Abstract
Dicyphus hesperus Knight (Heteroptera: Miridae) can contribute to the suppression of populations of Bemisia tabaci Gennadius (Hemiptera: Aleyrodidae) and Bactericera cockerelli Sulcer (Hemiptera: Psyllidae) in tomato. Nevertheless, the remaining levels of these pests could still be too high for the crop to tolerate. We thus tested here whether the combination of D. hesperus with the specialist parasitoids Eretmocerus eremicus Rose & Zolnerowich (Hymenoptera: Aphelinidae) (whitefly) and Tamarixia triozae (psyllid) can result in better pest control compared with methods based exclusively on single-species releases in tomato. We conducted two simultaneous experiments in tomato (‘Whitefly’ and ‘Psyllid’ Experiment), where we compared the effectiveness against B. tabaci and B. cockerelli in cages receiving releases of the predator or the specialist parasitoid alone, or in combination. Although all natural enemies reduced pest levels when released separately, the combination of D. hesperus with E. eremicus and D. hesperus with T. triozae resulted in better whitefly and psyllid control, respectively, compared with the separate releases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tomato crops in North America are often affected by the potato psyllid, Bactericera cockerelli Sulcer (Hemiptera: Psyllidae) and the sweetpotato whitefly, Bemisia tabaci Gennadius (Hemiptera: Aleyrodidae) (Butler and Trumble 2012a; Garzón-Tiznado et al. 2009). These two pests are able to cause direct damage that greatly decreases yield, but are even more important due to their efficiency as vectors of several plant viruses and bacteria (Butler and Trumble 2012a; Jones 2003). The potato psyllid transmits the bacterial pathogen Candidatus liberibacter solanacearum (syn. Ca. L. psyllaurous), which causes a disease referred to as ‘yellows’ in tomato (Munyaneza et al. 2007; Secor et al. 2009) and the sweetpotato whitefly is an effective vector of several plant viruses (Jones 2003). This is compounded by the fact that both pests develop rapidly and thus can produce several generations per growing season in warm climates (Cock 1993; Cranshaw 1994). Consequently, tolerable levels are virtually zero and growers often rely on frequent pesticide applications for their control needs. Such an intensive use is environmentally unfriendly, reduces food safety, has negative effects on human health (Pimentel and Burgess 2014) and favours the development of pesticide resistance, which ultimately makes chemical control ineffective. In fact, the development of pesticide resistance is already well documented in both the potato psyllid and the sweetpotato whitefly (Bass et al. 2015; Nauen and Denholm 2005; Palumbo et al. 2001). This is worsened by the limitations that customers, retailers and governments are placing on chemical control, which is reducing the list of allowed pesticides. Under this scenario, interest in other control methods such as integrated pest management (IPM) strategies, which provide additional control options to growers, is increasing.
IPM programmes prioritize implementation of biological, physical and cultural control methods over pesticide use. Regarding biocontrol of whitefly and psyllid in tomato, earlier research reported good establishment of the mirid predator Dicyphus hesperus Knight (Heteroptera: Miridae) and adequate control of the whitefly Trialeurodes vaporariorum Westwood (Hemiptera: Aleyrodidae) and other pests such as Frankliniella occidentalis (Thysanoptera: Thripiidae) or Tetranychus urticae Koch (Acari: Tetranychidae) (McGregor et al. 1999; Shipp and Wang 2006; Gillespie et al. 2007). More recently, B. tabaci and B. cockerelli have been added to the list of pests that can be suppressed by D. hesperus in tomato. Calvo et al. (2018) reported good developmental and reproductive rates for D. hesperus when reared on the sweetpotato whitefly and the potato psyllid, and Calvo et al. (2016) demonstrated that augmentation of D. hesperus suppressed both pests when they occurred either alone or together. Despite D. hesperus greatly suppressed whitefly and psyllid densities, these authors suggested that there was still room to increase the effectiveness against these pests, especially given the high efficiency of these two pests in transmitting different plant diseases. Combination with other natural enemies could enhance the effectiveness against these two pests, and here we tested whether the combination with the specialist parasitoids Tamarixia triozae (Burks) (Hymenoptera: Eulophidae) and Eretmocerus eremicus Rose & Zolnerowich (Hymenoptera: Aphelinidae) would result in better B. cockerelli and B. tabaci control, over the single release of either the parasitoids or the predator. We selected the primary parasitoid T. triozae as it has demonstrated some potential for psyllid control (Banks 2012; Butler and Trumble 2012b; Rojas et al. 2015; Workman and Whiteman 2009) and E. eremicus as it has been already successfully implemented for biocontrol of whitefly in tomato in North America (Greenberg et al. 2002; Hoddle and van Driesche 1999). Nevertheless, multispecies-based programmes can lead to different interactions (Straub et al. 2008), which are expected to benefit biological control if the species belong to different functional groups, i.e. species which do not share a resource/habitat and/or seasonal occurrence (Northfield et al. 2012). Contrarily, such predator-parasitoid combinations can result in negative effects if the released species interact through kleptoparasitism or intra-guild predation (IGP), among others. The consequences of IGP are predicted to depend on the relative rates of predation on parasitized versus unparasitized prey (Rosenheim 1998). When the predators prefer unparasitized prey, biological control is more effective when both the predator and parasitoid are released, than with the parasitoid alone (Heinz and Nelson 1996; Colfer and Rosenheim 2001; Bao-Fundadora et al. 2015). Contrarily, pests are released from regulation by a parasitoid when predation is higher on parasitized versus unparasitized prey (Snyder and Ives 2001).
