Abstract
The tomato–potato psyllid (TPP) Bactericera cockerelli, is a serious pest of solanaceous crops. Some populations are becoming pesticide-resistant, increasing the need for alternatives such as biological control (BC). This approach may be improved by combining different species of BC agents. We conducted three separate experiments to test four BC agents, either alone or combined with others: (1) A laboratory assay to test the effect of buckwheat (Fagopyrum esculentum) and alyssum (Lobularia maritima) flowers on the longevity of females of the parasitic wasp Tamarixia triozae; (2) A no-choice laboratory assay to investigate the consumption of B. cockerelli life stages by the predatory bug Engytatus nicotianae; (3) A cage experiment in a greenhouse to assess four natural enemy species against B. cockerelli on tomatoes: these were the predators Cleobora mellyi, Amblydromalus limonicus, E. nicotianae, and T. triozae. Access to buckwheat flowers allowed female T. triozae to live for an average of 10.9 days compared to 2.1 days with alyssum and 1.4 day with water but did not improve the BC of B. cockerelli. Adult E. nicotianae preyed on all offered B. cockerelli stages. In experiment 3, combinations of T. triozae with A. limonicus or E. nicotianae were not significantly better than single natural enemy species, except for the reduction of nymphal populations when A. limonicus and T. triozae were combined. Although there were few significant reductions in numbers of TPP when using natural enemy species combinations, some species showed good potential when used alone. We suggest testing earlier release of combinations of natural enemy for evaluate its impact on TPP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
“Biological control (BC) of invertebrate pests can be elegant, self-sustaining, non-polluting and cheap-when it works!” (Gurr et al. 2000). BC of glasshouse pests has been particularly successful, probably because of the enclosed nature of the crop, a relatively stable environment and limited potential for pests to arrive. The tomato–potato psyllid (TPP) Bactericera cockerelli Ŝulcer (Hemiptera: Triozidae) is a phloem-feeding insect native to North America and has been recognised as a severe pest of potatoes and tomatoes in its native range for over a hundred years (Butler and Trumble 2012). It was accidentally introduced into New Zealand in 2005 (Gill 2006), and is now a significant pest of field potatoes, greenhouse peppers and tomatoes, and tamarillos (Cyphomandra betacea) (Teulon et al. 2009). Adults and nymphs of B. cockerelli cause direct feeding damage (Sengoda et al. 2010), and vector a bacterial pathogen Candidatus Liberibacter solanacearum (CLso) that causes foliar damage, stunting, and reduced yields (Hansen et al. 2008; Liefting et al. 2008). CLso causes zebra chip disease in potatoes, a condition which manifests as brown patches and stripes in fresh tubers (Munyaneza et al. 2007; Munyaneza et al. 2015), and when such tubers are processed into chips and fried, the stripes become darker, causing consumers and processors to reject infected fresh tubers (Munyaneza 2012). CLso also causes early decline and death of infected plants and can lead to total crop losses (Sengoda et al. 2010).
Current management of TPP and CLso is based on frequent applications of broad-spectrum chemical insecticides (Guenthner et al. 2012), with up to 15 foliar applications each season in New Zealand potato crops (Anderson et al. 2013). Studies in the USA and Mexico show that some B. cockerelli populations have developed resistance to imidacloprid, a broad-spectrum neonicotinoid insecticide that has been widely used against the pest for many years in both countries (Cerna et al. 2013; Szczepaniec et al. 2019).
In Mexico, greenhouse trials for TPP biocontrol have involved the use of a single natural enemy species (Calvo et al. 2016; Perez-Aguilar et al. 2019), combined release of an entomopathogenic fungus and a parasitoid (Tamayo-Mejía et al. 2015), and combined release of two insect natural enemy species that attack different life stages of the pest (Ramírez-Ahuja et al. 2017). Tamayo-Mejía et al. (2015), combined Tamarixia triozae Burks (Hymenoptera: Eulophidae), a parasitic wasp native to parts of USA and Mexico, with Beauveria bassiana Balsamo (Vuillemin) (Ascomycetes: Hypocreales). This combination led to better control of TPP than when the two agents were released separately, even though adult T. triozae that emerged from B. bassiana-infected TPP nymphs had lower survival rates than did those emerging from non-infected nymphs. In the study by Ramírez-Ahuja et al. (2017) the zoophytophagous bug Dicyphus hesperus Knight (Heteroptera: Miridae) was combined with T. triozae, and although D. hesperus ate TPP nymphs containing T. triozae eggs, the combined effect of both natural enemy species led to additive reduction of TPP. The above examples indicate that despite possible negative natural enemy interactions that can disrupt BC (Denoth et al. 2002), the combined release of natural enemy species can provide improved control of TPP under greenhouse conditions. Four natural enemy species were selected for their biological control potential: the southern ladybird Cleobora mellyi Mulsant (Coleoptera: Coccinellidae), Amblydromalus limonicus (Garman & McGregor) (Acari: Phytoseiidae), T. triozae, and Engytatus nicotianae Koningsberger (Heteroptera: Miridae), commonly known as the tomato/tobacco mirid.
