Abstract
Amblyseius swirskii Athias-Henriot (Acari: Phytoseiidae) is a very efficient generalist predatory mite of Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) and Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae), worldwide released in horticultural greenhouses. Here, the toxicity of sulfoxaflor and other ten pesticides to A. swirskii adults when applied at their maximum field rate was assessed in the laboratory in terms of mortality and reproductive performance. The duration of the harmful activity when residues were aged under greenhouse was assessed for compounds not classified as harmless in the laboratory, based on the International Organization for Biological Control (IOBC) rules. Sulfoxaflor as well as flonicamid, flubendiamide, metaflumizone, methoxyfenozide, spiromesifen, and spirotetramat were harmless, emamectin was slightly harmful and abamectin, deltamethrin and spinosad were harmful. Emamectin was short-lived and abamectin, deltamethrin and spinosad were slightly persistent under our conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Almería, in South Eastern Spain, is one of the most important vegetable crop producers in Europe with 48,676 ha. The main crops in this area are tomato and sweet pepper (19 and 15% of the total growing area, respectively), followed by lettuce, zucchini, cucumber, melon and eggplant (MAGRAMA 2016a). The two main pests that threaten the production are the tobacco whitefly Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) and the western flower thrips Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae) (Robledo et al. 2009), both of which are remarkably polyphagous and included in the European and Mediterranean Plant Protection Organization (EPPO) A2 list of quarantine organisms for the EPPO region (EPPO 2015a, b). These pests are a limiting factor in the economic production of high-quality fruits because their damage causes considerable yield losses (Gómez et al. 2006; Oliveira et al. 2001). Bemisia tabaci is a polyphagous key pest complex of at least 28 species (De Barro et al. 2011) that is distributed throughout much of the world. The whiteflies cause economic damage to some crops due to phloem-feeding, excretion of honeydew that promotes the growth of blackish sooty moulds and the transmission of plant viruses (Colomer et al. 2011). Frankliniella occidentalis, which is currently the most important pest thrips in Europe, can cause direct damage by feeding on or ovipositing in developing fruits and leaves and indirect damage by virus transmission (Shipp et al. 2000).
Since the EU directive 2009/128/EC on the sustainable use of pesticides came into force in January 2014, Integrated Pest Management (IPM) has become mandatory in all European Union (EU) countries (OJEU 2009) encouraging more environmentally friendly alternatives to pesticides, such as biological control methods. Amblyseius swirskii (Athias-Henriot) (Acari: Phytoseiidae) is one of the most widely used natural enemies in the different horticultural crops of Almería (Amor et al. 2012). It is a very efficient generalist predator of B. tabaci and F. occidentalis (Calvo et al. 2011) and it can persist in the crops even at low pest densities (Colomer et al. 2011). However, despite its ability to currently control several pests, in simultaneous outbreaks in the crop, corrective insecticide treatments may be needed depending on the crop and on the growing season.
In order to guarantee the effectiveness of the IPM programs, pesticides applied nowadays in our modern crop production systems, must be respectful to natural enemies and pollinators and selective for human beings and the environment. Therefore, these pesticides must have new mode of actions that can delay the development of insecticide resistance (Biondi et al. 2012a; Roubus et al. 2014). This last premise is essential in horticultural crops because B. tabaci and F. occidentalis have developed resistance to a wide range of commonly used products (Bielza 2008; Fernández et al. 2009). In the EU, the authorized active ingredients (a.i.) considered the safest for agricultural use, are included in Annex I of the European directive 91/414/EEC and the list is updated regularly (MAGRAMA 2016b). The recently included insecticide sulfoxaflor, authorized in USA since 2013 (EPA 2013), is the first compound representative of the sulfoxamine insecticide class, which acts on insect nicotinic receptors (nAChRs) but in a different way than other pesticides (Sparks et al. 2013). This systemic insecticide exhibits a good potency against a broad range of sap-feeding insect species, including insecticide resistant populations of B. tabaci (Babcock et al. 2011), but the information on its selectivity to natural enemies is scarce.
Therefore, the objective of this study was to provide information on the toxic direct effect (mortality) and on the sublethal effects caused by sulfoxaflor to A. swirskii adults in order to assure a good performance of the natural enemy (Desneux et al. 2007) and to compare the results with other modern pesticides commonly used in Almería in horticultural crops, taking into account their different modes of action (IRAC 2016; MAGRAMA 2016b, c). To that end, effects of the fresh residues of the pesticides were firstly tested in the laboratory and secondly, and only for those causing any effect, a persistence test was also performed.