We therefore tested here whether or not the combination of the specialist parasitic wasps E. mundus and T. triozae with the generalist predator D. hesperus can result in improved sweetpotato and potato psyllid control over the single release of the predator. This was done in two experiments: (1) Whitefly experiment aimed at evaluating the effectiveness of single or joint releases of E. eremicus and D. hesperus against B. tabaci; and (2) Psyllid experiment focused on the evaluation of D. hesperus and T. triozae when released either alone or together against B. cockerelli. In both experiments the effectiveness was intended to be evaluated under a worst case scenario of rapid immigration of the pests into a tomato greenhouse.
Materials and methods
Pests, insects and supplemental food
The D. hesperus used in the assay was obtained from a rearing colony maintained on tomato and fed with Ephestia kuehniella Zeller (Lepidoptera: Pyralidae) eggs at 25 °C, 75% RH and 16:8 L:D photoperiod at the Koppert Mexico facilities located in Queretaro (Queretaro, Mexico). Bactericera cockerelli adults used to infest the tomato plants were collected from a mass rearing colony maintained on tomato plants and originally obtained from field samples from several locations within Mexico. Pupae of the parasitoid E. eremicus were obtained from the commercial product ERCAL™ (Koppert Biological Systems, Berkel en Rodenrijs, The Netherlands) and T. triozae specimens used in the assay were obtained from the commercial product TETRAPAR™ (Koppert México, Queretaro, Mexico). Eggs of E. kuehniella used as supplemental food during the experiment were supplied by Koppert Biological Systems in bottles containing 10 g of frozen eggs (ENTOFOOD™, Koppert Biological Systems, The Netherlands).
Experimental greenhouse
The experiments were conducted in a multi-tunnel greenhouse located in Amexe (Guanajuato, Mexico). Twenty-four walk-in cages were constructed inside the greenhouse to accommodate plants and isolate treatments. Each walk-in cage (1.5 × 2.5 × 3 m) was constructed of ‘anti-thrips’ polyethylene screen with 220 × 331 μm interstices and supported by heavy wires. Floors were covered with woven 2-mm-thick polyethylene cloth, and access to each cage was through a zippered doorway. The greenhouse was equipped with a system to control temperature and RH. Temperature and RH were monitored in four randomly selected walk-in cages with a HOBO H8 RH/Temp Loggers (Onset Computer, Bourne, MA, USA).
Experimental design and procedure
Initially, seeds of tomato cv. Merlice (De Riuter, St. Louis, Missouri, USA) were sown into 6 cm2 peat moss root cubes, and later transplanted simultaneously for both experiments into composted coconut fibre in 6.3 l white polyethylene flower pots, on 1st July 2015, when plants were at the five-leaf stage. Twelve plants were placed into each walk-in cage, and typical cultivation techniques for tomato cultivation were followed: plants were trained by the main stem to a black polyethylene string tied to a stainless-steel overhead wire. Secondary shoots were removed as required and each pot was supplied with a drip emitter delivering 2 l h−1, through which water and fertilizer were supplied as required.
Whitefly experiment
Four treatments were compared in a complete randomized block design with four replicates. Each block consisted of four adjacent walk-in cages (plots), each of which was randomly assigned to each treatment. Treatments were: (1) B. tabaci, (2) B. tabaci + D. hesperus, (3) B. tabaci + E. eremicus, and (4) B. tabaci + D. hesperus + E. eremicus. In all cages with B. tabaci, ten adults were released per plant for three weeks, beginning one day after transplanting for a total of thirty whitefly adults per plant. In all cages receiving D. hesperus, the predator was released at once one day after transplanting, at a rate of one D. hesperus per plant and at a sex ratio 1:1 (male:female). This release schedule for the whitefly was used to simulate a gradual but heavy immigration of the pest into the greenhouse. Timing and rate for the predator release were chosen based on previous studies that resulted in good whitefly control (Calvo et al. 2016).