Cleobora mellyi was introduced into New Zealand from Australia in the 1980s and 1990s for BC of the Eucalyptus tortoise beetle Paropsis charybdis Stål (Coleoptera: Chrysomelidae) (Baker et al. 2003), and a no-choice laboratory assay using potato leaf discs (O’Connell et al. 2012) showed that adults and 4th instar larvae of C. mellyi consumed up to 100 TPP nymphs in 24 h. A greenhouse cage trial further showed that C. mellyi reduced B. cockerelli populations on potato plants and kept them at a low density for up to seven weeks (Pugh et al. 2015). However, this has not been tested in field potatoes or greenhouse tomatoes.
Amblydromalus limonicus is a generalist predatory mite that was recently reported in New Zealand, and feeds and reproduces on a diet of eggs and first and second instar nymphs of TPP (Patel and Zhang 2017). It prefers TPP eggs and first instars (Xu and Zhang 2015), and it significantly suppressed TPP numbers on pepper plants but was less effective on tomatoes, perhaps due to the glandular trichomes of the latter (Kean et al. 2019). Tamarixia triozae is a parasitic wasp that was approved by the New Zealand Environmental Protection Agency (EPA) for importation and release in 2016, as a classical biocontrol agent of TPP in New Zealand. Females of this wasp obtain nutrients for oogenesis by feeding on first and second instar TPP nymphs, and preferentially parasitise fourth and fifth instars (Rojas et al. 2015). Engytatus nicotianae, the tomato/tobacco mirid, is an adventive species in New Zealand (Eyles and Schuh 2003). Nymphs and adults of E. nicotianae feed on whitefly nymphs (Thompson, personal communication), and have been observed feeding on the eggs and young nymphs of TPP in cultures (Olaniyan, personal observation).
A no-choice feeding assay was used to assess 24 h predation rates of male and female E. nicotianae on B. cockerelli eggs and first to fourth instar nymphs. Preliminary observations indicated that the fifth instar is rarely preyed on by E. nicotianae. A second experiment was conducted to investigate the effect of the nectar of flowers of buckwheat Fagopyrum esculentum Moench (Polygonaceae) cv. Katowase compared to that of alyssum Lobularia maritima L. cv. benthamii (Brassicaceae), on the longevity of female T. triozae. Floral nectar can increase the longevity and fecundity of parasitoids (Vattala et al. 2006).
Materials and methods
Insect cultures
All cultures were established in Lincoln University controlled temperature (CT) rooms set to temperature 24 ± 4 °C, 65–75% RH, and a L:D 16:8 h photoperiod. B. cockerelli, C. mellyi, E. nicotianae, and T. triozae were cultured on tomato (cv. Merlice) in cages (BugDorm 2120F; dimensions: W60 × D60 × H60 cm), BugDorm, Thailand. The side panels of the cage were made from 150 × 150 micro-mesh nylon with holes small enough to keep aphids, thrips, and parasitic wasps from escaping or entering the cage while allowing for ventilation.
Bactericera cockerelli adults were collected from hedges of African boxthorn, Lycium ferocissimum (Miers) (Solanaceae), in Canterbury and reared through overlapping generations for several months. Adults of C. mellyi were collected from resting sites in a forest of Eucalyptus nitens Deane & Maiden (Myrtaceae) near Blenheim, New Zealand, and transferred onto caged TPP-infested tomato plants with drinking water provided on dental rolls. Myzus persicae Sulzer (Hemiptera: Aphididae) was used as supplementary food for the ladybird when TPP numbers were low. The T. triozae culture was established with 40 adult wasps obtained from a colony at Plant and Food Research (https://www.plantandfood.co.nz/), Auckland, New Zealand, and reared on B. cockerelli nymphs on tomato, with drinking water provided as above.
Amblydromalus limonicus and E. nicotianae were supplied by BioForce Limited (https://www.bioforce.co.nz/), Karaka, Auckland, New Zealand. Once received, E. nicotianae was cultured on TPP-infested tomato plants for up to six weeks. Amblydromalus limonicus, packaged in a 1 l cardboard cylinder containing c. 15,000 mites in bran infested with Carpoglyphus lactis L. (Acari: Astigmata) as prey, was received two days before the experiment and stored at 15 °C and a L:D 16:8 photoperiod according to the supplier’s recommendation, until needed.