Materials and methods
Insects
The initial stock of A. swirskii was purchased from Koppert Biological Systems (La Mojonera, Spain) and reared on Carpoglyphus lactis (L.) (Acari: Carpoglyphidae) in a controlled environment chamber (Sanyo MLR-350, Madrid, Spain) at the standard conditions of 25 ± 2 °C, 80 ± 5% RH and a L:D 16:8 photoperiod. The colony of A. swirskii was synchronised prior to the assays to ensure that individuals were the same age and renewed periodically with individuals obtained from the commercial insectary.
Chemicals
Sulfoxaflor was tested at the rate recommended by the manufacturer (Dow AgroSciences Iberica S.A., Spain) as there are not commercial formulations available yet in Europe. The rest of selected insecticides, abamectin, emamectin, flonicamid, flubendiamide, metaflumizone, methoxyfenozide, spinosad, spiromesifen and spirotetramat, were tested at their maximum field recommended concentrations (MFRC) in accordance with the Spanish registration (MAGRAMA 2016c). Additionally, the neurotoxic pesticide deltamethrin, frequently applied in many horticultural crops and harmful to many natural enemies according to the IOBC database (IOBC 2016), was included in the study as a positive control (Table 1).
For the calculation of the pesticide amounts to be applied, the predicted initial environmental concentration PIEC (µg cm−2) = maximum field dose (g a.i. ha−1) /100 with a correction factor of deposits under field conditions, of 1.0 for arable crops, was used (Barrett et al. 1994). A water amount of 300 l ha−1 was also used in calculations. The negative controls were treated with distilled water alone.
Bioassays
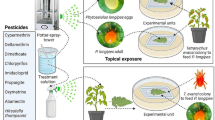
The effect of fresh pesticide residues on adults of A. swirskii was studied using a modification of the International Organization for Biological Control (IOBC) standard method developed for Psyttalia concolor (Szepl.) (Hymenoptera: Braconidae) (Jacas and Viñuela 1994). For the assessment of the duration of the harmful activity, we followed the method of van de Veire et al. (2004) with Encarsia formosa Gahan (Hymenoptera: Aphelinidae). The experiments were performed in a controlled environmental chamber under the conditions described above. The laboratory tests were conducted in two separate assays, with half of the pesticides and the negative and positive control, under the same environmental conditions than the rearing.
Adult exposure to fresh pesticide residues on glass surfaces
To evaluate the residual contact activity of the pesticides, the dismountable cages designed by Jacas and Viñuela (1994), and slightly modified by Bengochea et al. (2014), were used. The experimental units comprised two square glass plates (12 × 12 × 0.5 cm) and a round methacrylate frame (6 cm in diameter, 3.5 cm high) with several holes. These holes are covered by a mesh for ventilation, by a piece of plasticine for insect manipulation and by an hypodermic needle connected to a rubber tube that provided a flow of air produced by an aquarium pump to ensure forced ventilation. Two square glass plates per replicate were treated under the Potter precision tower (Burkard Manufacturing Co., Rickmansworth, UK) with a deposit of 1.5–2 mg cm−2 (1 ml, 55 kPa) at room temperature. As soon as the glass plates were dry, the cages were mounted with two crossed rubbers and 15 ♀ and 5 ♂ were introduced (optimal proportion based in our previous experience). The predatory mites were fed ad libitum with C. lactis eggs and nymphs. Five replicates were used for each treatment and the controls. Mortality was recorded 72 h after the exposure to pesticides. Survivors were used to assess reproduction and long-term mortality. Adults (3 ♀ and 1 ♂; again optimal proportion based in our experience) were transferred to ventilated plastic cages (12 cm in diameter, 5 cm high) with a piece of Whatman filter paper as the oviposition substrate. The mean oviposition (number of eggs per female per week), hatchability (hatching percentage) and the cumulative mortality (monitored daily) were recorded for seven days. Ten replicates were used per pesticide and control.