Adult whitefly to be released into cages were collected each week from a single colony cohort to assure homogeneity of age and sex ratio. They were later cooled briefly in a cold room at 8 °C for counting and then released into the designated walk-in cages at the above-mentioned rate. Adults of D. hesperus (less than three days old) were collected from a single colony cohort to assure homogeneity of age and were later cooled briefly in a cold room at 8 °C for counting and then released in designated walk-in cages as mentioned above. For all releases during the experiments, parasitoid pupae about to emerge were collected from the commercial products. Pupae were then counted and sexed before being released into designated walk-in cages at a sex ratio of 1:1. Eretmocerus eremicus neither parasitizes nor feeds on eggs or crawlers of B. tabaci, and thus E. eremicus releases began two weeks after the first whitefly release, coinciding with first availability of second instar nymphs of B. tabaci. Timing and rate for E. eremicus releases were chosen based in accordance with in field-release methods for other commercially available whitefly parasitoids (Stansly et al. 2005). Eggs of E. kuehniella were sprinkled once a week on all plants in cages receiving D. hesperus at a rate of 0.01 g cage−1, beginning just after the predator release, and for four weeks thereafter. Availability of whitefly nymphs was expected to be low during this period, and supplementary food was added to increase the likelihood of establishment due to the incapability of D. hesperus nymphs to reach maturity in the absence of prey (Sánchez et al. 2004).
Plants were monitored weekly for 14 weeks, beginning one week after transplanting. In each sampling, five plants were randomly selected in each walk-in cage, and then in each selected plant, one leaf was selected at random from the upper, one from the middle, and one from the bottom third of the plant. In all selected leaves, the number of whitefly nymphs, pupae, parasitized pupae and adults, as well as the number of mirid nymphs and adults were counted. In each case, leaves were turned carefully to count first whitefly and D. hesperus adults, and then the other insect stages, using a 15 × hand lens.
Psyllid experiment
Experimental design was the same as described above for the whitefly experiment, although treatments were: (1) B. cockerelli, (2) B. cockerelli + D. hesperus, (3) B. cockerelli + T. triozae, and (iv) B. cockerelli + D. hesperus + T. triozae. In all cages with B. cockerelli, one insect was released per plant for three weeks, beginning one day after transplanting for a total of three psyllid adults per plant. In all cages receiving D. hesperus, the predator was released at once one day after transplanting and at a rate of one D. hesperus per plant. This release schedule for B. cockerelli was used to simulate a gradual but heavy immigration of the pest into the greenhouse. Timing and rate for the predator release were chosen based on previous studies that resulted in good psyllid control (Calvo et al. 2016).
Psyllid adults to be released into cages were collected each week from a single colony cohort to assure homogeneity of age. They were later cooled briefly in a cold room at 8 °C for counting and then released into the designated walk-in cages at the above-mentioned rate at a sex-ratio 1:1 (male: female). Release of D. hesperus adults, and supplemental additions of E. kuehniella eggs, were carried out as mentioned above for the whitefly experiment. For all releases during the experiments, newly emerged T. triozae adults were used. Adults were cooled briefly in a cold room at 8 °C for counting before being released into designated walk-in cages at a sex ratio of 1:1. Tamarixia triozae neither parasitizes nor feeds on youngest stages of psyllid nymphs (Rojas et al. 2015; Yang et al. 2015), and thus T. triozae releases began two weeks after the first psyllid release, coinciding with first availability of second-third instar nymphs of B. cockerelli. Timing and rate for T. triozae releases were chosen based on previous studies where different rates for T. triozae releases were evaluated (Torres A; unpublished data).
Plants were monitored weekly for 14 weeks, beginning one week after transplanting. In each selected plant, one leaf was selected at random from the upper, one from the middle, and one from the bottom third of the plant. In all selected leaves, the number of whitefly nymphs, pupae, parasitized pupae and adults, as well as the number of mirid nymphs and adults were counted. In each case, leaves were turned carefully to count first psyllid and D. hesperus adults, and then the other insect stages, using a 15 × hand lens.
Ambient conditions
Mean daily temperature ranged from 18.3 ± 0.4 to 22.7 ± 0.5 °C and mean daily RH fluctuated from 61.5 ± 1.9 to 81.1 ± 1.3% during the experiments.
