020) Predation by Engytatus varians (Distant) (Hemiptera: Miridae) on Bactericera cockerelli (Sulcer) (Hemiptera: Triozidae) and two Spodoptera species. Bull Entomol Res 110(2):270–277
Ramírez-Ahuja ML, Rodríguez-Leyva E, Lomelí-Flores JR, Torres-Ruiz A, Guzmán-Franco AW (2017) Evaluating combined use of a parasitoid and a zoophytophagous bug for biological control of the potato psyllid, Bactericera cockerelli. Biol Control 106:9–15
Rojas P, Rodríguez-Leyva E, Lomeli-Flores JR, Liu TX (2015) Biology and life history of Tamarixia triozae, a parasitoid of the potato psyllid Bactericera cockerelli. BioControl 60:27–35
Rubio-Covarrubias OÁ, Almeyda-León IH, Ireta-Moreno J, Sánchez-Salas JA, Fernández-Sosa R, Borbón-Soto JT, Díaz-Hernández C, Garzón-Tiznado JA, Rocha-Rodríguez R, Cadena-Hinojosa MA (2006) Distribución de la punta morada y Bactericera cockerelli (Sulc) en las principales zonas productoras de papa en México. Agric Téc Méx 32(2):201–211
Sánchez JA, Gillespie DR, McGregor RR (2004) Plant preference in relation to life history traits in the zoophytophagous predator Dicyphus hesperus. Entomol Exp Appl 112(1):7–19
Sarkar SC, Milroy SP, Xu W (2022) Development and reproduction of a native generalist predator, Coccinella transversalis (Coleoptera: Coccinellidae), on the tomato potato psyllid, Bactericera cockerelli, with a greenhouse assay of biocontrol potential. Biol Control 176:105–108
Sarkar SC, Hatt S, Philips A, Akter M, Milroy SP, Xu W (2023a) Tomato potato psyllid Bactericera cockerelli (Hemiptera: Triozidae) in Australia: incursion, potential impact and opportunities for biological control. Insects 14(3):263
Sarkar SC, Milroy SP, Xu W (2023b) Potential of variegated lady beetle Hippodamia variegata in management of invasive tomato potato psyllid Bactericera cockerelli. Pest Manag Sci 79(2):821–832
Shipp JL, Wang K (2006) Evaluation of Dicyphus hesperus (Heteroptera: Miridae) for biological control of Frankliniella occidentalis (Thysanoptera: Thripidae) on greenhouse tomato. J Econ Entomol 99:414–420
SIAP (Servicio de Información Agroalimentaria Pesquera) (2021) Cierre de la producción agrícola (1980–2021). https://www.gob.mx/siap/acciones-y-programas/produccion-agricola-33119. Accessed 22 April 2023
SIAP (2022) Panorama agroalimentario 2022. https://drive.google.com/file/d/1jVWS4EFKK7HGwQOBpGeljUyaDT8X8Iyz/view. Accessed 20 June 2023
Silva DB, Bueno VHP, Calvo FJ, van Lenteren JC (2016) Do nymphs and adults of three neotropical zoophytophagous mirids damage leaves and fruits of tomato? Bull Entomol Res 107(2):200–207
Teulon DAJ, Workman PJ, Thomas KL, Nielsen MC (2009) Bactericera cockerelli: incursion, dispersal and current distribution on vegetable crops in New Zealand. N Z Plant Prot 62:136–144
Urbaneja-Bernat P, Mollá O, Alonso M, Bolkcmans K, Urbaneja A, Tena A (2015) Sugars as complementary alternative food for the establishment of Nesidiocoris tenuis in greenhouse tomato. J Appl Entomol 139(3):161–167
van Lenteren JC, Bolckmans K, Köhl J, Ravensberg WJ, Urbaneja A (2018) Biological control using invertebrates and microorganisms: plenty of new opportunities. BioControl 63:39–59
Veronesi ER, Olaniyan O, London H, Saville DJ, Wratten SD (2021) Potential inter-guild interactions to enhance biological control of Bactericera cockerelli on tomatoes: a laboratory and cage study. BioControl 66:343–353
Veronesi ER, Saville DJ, van Koten C, Wratten SD, Goldson SL (2022a) Potential of the mirid bug, Engytatus nicotianae, for the biological control of the tomato-potato psyllid in greenhouses. Crop Prot 156:105941
Veronesi ER, Wratten SD, van Koten C, Goldson SL (2022) Potential of the mirid bug Engytatus nicotianae, and the parasitic wasp Tamarixia triozae for the biological control of the tomato-potato psyllid; a cage greenhouse assay. NZ J Crop Hortic Sci. https://doi.org/10.1080/01140671.2022.2152843
Veronesi ER, Thompson ChJ, Goldson SL (2023) Insect biological control of the tomato-potato psyllid Bactericera cockerelli, a review. NZ J Crop Hortic Sci. https://doi.org/10.1080/01140671.2023.2229770
Xu Y, Zhang ZQ (2015) Amblydromalus limonicus: a “new association” predatory mite against an invasive psyllid (Bactericera cockerelli) in New Zealand. Syst Appl Acarol 20(4):375–382
Yang XB, Campos-Figueroa M, Silva A, Henne DC (2015) Functional response, prey preference, and mutual interference of the Tamarixia triozae (Hymenoptera: Eulophidae) on tomato and bell pepper. J Econ Entomol 108(2):414–424
Acknowledgements
We thank Koppert Biological Systems (El Marqués, Querétaro, Mexico) for providing the TETRAPAR product. Alejandro O. Guzmán-Pedraza received a student stipend from the Consejo Nacional de Ciencia y Tecnología, Mexico. We also thank Trevor Williams (Instituto de Ecología A.C., Xalapa, Veracruz, Mexico) for his support in the revision of this document. We acknowledge the comments of two anonymous reviewers, which have contributed to improve this manuscript.
Funding
This work was supported by the Coordinación de la Investigación Científica, Universidad Michoacana de San Nicolás de Hidalgo.
Author information
Authors and Affiliations
Contributions
AOG-P: Conceptualization, data curation, writing—original draft. AMM: Conceptualization, writing—review and editing. ÁR-A: Formal analysis, review and editing. LJP-C: Formal analysis, editing. SIM-A: Formal analysis, editing. JMC-Y: Data curation, editing. JIF: Data curation and review. SP: Conceptualization, formal analysis, writing—review and editing, funding acquisition.
Corresponding authors
Ethics declarations
Conflict of interest
There are no conflicts of interest associated with this manuscript.
Informed consent
No informed consent was required for this manuscript.
Research involving human and/or animals participants
No humans or animals (vertebrates) were used to produce this manuscript.
Additional information
Handling Editor: Josep Anton Jaques Miret
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guzmán-Pedraza, A.O., Martínez, A.M., Rebollar-Alviter, Á. et al. Efficacy of a mirid predator and an eulophid parasitoid to the tomato potato psyllid Bactericera cockerelli control on pepper plants. BioControl 69, 39–51 (2024). https://doi.org/10.1007/s10526-024-10240-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-024-10240-x