Persistence test
The duration of the harmful activity was assessed only for those compounds exhibiting any detrimental effect to the adults in the residual contact test in the laboratory. Potted sweet pepper plants (cv California Wonder, 30 cm high) were hand-sprayed until the point of run-off with each pesticide. The plants were maintained for residue aging in a greenhouse in Madrid (Central Spain) equipped with cooling and heating systems, in June 2013 (15-h light natural photoperiod). The amount of PAR (photosynthetic active radiation) and UV light present inside the greenhouse was measured using spectroradiometers (models BQM and UVM, respectively, Spectrum Technologies, Apogee Instruments, Logan, Utah, USA). Average environmental conditions during the residue aging were: 27.02 ± 0.2 °C, 45.62 ± 0.51% RH, UV radiation 27.88 ± 6.02 mol m−2s−1, PAR radiation 496.73 ± 117.24 mol m−2s−1, 15 h light natural photoperiod. As before, groups of A. swirskii adults (15 ♀ and 5 ♂) were exposed in the laboratory for 72 h to pepper leaves collected from the plants at 0, 4, 10, 21, and 31 days after pesticide treatment (DAT), using the ventilated plastic cages described above. Adult mortality was checked daily up to three days, and when the adult mortality was less than 25%, the survivors were used to study the possible alterations on reproduction following the methodology described in the former bioassay. Five replicates were used for each treatment and the controls. The trial was stopped when the pesticides were classified as harmless or up to one month after treatment (Hassan 1994).
Statistical analysis
Statistical analyses were performed using Statgraphics® Plus, version 5.0 (STSC 1897). The data were subjected to a one-way analysis of variance (ANOVA), and differences in means were determined (P < 0.05) by the least significant difference (LSD) multiple range test. When necessary to homogenize variances and/or correct deviations from normality, percentages values were transformed using the arcsine of the square root of the proportions. If any of the assumptions of the analysis of variance were violated after the appropriate transformations, the effect of treatments was analyzed by means of the non-parametric Kruskal–Wallis test.
The mortality data and the other recorded parameters were corrected with the Schneider–Orelli’s or the Abbott’s formula, respectively. Subsequently, once any of the parameters evaluated had been corrected, the pesticides were classified according the IOBC toxicity ratings. In the laboratory, the pesticides were classified as follows: 1 (harmless: <30%), 2 (slightly harmful: 30–79%), 3 (moderately harmful: 80–99%), and 4 (harmful: >99%). For the evaluation of the pesticide effects in the persistence test, the four toxicity categories of the IOBC/WPRS working group “Pesticides and Beneficial Organisms” for the extended laboratory were used in each aged residue to assess its harmfulness: 1 (harmless: <25%), 2 (slightly harmful: 25–50%), 3 (moderately harmful: 51–75%), or 4 (harmful: >75%). Pesticide persistence was categorised as follows: A (short-lived: <five days); B (slightly persistent: 5–15 days); C (moderately persistent: 16–30 days); and D (persistent: >30 days).
Results
Adult exposure to fresh pesticide residues on glass surfaces
The corrected lethal and sublethal effects caused by the pesticides to A. swirskii adults are presented in Table 2 and the IOBC toxicity ratings in Table 3. The adult mortality at 72 h in the untreated control (Table 2) averaged 5.0 ± 1.58 and 3.06 ± 1.25% in the two separate experiments. In the treated units, increases in mortality in comparison with the corresponding negative control ranged from 3.66 ± 1.47% in metaflumizone to 89.39 ± 3.68% in spinosad, being 19.53 ± 2.06% in sulfoxaflor (Table 2). Therefore, abamectin, deltamethrin and emamectin were categorised as slightly harmful (IOBC 2), spinosad as moderately harmful (IOBC 3) and sulfoxaflor and the rest of insecticides as harmless (IOBC 1) concerning this parameter (Table 3).
Sublethal effects were also detected in survivors (Table 2). The number of eggs produced per A. swirskii female per week were 4.32 ± 0.35 and 4.48 ± 0.30 in the two untreated controls (Table 2), whereas oviposition reductions ranging from 8.88 ± 3.41% in sulfoxaflor to 100% in abamectin and deltamethrin were detected. Concerning oviposition, emamectin was categorized as slightly harmful (IOBC 2), abamectin and deltamethrin harmful (IOBC 4) and the rest of insecticides, including sulfoxaflor, as harmless (IOBC 1) (Table 3). The hatching percentage was 100% in both untreated controls (Table 2). Despite of the reduction in oviposition caused by some treatments, none of the pesticide altered the egg hatch and all were categorised as harmless (IOBC 1) (Table 3).
For the long-term mortality following a 72 h pesticide exposure, phytoseiid mortality after seven days in the untreated control was 27.5 ± 8.70 and 17.5 ± 5.3% in the two separate experiments (Table 2). In the treated units, mortality increased from 1.82 ± 1.21% in sulfoxaflor and flubendiamide to 100% in abamectin (Table 2). Emamectin was categorised as slightly harmful (IOBC 2), deltamethrin as moderately harmful (IOBC 3), abamectin as harmful (IOBC 4) and sulfoxaflor and the rest of insecticides as harmless (IOBC 1) (Table 3).