Statistical analysis
Treatment effects on B. tabaci, B. cockerelli and D. hesperus were analysed using linear mixed effects models (α = 0.05), with time (weeks after first pest release) as a random factor nested in blocks to correct for pseudo-replication, due to repeated measurements (Crawley 2002) as in previous experiments with repeated measurements (see Messelink et al. 2008; Calvo et al. 2016). Treatments were compared, contingent on a significant model, through model simplification by combining treatments (Crawley 2002) and temporary effects on parasitized pupae were analysed with a two-way repeated measurement ANOVA (α = 0.05). Insect numbers per leaf were log(x + 1) transformed prior to analysis to stabilize error variance, although untransformed values are given in the text. The Abbott’s formula 100 × [(1 × (treated/control)] (Abbott 1925) and weekly means in each treatment were used to estimate the degree of nymphal whitefly and psyllid suppression obtained by the addition of the parasitoids and the predator, either alone or together.
Results
Whitefly experiment
Whitefly control
Populations of whitefly adults were suppressed in response to either single or joint predator and E. eremicus releases (Fig. 1a; F3,55 = 180.99; P < 0.001), although the combination of the parasitoid and D. hesperus was more effective (Table 1), with nearly 8-fold lower numbers of whitefly adults per leaf being recorded at the end of the experiment compared to cages with the pest only. Intermediate numbers of whitefly adults per leaf were recorded in cages receiving the predator only, whereas the highest numbers of whitefly adults per leaf, among treatments receiving natural enemies, were recorded in cages treated with the parasitoid only. In this latter treatment, the abundance of whitefly adults at the end of the experiment was slightly lower compared to cages with B. tabaci only. Dynamics of whitefly nymph plus pupa were similar to that observed for adults. Again, numbers of nymphs plus pupae per leaf were suppressed in all cages receiving the natural enemies, compared to cages with whitefly only (Fig. 1b; F3,55 = 147.30; P < 0.001), with the combination of D. hesperus and E. eremicus providing the bests results (Table 1). In cages receiving both the predator and the parasitoid, whitefly nymph plus pupa numbers remained nearly constant over the entire experimental period, and the degree of pest suppression amounted to 76%. In cages treated with the predator only, the abundance of nymphs plus pupae of B. tabaci was higher than in cages receiving the combination, and consequently the degree of pest suppression was lower (67%). The parasitoid alone was the least effective method in controlling whitefly nymphs and pupae, and therefore pest numbers increased more rapidly and were the significantly greatest among all treatments receiving natural enemies. As a consequence, the degree of pest (nymphs and pupae) suppression was also the lowest (40%).
Natural enemies
In all cages receiving the predator, similar and progressively higher numbers of nymphs plus adults of D. hesperus per leaf were recorded during the whole experiment, thus reflecting no effects on the predator populations in response to the supplemental releases of E. eremicus (Fig. 2a; F1,55 = 0.275; P = 0.602). Contrarily, far fewer whitefly pupae parasitized by E. eremicus were found in cages receiving D. hesperus and E. eremicus compared to cages treated with the parasitoid only (Fig. 2b; F1,55 = 70.10; P < 0.001). Differences were greatest during the last half of the experiment, when the number of parasitized whitefly pupae per leaf reached the highest levels and increased more rapidly in cages treated with the parasitoid only than in cages with the predator and the parasitoid (treatment: F1,119 = 32.42; P < 0.001; time: F13,1547 = 11.59; P < 0.001; treatment × time: F13,1547 = 8.32; P < 0.001).
Psyllid experiment
Psyllid control
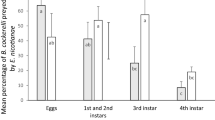
Psyllid adults were suppressed in response to all treatments (F3,55 = 98.01; P < 0.001), with the combination of D. hesperus and T. triozae being the most effective in reducing adult pest levels (Fig. 3a; Table 1). Consequently, almost no pest adults were found at the end of the experiment in response to the joint release of D. hesperus and T. triozae, whereas ca. 70 adults per leaf were recorded in cages with the pest only. The predator and parasitoid singly were less effective than their combination against psyllid adults, though provided similar and intermediate results. Psyllid nymphs plus pupae numbers increased progressively throughout the experiment in cages with the pest only (Fig. 3b), but were significantly suppressed by all treatments (F3,55 = 77.58; P < 0.001; Table 1). Again, the combination of the predator and the parasitoid was the most effective treatment, and was able to keep nymph plus pupa levels nearly constant over the entire experiment. Thus, ca. 100-fold lower nymph plus pupa psyllid numbers were recorded at the end of the experiment compared to those recorded in cages with the pest only. This represented a level of 88% pest suppression, which is higher than the 54 and 48% estimated in cages receiving the predator and the parasitoid separately, respectively, which again provided similar and intermediate results.