Plants
Two-week-old unsprayed tomato seedlings (cv. Merlice) were purchased from Zealandia Horticulture Ltd. (https://www.zealandia.co.nz/), Christchurch, New Zealand. At the Lincoln University plant nursery, the seedlings were transplanted singly into 2 l pots containing potting mix taken from a 500 l batch comprising 400 l composted bark, 100 l pumice, 1500 g Osmocote Exact® Standard 16-3.9-10 + TE (3–4 month release), 500 g horticultural lime and 500 g Osmocote Total Hydraflow (12 month release). This potting mix was used to grow buckwheat and alyssum from seeds supplied by Kings Seeds (https://www.kingsseeds.co.nz/), Katikati, New Zealand. Sowings were made fortnightly to ensure a steady supply of plants in flower throughout the experiments.
Experiment set-up
Effect of floral nectar on the longevity of female T. triozae
Only female T. triozae (≤ two days old) were used in this bioassay that was conducted in a CT room; temperature 26 ± 1 °C and a L:D 16:8 photoperiod. Experimental arenas were 90 mm diameter Petri dishes, each with a piece of nylon mesh glued over its top with liquid silicon to confine the wasps while allowing for ventilation. Two holes, each about 7 mm in diameter, were made on opposite sides of the walls of each dish. Excised flowers (either buckwheat or alyssum) with the stalks inserted in a wet dental roll wrapped in Parafilm (to retain moisture and keep the flowers fresh) were inserted through one of the holes. The use of excised flowers was justified because nectar content and production are not significantly altered in excised buckwheat flowers (Wade and Wratten 2007). Once flowers were inserted, a female T. triozae was released into the Petri dish through the opposite hole, which was plugged as above. A control (water only) treatment, with both holes in the sides of the Petri dish plugged with dental rolls soaked in water, was included. The water-only rolls in the three treatments were replaced daily, whereas flowers in the alyssum and buckwheat treatments were replaced with fresh ones every second day. There were 20 wasps per treatment, i.e., buckwheat (20), alyssum (20), water only (20), making a total of 60 arenas arranged in a completely randomised design. Each wasp was checked daily, its status determined as alive or dead. Time to death (longevity) in days was also recorded for wasps that died.
Laboratory consumption rate assay of E. nicotianae on B. cockerelli
The assay was set up as a randomised complete block design with twelve treatments, each replicated in eight 24 h blocks in a CT room set to 26 ± 1 °C, 65–75% RH, and a L:D 16:8 photoperiod. Treatments comprised either eggs, first and second instars (five of each instar), third instar, or fourth instar B. cockerelli nymphs in feeding arenas to which a female, male or no adult E. nicotianae was added. Fifth instar nymphs were not included in this study due to the high chance of their becoming adults during the experiment and because preliminary essays showed that the fifth instar is rarely preyed on by E. nicotianae. Treatment structure was therefore a 4 (B. cockerelli life stages) × 3 (female, male or no adult E. nicotianae) factorial design. Arenas were 90 mm vented Petri dishes, with a piece of 10 cm diameter filter paper cut to fit into each dish. A small, fully expanded tomato leaflet was excised from the growing tip of a tomato plant, and its petiole was wrapped in a piece of moistened cotton wool inserted into a 0.5 ml Eppendorf tube to keep the leaflet fresh. Ten individuals each, of the B. cockerelli nymphal groups (see above) were transferred to each tomato leaflet using a fine painter’s brush. For eggs, tomato leaflets on which TPP eggs had been laid over a 48 h period were excised from whole plants. Excess eggs were removed under a microscope to leave 20 eggs per leaflet. One male or female E. nicotianae was then introduced into each feeding arena and a lid placed over the Petri dish. The four controls, one for each B. cockerelli treatment, were included in each block to monitor mortality in the absence of E. nicotianae. After 24 h, each leaflet was examined under a dissecting microscope and the number of the particular B. cockerelli life stage (for that treatment) that were alive, was counted to determine the percentage consumed by E. nicotianae.