Persistence test
The duration of the harmful activity to A. swirskii adults when pesticide residues were aged in potted sweet pepper plants under greenhouse in central Spain varied with the pesticide tested (Table 4). Emamectin caused less than 25% mortality and no sublethal effects at 4 DAT and it was classified as short-lived (IOBC class A). All the other pesticides (abamectin, deltamethrin and spinosad), reduced their toxicity after ten days, and therefore they were classified as slightly persistent (IOBC class B) under our conditions.
Discussion
The exposure of the predatory mite A. swirskii adults to fresh residues of sulfoxaflor on glass plates in the laboratory at the recommended rate by the manufacturer did not cause any effect and the insecticide was classified as harmless (IOBC 1). To our knowledge, there is no information on the effects of the novel systemic pesticide sulfoxaflor on the predatory mite A. swirskii. In our study, the insecticide was safe to the natural enemy as it was to L3 larvae of Chrysoperla carnea (Stephens) (Neuroptera: Chrysopidae) and adults of Adalia bipunctata (L.) (Coleoptera: Coccinellidae) after contact exposure for 72 h (Garzón et al. 2015). However, the insecticide is detrimental to some developmental stages of other natural enemies: slightly harmful to C. carnea adults, harmful to L4 larvae of A. bipunctata (Garzón et al. 2015) and adults of Nesidiocoris tenuis Reuter and Macrolophus basicornis (Stal) (Hemiptera: Miridae) (Wanumen et al. 2016). The last authors reported that, for M. basicornis, its toxicity was higher under extended laboratory and semi-field conditions than in the laboratory, suggesting that the zoophytophagous feeding of the family Miridae allows the individuals to acquire some pesticide while sucking on the plant, increasing the residual contamination from walking on the residues.
Similarly, flonicamid, flubendiamide, metaflumizone, methoxyfenozide, spiromesifen and spirotetramat applied at their MFRC registered in Spain were also harmless (IOBC 1) to A. swirskii adults. Roditakis et al. (2014) also found that flonicamid did not cause any mortality to the phytoseiid mite in the laboratory even when they were treated at a concentration twice (125 mg a.i. l−1) that used in our trials (60 mg a.i. l−1). However, in contrast to our results, these authors reported a reduction in egg hatching. The different pesticide exposure and the higher concentration applied might have accounted for the differences. Under field conditions, Colomer et al. (2011) also showed the compatibility of this insecticide with established populations of A. swirskii in sweet pepper commercial greenhouses of South Eastern Spain.
Flubendiamide was safe to A. swirskii adults, as it was to a large number of natural enemies, including the predatory mites Amblyseius cucumeris (Oudemans) and Phytoseiulus persimilis Athias-Henriot (Acari: Phytoseiidae) (Tohnishi et al. 2005). The insecticide was even compatible with all developmental stages of phytoseiid mites when it was applied at high rates (up to 987.4 mg a.i. l−1), as reported by Lefebvre et al. (2011) and Beers and Schmidt (2014) in Galendromus occidentalis (Nesbitt) and Lefebvre et al. (2012) in Amblyseius fallacis (Garman). In contrast, Doker et al. (2015) reported a significant reduction in oviposition of Iphiseius degenerans (Berlese) (Acari: Phytoseiidae) at the same concentration than the one used in our work.
Gradish et al. (2011) have previously reported the compatibility of A. swirskii and the same metaflumizone formulation used in our work but applied at lower rates (69 and 138 and 240 mg a.i. l−1), when adults were exposed to fresh residues on bean leaves. In our study, the only effect detected on A. swirskii adults was a small significant increment in the long-term mortality. Because the percentage increase was under 30%, the pesticide was classified as harmless.
Methoxyfenozide, which is considered as environmentally friendly and highly selective for arthropods except lepidopteran pests (Carlson et al. 2001; Smagghe et al. 2013), was harmless to A. swirskii in the lab. This is consistent with the results of other authors on different phytoseiid mites: A. fallacis, Amblyseius californicus (McGregor), G. occidentalis (Nesbitt) and Kampimodromus aberrans (Oudemans) (Acari: Phytoseiidae) (Bostanian et al. 2010; Tirello et al. 2013; van de Veire and Tirry 2003). In commercial greenhouses it was also compatible with established populations of A. swirskii (Colomer et al. 2011).