Natural enemies
Dynamics of D. hesperus populations were similar in all treatments with predator release (Fig. 4a). The abundance of the predator remained nearly constant during the first weeks and increased rapidly during the last third of the experiment. Nevertheless, more predators were founded in cages receiving D. hesperus and T. triozae in several weeks during the experiment, which resulted in a significantly greater abundance (F1,55 = 7.851; P = 0.007). In cages with T. triozae only, the number of parasitized psyllid pupae increased progressively over the first weeks of the experiment and levelled off at values ca. 20 parasitized pupae per leaf, and this up to the end of the experiment (Fig. 4b). Contrarily, in cages also receiving the predator, the number of parasitized psyllid pupae was much lower throughout the experiment, and thus significantly lower (F1,55 = 3272.4; P < 0.001). Consequently, density of parasitized pupae varied over time and in response to the combination with D. hesperus (treatment: F1,119 = 24.02; P < 0.001; time: F13,1547 = 5.86; P < 0.001; treatment × time: F13,1547 = 3.12; P < 0.001).
Discussion
All natural enemies established successfully during the experiment. Here, D. hesperus was able to develop and reproduce on tomato with either the sweetpotato whitefly or the potato psyllid as prey and supplemental addition of E. kuehniella eggs at the beginning of the experiments. This correlates with earlier results conducted on tomato infested with these two pests (Calvo et al. 2016). This extends the list of pests on which the predator is able to establish successfully on tomato, which already included other important pests such as F. occidentalis or T. urticae (McGregor et al. 1999; Shipp and Wang 2006; Gillespie et al. 2007). Regarding the parasitoids, we found good numbers of parasitized pupae, which reveals the capability of the parasitic wasps to perform well on tomato, as was also demonstrated by earlier studies (Banks 2012; Butler and Trumble 2012b; Rojas et al. 2015; Workman and Whiteman 2009; Greenberg et al. 2002; Hoddle and van Driesche 1999). Interestingly, although the predator had a lower availability of unparasitized hosts when combined with the parasitoids, we found no effects on predator populations in response to the release of E. eremicus, whereas the combination with T. triozae did affect the abundance of the predator. On average, we found more parasitized psyllid pupae than parasitized whitefly pupae, and consequently more unparasitized prey would have been available in the whitefly experiment. Additionally, if the rate of predation on parasitized whiteflies was higher than on parasitized psyllids, then the predator would still have more suitable prey in the whitefly experiment, all of which could explain the above-mentioned effects on the predator populations. However, the combination with the predator had a big impact on both parasitoids, as numbers of parasitized pupae were much lower in cages receiving both the predator and the parasitoid, compared to cages with the parasitoids alone. Beside the effects of direct predation by the predator on the parasitoid, which we discuss later more in depth and could be insignificant on a greenhouse level, the resource competition could explain such phenomena. The predator predates earlier pest stages than those that are preferred by the parasitoids for parasitization by either E. eremicus or T. triozae (Ramírez-Ahuja et al. 2017; Yang et al. 2015; Vet and van Lenteren 1981), which reduces the number of future hosts for the parasitoid. This could however be a temporary effect mediated by the abundance of the predator or the presence of an alternative prey, as abundance of parasitized pupae of either whitefly of psyllid varied over time and in response to D. hesperus release. Immediately after the release (short-term), before the predator gets established in the crop thus it is still at low levels and more nymphs escape predation, the parasitoid would have the chance to increase in numbers. Addition of E. kuehniella eggs could have also reduced the predation on parasitized hosts during the first weeks after the release. Contrarily, once the predator is established (mid- to long-term), the higher predator density would result in higher pest predation, thus reducing the number of future hosts for the parasitoid and ultimately resulting in lower parasitoid numbers. This correlates with the dynamics observed during our study. During the first weeks of the experiment, on average we found similar densities of parasitized pupae of psyllid and whitefly in cages with joint parasitoid and predator releases, and cages with the parasitoids only. After that, as the abundance of the predator increased, we found progressively greater differences in numbers of parasitized hosts between treatments with the parasitoid and the parasitoid and the predator together.