Greenhouse cage experiment
This experiment was a randomised complete block with eight treatments and six blocks. Caged tomato plants were assigned to one of eight treatments: (1) Control; (2) T. triozae (one male and two females); (3) E. nicotianae (one pair of adults); (4) E. nicotianae (one pair of adults) + T. triozae (one male and two females); (5) A. limonicus (ca. 50 individuals applied by sprinkling approximately 3 ml of the bran containing the mite); (6) A. limonicus [ca. 50 individuals applied as in (5) above] + T. triozae (one male and two females); (7) one pair of adult C. mellyi; (8) one male and two females T. triozae + 1 flowering buckwheat plant. Cleobora mellyi was not combined with any of the other species because of its voracity (O’Connell et al. 2012). With this set of treatments, the interaction between the presence/absence of T. triozae and of E. nicotianae can be examined using a 2 × 2 factorial structure involving treatments 1–4. Similarly, the interaction between the presence/absence of T. triozae and of A. limonicus can be examined using a 2 × 2 factorial structure involving treatments 1, 2, 5 and 6.
Before the introduction of biological control agents (BCAs), a, mating pair of eight-day-old adult B. cockerelli was released into each of the 48 cages (including control cages) at seven day intervals for three consecutive weeks. This allowed time for oviposition and completion of one generation and was meant to simulate a heavy infestation of the pest. BCAs were then added to each cage based on the randomly generated treatment assignations. In the Tamarixia + buckwheat treatment, buckwheat plants were replaced with younger ones three weeks after the first release of BCAs. At the same time, the second release of BCAs was carried out for all treatments following the protocol used during the first release.
Bactericera cockerelli numbers (eggs, nymphs, adults) on the tomato plants in the greenhouse cage experiment were assessed twice: three weeks after the first release of BCAs (three weeks) and three weeks after the second release (week 6). In week 3, a sample of four leaflets was collected from each plant: one from the top, two from the middle and one from the bottom third of the plant, and the number of eggs, nymphs and adults of B. cockerelli on each leaflet was counted, recorded separately, and averaged prior to statistical analysis. In week 6, the sample size was increased to 16 leaflets per plant (four on top, eight in the middle and four at the bottom third of the plant) to reduce variability in the average count per cage data. At the end of the experiment, plants and cages were checked for natural enemies to determine their survival (actual counts were made only for C. mellyi, which is large and easy to see and count).
Plants were irrigated via an Orbit® irrigation timer attached to a 19 mm polyethylene lateral line supplying water to 2 l h−1 drippers inserted through a small hole made in the back of each cage, which was plugged using paper towels, and sealed with cellulose tape to prevent escape or ingress of insects. A data logger EasyLog (EL-USB-2-LCD+) (Lascar Electronics, UK) was placed in one randomly selected cage to record temperature and RH at 15 min intervals. The temperature in the experimental cages over the six weeks ranged from 10 to 37 °C, with a mean of 19.9 °C ± 5.62 (SD). The mean RH was 87. 4% ± 11.05, and ranged from 44 to 101.5%. On days when the daytime temperature in the greenhouse exceeded 28 °C, the floor was hosed down for evaporative cooling. From the time BCAs were first introduced, the experiment lasted six weeks (March 1–April 12, 2018) and was terminated when the plants became too tall for the cages.
Data analysis
The numbers of B. cockerelli of the particular life stage consumed by E. nicotianae in each treatment of the laboratory consumption rate assay were counted and converted to a percentage of the number initially provided. There was zero consumption in all of the controls, so these data were excluded from the statistical analysis. The remaining percentage data (the response variable) met the ANOVA assumptions of normality and homogeneity of variance. Normality was checked by examining a histogram of the ANOVA residuals and a quantile–quantile plot of the residual quantiles versus the quantiles of a normal distribution. Homogeneity of variance was checked by examining a plot of the residuals versus the fitted values. As appropriate for a randomized complete block design, the ANOVA model included a term for blocks, and the treatment structure was a 2 × 4 factorial design as detailed above, with “Engytatus” (male or female) and “TPP life stage” (eggs, first and second instar, third instar, fourth instar) as the factors. Means were separated using the unprotected least significant difference (LSD) procedure at p < 0.05. This multiple comparison procedure (MCP) was chosen since it is the only “consistent” MCP and for other reasons as outlined in Saville (1990, 2015). The T. triozae longevity data were subjected to one-way ANOVA, and a 95% confidence interval for the true mean of each treatment was calculated for each treatment separately.
The average B. cockerelli (eggs, nymphs, adults) per cage count data from the greenhouse experiment were normalised using a square-root transformation and subjected to two-way (blocks and treatments) ANOVA that included 2 × 2 factorial contrasts (Cochran and Cox 1957; Saville and Wood 1991) as detailed above. Two sets of 2 × 2 factorial contrasts were set up: the first set used treatments 1–4 and the second set used treatments 1, 2, 5 and 6. Means were compared using these contrasts or the unprotected least significant difference (LSD) procedure at p < 0.05 (Saville 2015). In terms of presentation of results, LSD bars are given as well as SE bars since the LSD provides the most succinct and convenient summary of the statistical analysis, and for other reasons as detailed in Saville and Rowarth (2008).