Spiromesifen was also harmless even though it significantly increased direct mortality, decreased oviposition and increased the long-term mortality of A. swirskii adults. The susceptibility of predatory mites to this insecticide seems to be highly variable depending on the different physiological characteristics and/or behavioural habits of the species (Cloyd et al. 2006). As such, Cheon et al. (2008) did not detect any lethal effect on Neoseiulus womersleyi Schicha when females were treated with 100 mg a.i. l−1, and Cloyd et al. (2006) reported that it was safe to A. californicus and harmful to P. persimilis (15 and 31 ml commercial product l−1). In contrast, a concentration of 76.2 mg a.i. l−1 reduced the oviposition of G. occidentalis females after contact with pesticide residues (Saenz de Cabezón and Zalom 2007).
Spirotetramat, classified as a “reduced risk pesticide” by the USA Environmental Protection Agency (EPA 2008), was selective for A. swirskii adults. The phytoseiid mite G. occidentalis appears to be even more tolerant to the product than A. swirskii because a concentration of 164 mg a.i. l−1 (two-fold higher than that used in the present work) did not cause any effect (Beers and Schmidt 2014). However, a concentration of 328 mg a.i. l−1 significantly increased the predatory mite mortality (Beers and Schmidt 2014).
The rest of the studied pesticides caused some deleterious effects to the predatory mite in the laboratory. Emamectin was classified as slightly harmful (IOBC 2) due to the lethal and sublethal effects caused to A. swirskii adults. Similarly, under semi-field and field conditions, Amor et al. (2012) reported that the predatory mite was affected by a concentration slightly higher (14.25 mg a.i. l−1) than that used in our trials (12.83 mg a.i. l−1). Based on the scarce information in the literature, other phytoseiid mites such as A. cucumeris and Euseius victoriensis (Womersley) are equally affected by emamectin, as shown by Kim et al. (2005) and Bernard et al. (2010).
Abamectin and deltamethrin were harmful (IOBC 4) in our trials due to the direct mortality caused to A. swirskii adults at 72 h and the total inhibition of oviposition, because survivors exhibited an impaired mobility with desynchronized movements. Seven days after exposure, mortality was higher than 95% in both pesticides. In agreement with our findings, several authors have already reported that fresh residues of abamectin are highly toxic to the majority of the phytoseiid mites in the laboratory and cause increases in mortality and reductions in longevity and oviposition to P. persimilis, A. cucumeris, A. fallacis and Amblyseius degenerans Berlese (Bostanian and Akalach 2006; Gentz et al. 2010; Kim et al. 2005; Sterk et al. 2003). Similarly, deltamethrin, a broad-spectrum insecticide, is toxic to a wide range of natural enemies including the predatory mites (Jansen 2010). As such, Bonafos et al. (2007) reported 100% mortality of Amblyseius andersoni (Chant) when both the phytoseiids and a bean leaf disk were treated at concentrations slightly higher than ours (17.5 mg a.i. l−1) under the Potter precision tower. The harmfulness was also observed in field conditions based on results of Rodrigues et al. (2002) with established populations of Amblyseius barkeri Hughes, Euseius stipulatus (Athias-Henriot), Euseius finlandicus (Oudemans) and Typhlodromus pyri Scheuten (Acari: Phytoseiidae) in apple orchards of northern Portugal.
Spinosad, authorized in organic farming in European member states after its inclusion in Annex II of the EU council regulation 2092/91 in 2008 (OJEC 2008), was the insecticide with the strongest direct effect on A. swirskii. Rahman et al. (2011) also reported that the pesticide was very toxic to the phytoseiids A. cucumeris, Amblyseius montdorensis (Schicha) and the hypoaspid Hypoaspis miles (Berlese) at a concentration lower than that used in our trials (96 mg a.i. l−1). In the review of Biondi et al. (2012b), however, contradictory results are presented concerning this pesticide, which is more effective by ingestion than by contact. Some phytoseiids are very much affected even in the field or the greenhouse (T. pyri, A. cucumeris and Euseius tularensis Congdon) while not acute toxicity or detrimental effects on the survival, predation and reproduction were detected in others (A. cucumeris and P. persimilis). The different formulations, concentrations and frequency of treatment in the field might have accounted for the results.