One important topic in biological control is whether agents should be introduced singly, to allow the effect of each released species separately, or if several species should be introduced at the same time and place to speed the establishment of the natural enemy complex. Single species-based programmes are often simpler and cheaper, and in the majority of cases successful control of insect pests is achieved by a single agent (Myers et al. 1989; Calvo et al. 2009, 2012a, b, 2016). The psyllid parasitoid, T. triozae and the predator separately were equally effective against the potato psyllid, whereas E. eremicus alone was less effective at controlling B. tabaci than the predator alone. This can be explained by differences in reproductive, development and predation/parasitism rates, between both parasitoids and the predator, on each prey/host-plant system. Estimates for the intrinsic rate of increase for D. hesperus on tomato are comparable to those for E. ermicus, but lower than those reported for T. triozae (Headrick et al. 1999; Rojas et al. 2015; Calvo et al. 2018). Nevertheless, D. hesperus consumes slightly more than 300 psyllid nymphs during its life time, which is similar to the quantity of hosts killed by T. triozae by parasitization and host-feeding (Calvo et al. 2018; Rojas et al. 2015; Cerón-González et al. 2014). A similar killing rate would therefore result in a similar control capacity, as observed in our study. Conversely, D. hesperus kills 23.7–43.2 whitefly nymphs per day and is able to survive for ca. 80 days on tomato (McGregor et al. 1999; Sánchez et al. 2004), whereas E. eremicus lays a lifetime average of 22.9 eggs on cotton, and 23.1 eggs on sweetpotato (Headrick et al. 1999). The higher rate of predation of D. hesperus than parasitization rate of E. eremicus, and similar growth rate would therefore explain the greater effectiveness of the predator observed in our study. Nevertheless, in our study when both types of natural enemies (specialist parasitoids and the predator) were concomitantly released, it resulted in better whitefly and potato psyllid control than the release of either the parasitoids or the predator separately, and thus constitutes an example of increased pest control through the establishment of a natural enemy complex that increases biodiversity and thus combines different mode of actions. Such improved pest control should have been in response to the complementarity (i.e. additive effect) of the specialist parasitoids and the predator and consequently would suggest little effect of IGP by D. hesperus on the specialists T. triozae and E. eremicus, and insignificant effects of other possible negative interactions among the species on the regulation of the pests (Straub et al. 2008; Northfield et al. 2012; Rosenheim 1998). This is confirmed by Ramírez-Ahuja et al. (2017), who reported that D. hesperus has a strong preference for unparasitized over parasitized psyllid nymphs and prefers second-third instar nymphs for predation, whereas T. triozae prefers older stages for parasitization and second-third instar nymphs for predation (Yang et al. 2015), which resulted in an increased mortality when D. hesperus and T. triozae were released together over the release of both separately. Similarly, McGregor and Gillespie (2005) observed that D. hesperus females readily fed upon larvae and pupae of the specialist whitefly parasitoid, Encarsia formosa Gahan (Hymenoptera: Aphelinidae), although they found no preference of the predator for parasitized whitefly pupae and no apparent effects of IGP by D. hesperus on whitefly control. They thus concluded that IGP by D. hesperus on E. formosa had no adverse effect on biological control of greenhouse whitefly. We are not aware of any study evaluating the IGP by D. hesperus on E. eremicus, but we could expect a similar behavior to that observed on E. formosa, as E. eremicus is biologically comparable.
Overall, our results suggest that the most effective tactic among those tested here, i.e. the combination of both the specialist parasitoids and the predator against either B. tabaci or B. cockerelli, would result in better pest control. Implementation of this method would increase the effectiveness and reliability of biological control based programmes for whitefly and potato psyllid control in tomato over the single release of either the parasitoids or the predator. Nevertheless, this strategy should be tested under larger scale and realistic greenhouse conditions before it can be recommended with confidence for commercial tomato crops.
References
Abbott WA (1925) A method to computing the effectiveness of an insecticide. J Econ Entomol 18:265–267