Results
Engytatus nicotianae consumption rates on B. cockerelli
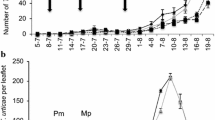
Male and female E. nicotianae preyed on all the life stages of B. cockerelli provided. The mean TPP (all stages) consumption rate of E. nicotianae over 24 h was 34.6% and 43.2% for males and females, respectively. The difference between male and female consumption rates was not statistically significant (p = 0.16). However, TPP life stage had a statistically significant effect on predation by E. nicotianae (F3, 48 = 8.17; p < 0.001). Unprotected LSD (5%) showed this difference to be due to lower consumption rates of fourth instar TPP nymphs compared to the other three life stages. The interaction between life stage and the proportion of B. cockerelli consumed by male and female E. nicotianae was also significant (F3, 48 = 3.32; p < 0.05). Females fed on a significantly higher proportion of third instar nymphs than did males while males consumed more eggs than females, although the latter difference was not statistically significant (Fig. 1).
Mean predation rates (+ SE) of male and female E. nicotianae on B. cockerelli life stages. The vertical bar is the least significant difference (LSD) at p = 0.05. Any difference between two means which exceeds this (5%), is statistically significant at p = 0.05. These differences are also indicated using lettering; means with no letters in common differ significantly at p < 0.05
Floral nectar and longevity of female T. triozae
The mean longevity ± 95% confidence intervals were 1.35 ± 0.33, 2.05 ± 0.59 and 10.9 ± 3.38 days for T. triozae given water, alyssum and buckwheat flowers, respectively. Female T. triozae provided with buckwheat flowers lived around five times longer then those given alyssum flowers, and eight times as long then those given only water. Maximum survival periods for T. triozae fed with buckwheat, alyssum, or water were 29, five, and three days, respectively (Fig. 2).
Natural enemy treatments and B. cockerelli
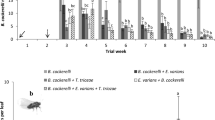
At week 3, comparison of the √ mean number of B. cockerelli per leaflet using the unprotected LSD at 5% level of significance showed that the mean number of eggs per leaflet for C. mellyi was significantly lower than that in the control (Fig. 3a). The mean number of nymphs per leaflet for T. triozae, A. limonicus, and the A. limonicus + T. triozae combination also differed significantly from that in the control (Fig. 3b). There was also a significant difference in the mean number of adults per leaflet between A. limonicus and the control (Fig. 3c). At week 6 there were no statistical differences in TPP numbers between the control and the other treatments (Table 1). The factorial contrasts indicated that at both weeks 3 and 6, there were no significant interactions between T. triozae and either E. nicotianae or A. limonicus, though some interactions were close to 5% significant. With respect to natural enemy survival, T. triozae was recovered as adults and parasitised TPP nymphs from all the treatments that involved the parasitoid, and was too numerous to be counted. Cleobora mellyi also survived in all six cages (replicates) where it had been introduced (all four were alive in five of the cages and two were alive in one cage). No live E. nicotianae or A. limonicus were recovered by the end of the experiment (week 6).
Effect of biocontrol agents on B. cockerelli populations on tomato leaflets after three weeks (week 3). Mean square root number per leaflet (+ SE) of a eggs, b nymphs and c adults. The vertical bar is the least significant difference (LSD) at p = 0.05. Differences which exceed the LSD are statistically significant at p = 0.05. These differences are also indicated using lettering; means with no letters in common differ significantly at p < 0.05. To back-transform the means, simply square them. These back-transformed numbers per leaflet, from left to right in each graph, are a 15.4, 4.8, 9.7, 6.1, 1.5, 3.2, 0.4 and 5.2 eggs, b 7.9, 0.5, 3.6, 2.9, 1.7, 0.2, 3.0 and 3.1 nymphs and c 2.0, 0.6, 1.6, 1.3, 0.1, 0.8, 0.9 and 0.5 adults per leaflet
Discussion
Due to the problems associated with pesticide use, there is increasing emphasis on biological control as a sustainable pest management strategy in field and greenhouse crops. Under experimental greenhouse conditions in Mexico, synergies between two natural enemy species improved biological control of B. cockerelli on tomatoes (Tamayo-Mejía et al. 2015; Ramírez-Ahuja et al. 2017). In the current study, we also investigated whether releases of two natural enemy species (although these differed from those used in Mexico) would have improved B. cockerelli biological control.