The duration of the residual toxicity of those pesticides classified as not harmless in the laboratory (abamectin, deltamethrin, emamectin and spinosad) when the residues were aged under a greenhouse in central Spain was moderate. Emamectin was short-lived and consequently compatible with A. swirskii adults (IOBC A) because at 4 DAT its residues were not harmful. Similarly, field trials with A. swirskii on pepper plants under unspecified climatological conditions, showed that three-, seven- and 14-day-old residues were harmless to the predatory mite, because this pesticide quickly degrades on the leaf surface (Amor et al. 2012).
The other three studied pesticides abamectin, deltamethrin and spinosad were ranked as slightly persistent (IOBC B) because their harmful activity lasted longer than five days. The primary degradation mechanism of all of these pesticides is photolysis (Liu et al. 2010; van de Veire et al. 2004). Hence, the differences in their harmful activity to beneficial fauna are influenced by the light intensity and UV radiation during the aging of the residue. Thus, there is contradictory information in the literature on the duration of the harmful activity of these pesticides to different natural enemies apart from those linked to the species and/or developmental stage studied and the plant chosen for the application of the pesticides.
Nadimi et al. (2011) and van de Veire et al. (2001) have reported that abamectin had a slight persistent effect (IOBC B) on A. californicus and P. persimilis protonymphs, which was consistent with our findings, though protonymphs were more sensitive to pesticides than the adults. In contrast, Blümel and Hausdorf (2002) observed that zero-, three- and ten day old residues aged in bean plants rapidly killed 100% of P. persimilis protonymphs. In contrast to our results (spinosad was slightly persistent to A. swirskii adults), this insecticide was ranked as short-lived (IOBC A) to the closely related species A. californicus (van de Veire et al. 2001), when the residues were aged in sweet pepper plants. None of these authors provided information on the amount of light or UV radiation during the aging of the pesticide residues. However, van de Veire et al. (2004) reported that the persistence of abamectin and spinosad to Encarsia formosa Gahan was higher in European locations with lower light intensity.
Deltamethrin was classified as moderately persistent (IOBC C) to E. finlandicus protonymphs in semi-field tests performed in plum orchards in Greece under unspecified climatological conditions (Broufas et al. 2008), while it was slightly persistent (IOBC B) to A. swirskii adults in our trials in Central Spain in June. Apart from the differences linked to the different developmental stages and species studied, the amount of UV radiation and the number of day hours must have played a role on the photolytic degradation, which seems to have been slower in Greece. In summary, sulfoxalfor as well as flubendiamide, flonicamid, metaflumizone, methoxyfenozide, spiromesifen and spirotetramat are compatible with A. swirskii. Abamectin, deltamethrin, emamectin and spinosad can also be recommended for use in IPM programs if appropriate safety deadlines are used before the natural enemy release.
References
Amor F, Medina P, Bengochea P, Cánovas M, Vega P, Correia R, García F, Gómez M, Budia F, Viñuela E, López J (2012) Effect of emamectin benzoate under semi-field and field conditions on key predatory biological control agents used in vegetable greenhouse. Biocontrol Sci Technol 22(2):219–232
Babcock JM, Gerwick CB, Huang JX, Loso MR, Nakamura G, Nolting SP, Rogers RB, Sparks TC, Thomas J, Whatson GB, Zhu Y (2011) Biological characterization of sulfoxaflor, a novel insecticide. Pest Manag Sci 67(3):328–334
Barrett KI, Grandy N, Harrison EG, Hassan SA, Oomen P (1994) Guidance document on regulatory testing procedures for pesticides with non-target arthropods. SETAC, Belgium
Beers EH, Schmidt RA (2014) Impacts of orchard pesticides on Galendromus occidentalis: lethal and sublethal effects. Crop Prot 56:16–24