Banks E (2012) Potato zebra chip disease: a potential threat to processing & fresh market crop. Hortic Matters 13(10):6–7
Bao-Fundadora L, Ramírez-Romero R, Sánchez-Hernández CV, Sánchez-Martínez J, Desneux N (2015) Intraguild predation of Geocoris punctipes on Eretmocerus eremicus and its influence on the control of the whitefly Trialeurodes vaporariorum. Pest Manag Sci 72:1110–1116
Bass C, Denholm I, Williamson MS, Nauen R (2015) The global status of insect resistance to neonicotinoid insecticides. Pest Biochem Physiol 121:78–87
Butler CD, Trumble JT (2012a) The potato psyllid, Bactericera cockerelli (Sulc) (Hemiptera: Triozidae) life history, relationship to plant diseases, and management strategies. Terrest Arthropod Rev 5:87–111
Butler CD, Trumble JT (2012b) Identification and impact of natural enemies of Bactericera cockerelli (Hemiptera: Triozidae) in Southern California. J Econ Entomol 105:1509–1519
Calvo FJ, Bolckmans K, Belda JE (2009) Development of a biological control-based IPM method for Bemisia tabaci for protected sweet pepper crops. Entomol Exp App 133:9–18
Calvo FJ, Bolckmans K, Belda JE (2012a) Release rate for a pre-plant application of Nesidiocoris tenuis for Bemisia tabaci control in tomato. BioControl 57:809–817
Calvo FJ, Lorente MJ, Stansly PA, Belda JE (2012b) Preplant release of Nesidiocoris tenuis and supplementary tactics for control of Tuta absoluta and Bemisa tabaci in greenhouse tomato. Entomol Exp App 143:111–119
Calvo FJ, Torres-Ruiz A, Velázquez-González JC, Rodriguez-Leyva E, Lomeli-Flores JR (2016) Evaluation of Dicyphus hesperus for biological control of sweetpotato whitefly and potato psyllid on greenhouse tomato. BioControl 61:415–424
Calvo FJ, Torres A, González EJ, Velázquez MB (2018) The potential of Dicyphus hesperus as a biological control agent of potato psyllid and sweetpotato whitefly in tomato. Bull Entomol Res. https://doi.org/10.1017/S0007485318000020
Cerón-González C, Lomeli-Flores JR, Rodríguez-Leyva E, Torres-Ruíz A (2014) Fertility and feeding of Tamarixia triozae (Hymenoptera: Eulophidae) on potato psyllid Bactericera cockerelli. Revista Mexicana de Ciencias Agrícolas 5:893–899
Cock MJW (1993) Bemisia tabaci: an update 1986–1992 on the cotton whitefly with an annotated bibliography. FAO Publishing. CAB International Institute of Biological Control, Ascot
Colfer RG, Rosenheim JA (2001) Predation on immature parasitoids and its impact on prey suppression. Oecologia 126:292–304
Cranshaw WS (1994) The potato (tomato) psyllid, Paratrioza cockerelli (Sulc), as a pest of potatoes. In: Zehnder GW, Powelson RK, Jansson RK, Raman KW (eds) Advances in potato biology and management. APS Press, St. Paul, pp 83–95
Crawley MJ (2002) Statistical computing. An introduction to data analysis using S-plus. Wiley, Chichester
Garzón-Tiznado JA, Cárdenas-Valenzuela OG, Bujanos-Muñiz R, Marín-Jarillo A (2009) Association of Hemiptera: Triozidae with the disease ‘‘permanente del tomate’’ in Mexico. Agricultura Técnica en México 35:61–72
Gillespie DR, McGregor RR, Sánchez JA (2007) Dicyphus hesperus (Hemiptera: Miridae) as a success story in development of endemic natural enemies as biological control agents. In: Vincent CM, Goettel M, Lazarovits G (eds) Case studies in biological control: a global perspective. CABI Publishing, Oxfordshire, pp 128–135
Greenberg SM, Jones WA, Liu TX (2002) Interactions among two species of Eretmocerus (Hymenoptera: Aphelinidae), two species of whiteflies (Homoptera: Aleyrodidae), and tomato. Biol Control 31(2):397–402
Headrick DH, Bellows TS, Perring TM (1999) Development and reproduction of a population of Eretmocerus eremicus (Hymenoptera: Aphelinidae) on Bemisia argentifolii (Homoptera: Aleyrodidae). Environ Entomol 28(2):300–306
Heinz KM, Nelson JM (1996) Interspecific interactions among natural enemies of Bemisia in an inundative biological control program. Biol Control 6:384–393
Hoddle MS, van Driesche RG (1999) Evaluation of Eretmocerus eremicus and Encarsia formosa (Hymenoptera: Aphelinidae) Beltsville strain in commercial greenhouses for biological control of Bemisia argentifolii (Homoptera: Aleyrodidae) on colored poinsettia plants. Florida Entomol 82:556–569
Jones RD (2003) Plant viruses transmitted by whiteflies. Eur J Plant Pathol 109:195–219
McGregor RR, Gillespie DR (2005) Intraguild predation by the generalist predator Dicyphus hesperus on the parasitoid Encarsia formosa. Biocontrol Sci Techol 15(3):219–227
McGregor R, Gillespie D, Quiring D, Foisy M (1999) Potential use of Dicyphus hesperus Knight (Heteroptera: Miridae) for biological control of pests of greenhouse tomatoes. Biol Control 16:104–110