The natural enemy treatments (single and combined) had few statistically significant effects on the mean number of B. cockerelli eggs, nymphs, or adults per leaflet. This outcome may have been, in part, due to the high level of variability, and more likely because the pest population was already too high before the introduction of natural enemies. Although C. mellyi significantly reduced the number of TPP eggs in week 3, the previously demonstrated voracity of this ladybird on TPP nymphs on potato leaf discs in that case (O’Connell et al. 2012) was not evident in this study. A possible explanation is that C. mellyi is not adapted to the sticky trichomes on the tomato plants, which probably hampered its movements and attack rates. Cleobora mellyi was often observed resting on the top or bottom of cages rather than on the plants, and it laid most of its egg batches on the plastic panels of the cage with only a few being laid on tomato plants (Olaniyan, personal observation). This combination of behaviours needs to be tested through further experimentation.
Engytatus nicotianae preyed on all the TPP life stages, which, for the first time, confirmed previous anecdotal reports and justified the bug’s inclusion in the greenhouse cage experiment. The results of the consumption assay showed that male and female E. nicotianae prefer different life stages of TPP as prey, and suggests that there may be minimal competition between the sexes, which could lead to complementary control of TPP. Unlike C. mellyi, the mobility of E. nicotianae on whole tomato plants was not likely to have been affected by the glandular trichomes, since all mirid bugs in its tribe (Dicyphini) have specialised tarsi that enable them to move freely on the surface of plants with glandular trichomes (Schuh and Slater 1995). Despite this potential advantage, neither the release of E. nicotianae alone nor the combined release of this species with T. triozae led to a significant suppression of B. cockerelli populations as indicated by the results from experiment three.
Tamarixia triozae is considered the most important parasitoid of B. cockerelli and when released alone, it provided a significant reduction of TPP nymphs in week 3 of the greenhouse cage experiment. This was not surprising since females of this synovigenic parasitoid host feed on younger nymphs and parasitise fourth and fifth instars. When T. triozae was released with A. limonicus, which feeds on eggs and first and second instars, there was a further reduction in B. cockerelli nymphal populations. Also, fewer B. cockerelli eggs were found when these two BCAs were combined, compared to when T. triozae was released alone, which suggests complementarity between these two enemy species that could be exploited for TPP biocontrol. Separate release of A. limonicus resulted in the lowest number of B. cockerelli adults and significantly reduced egg numbers. However, these impacts did not persist up to week 6, and no live mites were recovered at the end of the experiment.
The results of the T. triozae longevity assay showed an eightfold increase in the mean longevity of female T. triozae that were given buckwheat flowers compared to water only, and a fivefold increase compared to the wasps that were provided with alyssum flowers. This result is consistent with previous work, which showed that, compared to alyssum, buckwheat nectar has a stronger positive effect on the longevity of some other parasitoid species (Araj et al. 2006; Vattala et al. 2006). Further, in another study to assess parasitoid foraging success on flowers of different species (Patt and Rohrig 2017), the floral nectaries of alyssum were found to be only partially accessible to Tamarixia radiata Waterston (Hymenoptera: Eulophidae) (Patt & Rohrig, 2017) that is congeneric, and of similar size to T. triozae. The mean longevity of female T. triozae given only water in this study was 1.35 days, and 1.7 days in Rojas et al. (2015), and these results strongly suggest that female T. triozae need an alternative energy source if they are to be released prophylactically in greenhouses before B. cockerelli infestation. In terms of the impact of potential biological control agents on pathogen transmission by TPP, there is no evidence that the tested biocontrol agents can play an active role in preventing the immediate transmission of the disease if the initial B. cockerelli female that laid the eggs was infected. However, the removal of some of the TPP eggs and nymphs could help reduce the spread of the pathogen by reducing the numbers of infected offspring leaving the plant and dispersing to new ones.
Of the BCA treatments in this study, the combination A. limonicus + T. triozae, and separate release of T. triozae gave the highest reduction in B. cockerelli nymph numbers, with A. limonicus also showing promise for TPP egg population reduction. Engytatus nicotianae consumption rate on B. cockerelli warrants further investigation of this bug as a BCA of the pest. Further experiments in larger cages and a higher starting density of the pest, as well as introducing the natural enemy treatments earlier (such as one week after the introduction of the pest) would allow for a better assessment of the potential of these natural enemies against B. cockerelli.