Bengochea P, Sánchez-Ramos I, Saelices R, Amor F, del Estal P, Viñuela E, Adán A, López A, Budia F, Medina P (2014) Is emamectin benzoate effective against the different stages of Spodoptera exigua (Hübner) (Lepidoptera, Noctuidae)? Irish J Agric Food Res 53:37–49
Bernard MB, Cole P, Kobelt A, Horne PA, Altmann J, Wratten SD, Yen AL (2010) Reducing the impact of pesticides on biological control in australian vineyards: pesticide mortality and fecundity effects on an indicator species, the predatory mite Euseius victoriensis (Acari: Phytoseiidae). J Econ Entomol 103(6):2061–2071
Bielza P (2008) Insecticide resistance management strategies against the western flower thrips, Frankliniella occidentalis. Pest Manag Sci 64:1131–1138
Biondi A, Desneux N, Siscaro G, Zappalà L (2012a) Using organic-certified rather than synthetic pesticides may not be safer for biological control agents: selectivity and side effects of 14 pesticides on the predator Orius laevigatus. Chemosphere 87(7):803–812
Biondi A, Mommaerts V, Smagghe G, Viñuela E, Zappalà L, Desneux N (2012b) Non-target impact of spinosyns on beneficial arthropods, a review. Pest Manag Sci 68(12):1523–1536
Blümel S, Hausdorf H (2002) Results of the 8th and 9th IOBC Joint Pesticides Testing Programme: persistence test with Phytoseiulus persimilis Athias-Henriot (Acari: Phytoseiidae). IOBC/WPRS Bull 25(11):43–51
Bonafos R, Serrano E, Auger P, Kreiter S (2007) Resistance to deltamethrin, lambda-cyhalothrin and chlorpyriphos-ethyl in some populations of Typhlodromus pyri Scheuten and Amblyseius andersoni (Chant) (Acari: Phytoseiidae) from vineyards in the south-west of France. Crop Prot 26:169–172
Bostanian NJ, Akalach M (2006) The effect of indoxacarb and five other insecticides on Phytoseiulus persimilis (Acari: Phytodeiidae), Amblyseius fallacis (Acari: Phytoseiidae) and nymphs of Orius insidiosus (Hemiptera: Anthocoridae). Pest Manag Sci 62:334–339
Bostanian NJ, Hardman JM, Thistlewood HA, Racette G (2010) The response of Neoseiulus fallacies (Garman) and Galendromus occidentalis (Nesbitt) (Acari: Phytoseiidae) to six reduced risk insecticides in Canada. IOBC/WPRS Bull 55:73–77
Broufas GD, Pappas ML, Vassiliou G, Koveos DS (2008) Toxicity of certain pesticides to the predatory mite Euseius finlandicus (Acari: Phytoseiidae). IOBC/WPRS Bull 35:85–91
Calvo FJ, Bolckmans K, Belda JE (2011) Control of Bemisia tabaci and Frankliniella occidentalis in cucumber by Amblyseius swirskii. BioControl 56(2):185–192
Carlson GR, Dhadialla TS, Hunter R, Jansson RK, Jany CS, Lidert Z, Slawecki RA (2001) The chemical and biological properties of methoxyfenozide, a new insecticidal ecdysteroid agonist. Pest Manag Sci 57:115–119
Cheon GS, Paik CH, Kim SS (2008) Selective toxicity of three acaricides to the predatory mite Neoseiulus womersleyi and its prey Tetranychus urticae (Acari: Phytoseiidae, Tetranychidae). Korean J Pestic Sci 12:249–255
Cloyd RA, Galle CL, Keith S (2006) Compatibility of three miticides with the predatory mites Neoseiulus californicus McGregor and Phytoseiulus persimilis Athias-Henriot (Acari: Phytoseiidae). HortScience 41(3):707–710
Colomer I, Aguado P, Medina P, Heredia RM, Fereres A, Belda JE, Viñuela E (2011) Field trial measuring the compatibility of methoxyfenozide and flonicamid with Orius laevigatus Fieber (Hemiptera: Anthocoridae) and Amblyseius swirskii (Athias-Henriot) (Acari: Phytoseiidae) in comercial pepper greenhouse. Pest Manag Sci 67:1237–1244
De Barro PJ, Liu SS, Boykin LM, Dinsdale AB (2011) Bemisia tabaci: a statement of species status. Annu Rev Entomol 56:1–19
De Cabezón Sáenz, Irigaray FJ, Zalom FG (2007) Selectivity of acaricide exposure on Galendromus occidentalis reproductive potential. Biocontrol Sci Techn 17(5):541–546
Desneux N, Decourteye A, Delpuech JM (2007) The sublethal effects of pesticides on beneficial arthropods. Annu Rev Entomol 52:81–106