Messelink GJ, van Maanen R, van Steenpaal SEF, Janssen A (2008) Biological control of thrips and whiteflies by a shared predator: two pests are better than one. Biol Control 44:372–379
Munyaneza JE, Crosslin JM, Upton JE (2007) Association of Bactericera cockerelli (Homoptera: Psyllidae) with “Zebra Chip”, a new potato disease in Southwestern United States and Mexico. J Econ Entomol 100:656–663
Myers J, Charlene H, Kovacs E (1989) How many insect species are necessary for the biological control of insects? Environ Entomol 18(4):541–547
Nauen R, Denholm I (2005) Resistance of insect pests to neonicotinoid insecticides: current status and future prospects. Arch Insect Biochem Physiol 58:200–215
Northfield TD, Crowder DW, Jabbour R, Snyder WE (2012) Natural enemy functional identity, trait-mediated interactions and biological control. In: Ohgushi T, Schmitz O, Holt RD (eds) Trait-mediated indirect interactions: ecological and evolutionary perspectives. Cambridge University Press, New York, pp 450–465
Palumbo JC, Horowitz AR, Prabhaker N (2001) Insecticidal control and resistance management for Bemisia tabaci. Crop Protect 20:739–765
Pimentel D, Burgess M (2014) Environmental and economic costs of the application of pesticides primarily in the United States. In: Pimentel D, Peshin R (eds) Integrated pest management. Springer, Dordrecht, pp 47–71
Ramírez-Ahuja ML, Rodríguez-Leyva E, Lomeli-Flores JR, Torres-Ruíz A, Guzmán-Franco AW (2017) Evaluating combined use of a parasitoid and a zoophytophagous bug for biological control of the potato psyllid, Bactericera cockerelli. Biol Control 106:9–15
Rojas P, Rodríguez-Leyva E, Lomeli-Flores JR, Liu TX (2015) Biology and life history of Tamarixia triozae (Hymenoptera: Eulophidae), a parasitoid of Bactericera cockerelli (Hemiptera: Triozidae). BioControl 60:27–35
Rosenheim JA (1998) Higher-order predators and the regulation of insect herbivore populations. Annu Rev Entomol 43:421–447
Sánchez JA, Gillespie DR, McGregor RR (2004) Plant preference in relation to life history traits in the zoophytophagous predator Dicyphus hesperus. Entomol Exp App 112:7–19
Secor GA, Rivera VV, Abad JA, Lee IM, Clover GRG, Liefting LW, Li X, de Boer SH (2009) Association of ‘Candidatus Liberibacter solanacearum’ with zebra chip disease of potato established by graft and psyllid transmission, electron microscopy, and PCR. Plant Dis 93(6):574–583
Shipp JL, Wang K (2006) Evaluation of Dicyphus hersperus (Heteroptera: Miridae) for biological control of Frankliniella occidentalis (Thysanoptera: Thripidae) on greenhouse tomato. J Econ Entomol 99(2):414–420
Snyder WE, Ives AR (2001) Generalist predators disrupt biological control by a specialist parasitoid. Ecology 82:705–716
Stansly PA, Calvo FJ, Urbaneja A (2005) Release rates for control of Bemisia tabaci (Homoptera: Aleyrodidae) biotype “Q” with Eretmocerus mundus (Hymenoptera: Aphelinidae) in greenhouse tomato and pepper. Biol Cont 35:124–133
Straub CS, Finke DL, Snyder WE (2008) Are the conservation of natural enemy biodiversity and biological control compatible goals? Biol Control 45:225–237
Vet LM, van Lenteren JC (1981) The parasite-host relationship between Encarsia formosa Gah. (Hymenoptera:Aphelinidae) and Trialeurodes vaporariorum (Westw.) (Homoptera: Aleyrodidae). A composition of three Encarsia spp. and one Eretmocerus sp. to estimate their potentialities in controlling whitefly on tomatoes in greenhouses with a low temperature regime. J App Entomol 91:327–348
Workman PJ, Whiteman SA (2009) Importing Tamarixia triozae into containment in New Zealand. N Z Plant Protect Soc 62:136–144
Yang XB, Campos-Figueroa M, Silva A, Henne DC (2015) Functional response, prey stage preference, and mutual interference of the Tamarixia triozae (Hymenoptera: Eulophidae) on tomato and bell pepper. J Econ Entomol 108:414–424
Acknowledgements
Authors thank Elizabeth Julieta González-Jaime (R&D Koppert Mexico SA de CV) for technical assistance. The authors also thank anonymous reviewers for providing valuable and constructive remarks. Special thanks to Karen Girard (R&D, Koppert Biological Systems, UK) for reviewing the manuscript and provide valuable remarks. This work was partially supported by the National Council of Science and Technology (CONACYT) [Grant Number PEI-CONACYT-223073_2015].
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Dirk Babendreier.
Rights and permissions
About this article
Cite this article
Calvo, F.J., Velázquez-González, J.C., Velásquez-González, M.B. et al. Supplemental releases of specialist parasitic wasps improve whitefly and psyllid control by Dicyphus hesperus in tomato. BioControl 63, 629–639 (2018). https://doi.org/10.1007/s10526-018-9898-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-018-9898-0