References
Anderson JAD, Walker GP, Alspach PA, Jeram M, Wright PJ (2013) Assessment of susceptibility to zebra chip and Bactericera cockerelli of selected potato cultivars under different insecticide regimes in New Zealand. Am J Potato Res 90:58–65
Araj S, Wratten SD, Lister AJ, Buckley H (2006) Floral nectar affects longevity of the aphid parasitoid Aphidius ervi and its hyperparasitoid Dendrocerus aphidum. N Z Plant Prot 59:178–183
Baker SC, Elek JA, Bashford R, Paterson SC, Madden J, Battaglia M (2003) Inundative release of coccinellid beetles into eucalypt plantations for biological control of chrysomelid leaf beetles. Agric For Entomol 5:97–106
Butler CD, Trumble JT (2012) The potato psyllid, Bactericera cockerelli (Sulc) (Hemiptera: Triozidae): life history, relationship to plant diseases, and management strategies. Terr Arthropod Rev 5:87–111
Calvo FJ, Torres-Ruiz A, Velazquez-Gonzalez JC, Rodriguez-Leyva E, Lomeli-Flores JR (2016) Evaluation of Dicyphus hesperus for biological control of sweet potato whitefly and potato psyllid on greenhouse tomato. BioControl 61:415–424
Cerna E, Ochoa Y, Aguirre LA, Flores M, Landeros J (2013) Determination of insecticide resistance in four populations of potato psillid Bactericera cockerelli (Sulc.) (Hemiptera: Triozidae). Phyton Int J Exp Bot 82:63–68
Cochran WG, Cox GM (1957) Experimental designs, 2nd edn. John Wiley & Sons
Denoth M, Frid L, Myers JH (2002) Multiple agents in biological control: improving the odds? Biol Control 24:20–30
Eyles AC, Schuh RT (2003) Revision of New Zealand Bryocorinae and Phylinae (Insecta: Hemiptera: Miridae). N Z J Zool 30:263–325
Gill G (2006) Tomato psyllid detected in New Zealand. Biosecurity (69):10–11
Guenthner J, Goolsby J, Greenway G (2012) Use and cost of insecticides to control potato psyllids and zebra chip on potatoes. Southwest Entomol 37:263–270
Gurr GM, Wratten SD, Barbosa P (2000) Success in conservation biological control of arthropods. In: Biological control: measures of success. Springer, Dordrecht, pp 105–132
Hansen AK, Trumble JT, Stouthamer R, Paine TD (2008) A new huanglongbing species, “Candidatus Liberibacter psyllaurous”, found to infect tomato and potato, is vectored by the psyllid Bactericera cockerelli (Sulc). Appl Environ Microbiol 74:5862–5865
Kean AM, Nielsen M-C, Davidson MM, Butler RC, Vereijssen J (2019) Host plant influences establishment and performance of Amblydromalus limonicus, a predator for Bactericera cockerelli. Pest Manag Sci 75:787–792
Liefting L, Perez-Egusquiza Z, Clover G, Anderson J (2008) A new ‘Candidatus Liberibacter’ species in Solanum tuberosum in New Zealand. Plant Dis 92:1474
Munyaneza JE (2012) Zebra chip disease of potato: biology, epidemiology, and management. Am J Potato Res 89:329–350
Munyaneza JE (2015) Zebra chip disease, Candidatus Liberibacter, and potato psyllid: a global threat to the potato industry. Am J Potato Res 92:230–235
Munyaneza JE, Crosslin JM, Upton JE (2007) Association of Bactericera cockerelli (Homoptera: Psyllidae) with “Zebra Chip,” a new potato disease in southwestern United States and Mexico. J Econ Entomol 100:656–663
O’Connell DM, Wratten SD, Pugh AR, Barnes AM (2012) ‘New species association’ biological control? Two coccinellid species and an invasive psyllid pest in New Zealand. Biol Control 62:86–92
Patel K, Zhang ZQ (2017) Prey preference and reproduction of predatory mites, Amblybromalus limonicus and Neoseiulus cucumeris, on eggs and 1st instar nymphs of the tomato/potato psyllid. Int J Acarol 43:468–474
Patt JM, Rohrig E (2017) Laboratory evaluations of the foraging success of Tamarixia radiata (Hymenoptera: Eulophidae) on flowers and extrafloral nectaries: potential use of nectar plants for conservation biological control of Asian citrus psyllid (Hemiptera: Liviidae). Fla Entomol 100:149–156
Perez-Aguilar DA, Martinez AM, Vinuela E, Figueroa JI, Gomez B, Morales SI, Tapia A, Pineda S (2019) Impact of the zoophytophagous predator Engytatus varians (Hemiptera: Miridae) on Bactericera cockerelli (Hemiptera: Triozidae) control. Biol Control 132:29–35
Pugh AR, O’Connell DM, Wratten SD (2015) Further evaluation of the southern ladybird (Cleobora mellyi) as a biological control agent of the invasive tomato–potato psyllid (Bactericera cockerelli). Biol Control 90:157–163
de Ramírez-Ahuja ML, Rodríguez-Leyva E, Lomeli-Flores JR, Torres-Ruiz A, Guzmán-Franco AW (2017) Evaluating combined use of a parasitoid and a zoophytophagous bug for biological control of the potato psyllid, Bactericera cockerelli. Biol Control 106:9–15
Rojas P, Rodriguez-Leyva E, Lomeli-Flores JR, Liu TX (2015) Biology and life history of Tamarixia triozae, a parasitoid of the potato psyllid Bactericera cockerelli. BioControl 60:27–35
Saville DJ (1990) Multiple comparison procedures: the practical solution. Am Stat 44:174–180
Saville DJ (2015) Multiple comparison procedures—cutting the Gordian knot. Agron J 107:730–735
Saville DJ, Wood GR (1991) Statistical methods: the geometric approach. Springer, New York
Saville DJ, Rowarth JS (2008) Statistical measures, hypotheses, and tests in applied research. J Nat Resour Life Sci Educ 37:74–82
Schuh RT, Slater JA (1995) True bugs of the world (Hemiptera: Heteroptera): classification and natural history. Comstock Publisher Associates, Ithaca
Sengoda VG, Munyaneza JE, Crosslin JM, Buchman JL, Pappu HR (2010) Phenotypic and etiological differences between psyllid yellows and zebra chip diseases of potato. Am Potato J 87:41–49
Szczepaniec A, Varela KA, Kiani M, Paetzold L, Rush CM (2019) Incidence of resistance to neonicotinoid insecticides in Bactericera cockerelli across Southwest U.S. Crop Prot 116:188–195
Tamayo-Mejía F, Tamez-Guerra P, Guzmán-Franco AW, Gomez-Flores R (2015) Can Beauveria bassiana Bals. (Vuill) (Ascomycetes: Hypocreales) and Tamarixia triozae (Burks) (Hymenoptera: Eulophidae) be used together for improved biological control of Bactericera cockerelli (Hemiptera: Triozidae)? Biol Control 90:42–48
Teulon DAJ, Workman PJ, Thomas KL, Nielsen MC (2009) Bactericera cockerelli: incursion, dispersal and current distribution on vegetable crops in New Zealand. N Z Plant Prot 62:136–144
Vattala HD, Wratten SD, Phillips CB, Wäckers FL (2006) The influence of flower morphology and nectar quality on the longevity of a parasitoid biological control agent. Biol Control 39:179–185
Wade MR, Wratten SD (2007) Excised or intact inflorescences? Methodological effects on parasitoid wasp longevity. Biol Control 40:347–354
Xu Y, Zhang Z-Q (2015) Amblydromalus limonicus: a “new association” predatory mite against an invasive psyllid (Bactericera cockerelli) in New Zealand. Syst Appl Acarol 20:375–382
Acknowledgements
This work was supported by a grant from the New Zealand Agricultural and Marketing Research and Development Trust (AGMARDT) (Grant Number A17022). Supplementary funding was provided by Tomatoes New Zealand and Vegetables New Zealand. We thank Dr. Gonzalo Avila and Frances MacDonald (Plant and Food Research, Auckland) for providing us with T. triozae, Mike and Kristen Gerard for the C. mellyi, and Chris and John Thompson of BioForce Ltd. for the supply of A. limonicus and E. nicotianae. The authors are also grateful to Brent Richards (Lincoln University Plant Nursery) for technical assistance, and to Sherry Hanna, Jacqueline Bennet, Kate Scanlan (Lincoln University), and Alexander Zhou (Burnside High School), who helped to count B. cockerelli on tomato leaflet samples.
Author information
Authors and Affiliations
Contributions
All authors participated equally in the conception and design of the study. OO and ERV conducted the E. nicotianae feeding assay, and OO and HL conducted the greenhouse experiment; DJS analysed the data from both experiments. OO and SDW designed the T. triozae longevity assay; OO conducted the assay and analysed the data. All authors participated in the preparation and review of drafts of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
We have no potential conflicts of interest.
Ethical approval
Our research involved only insects and plants.
Additional information
Handling Editor: Dirk Babendreier.
Rights and permissions
About this article
Cite this article
Veronesi, E.R., Olaniyan, O., London, H. et al. Potential inter-guild interactions to enhance biological control of Bactericera cockerelli on tomatoes: a laboratory and cage study. BioControl 66, 343–353 (2021). https://doi.org/10.1007/s10526-020-10074-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-020-10074-3