Doker I, Pappas ML, Samaras C, Triantafyllou A, Kazak C, Broufas GD (2015) Compatibility of reduced-risk insecticides withy the non-target predatory mite Iphiseius degenerans (Acari: Phytoseiidae). Pest Manag Sci 71(9):1267–1273
EPA (Environmental Protection Agency) (2008) EPA spirotetramat fact sheet. http://www.thebeeyard.org/wp-content/uploads/2010/03/plugin-spirotetramat.pdf. Cited 9 March 2016
EPA (Environmental Protection Agency) (2013) Pesticide news story: The EPA’s final decision on the new active ingredient sulfoxaflor. https://archive.epa.gov/pesticides/news/web/html/sulfoxaflor-decision.html. Cited 21 March 2016
EPPO (European and Mediterranean Plant Protection Organization) (2015a) Data sheets on quarantine pests: Bemisia tabaci. http://www.eppo.int/QUARANTINE/data_sheets/insects/BEMITA_ds.pdf. Cited 9 March 2016
EPPO (European and Mediterranean Plant Protection Organization) (2015b) Data sheets on quarantine pests: Frankliniella occidentalis. http://www.eppo.int/QUARANTINE/data_sheets/insects/FRANOC_ds.pdf. Cited 9 March 2016
Fernández E, Grávalos C, Haro PJ, Cifuentes D, Bielza P (2009) Insecticide resistance status of Bemisa tabaci Q-biotype in south-eastern Spain. Pest Manag Sci 65:885–891
Garzón A, Medina P, Amor F, Viñuela E, Budia F (2015) Toxicity and sublethal effects of six insecticides to last instar larvae and adults of the biocontrol agents Chrysoperla carnea (Stephens) (Neuroptera: Chrysopidae) and Adalia bipunctata (L.) (Coleoptera: Coccinellidae). Chemosphere 132:87–93
Gentz MC, Murdoch G, King GF (2010) Tandem use of selective insecticides and natural enemies for effective reduced-risk pest management. Biol Control 52(3):208–215
Gómez M, Garcia F, GreatRex R, Lorca M, Serna A (2006) Preliminary field trials with the synthetic sexual aggregation pheromone of Frankliniella occidentalis on protected pepper and tomato crops in south-east Spain. IOBC/WPRS Bull 29(4):153–158
Gradish AE, Scott-Dupree CD, Shipp L, Harris CR, Ferguson G (2011) Effect of reduced risk pesticides on greenhouse vegetable arthropod biological control agents. Pest Manag Sci 67:82–86
Hassan SA (1994) Activities of the IOBC/WPRS working group Pesticides and Beneficial Organisms. IOBC/WPRS Bull 17:1–5
IOBC (International Organization for Biological Control) (2016) IOBC Pesticide Side Effect Database. http://www.iobc-wprs.org/restricted_member/toolbox.cfm. Cited 3 March 2016
IRAC (Insecticide Resistance Action Committee) (2016) Mode of action classification scheme (version 8.0). http://www.irac-online.org/documents/moa-classification/. Cited 10 Feb 2016
Jacas J, Viñuela E (1994) Analysis of a laboratory method to test the effects of pesticides on adult females of Opius concolor (Hymn.: Braconidae), a parasitoid of the olive fruit fly Bactrocera oleae (Dip.: Tephritidae). Biocontrol Sci Technol 4:147–154
Jansen JP (2010) Beneficial arthropods and pesticides: building selectivity lists for IPM. IOBC/WPRS Bull 55:23–47
Kim SS, Seo SG, Park JD, Kim SG, Kim DI (2005) Effects of selected pesticides on the predatory mite, Amblyseius cucumeris (Acari: Phytoseiidae). J Entomol Sci 40(2):107–114
Lefebvre M, Bostanian NJ, Thistlewood HMA, Mauffette Y, Racette G (2011) A laboratory assessment of the toxic attributes of six ‘reduced risk insecticides’ on Galendromus occidentalis (Acari: Phytoseiidae). Chemosphere 84:25–30
Lefebvre M, Bostanian NJ, Mauffette Y, Racette G, Thistlewood HA, Hardman JM (2012) Laboratory-based toxicological assessments of new insecticides on mortality and fecundity of Neoseiulus fallacis (Acari: Phytoseiidae). J Econ Entomol 105(3):866–871
Liu P, Liu Y, Liu Q, Liu J (2010) Photodegradation mechanism of deltamethrin and fenvalerate. J Environ Sci 22(7):1123–1128
MAGRAMA (Ministry of Agriculture, Food and environment) (2016a) Annual survey directory 2012. Madrid. http://www.magrama.gob.es/estadistica/pags/anuario/2012/AE_2012_Completo.pdf. Cited 22 Feb 2016 (in Spanish)
MAGRAMA (Ministry of Agriculture, Food and environment) (2016b) European community list of active substances included, excluded and under evaluation. http://www.mapama.gob.es/agricultura/pags/fitos/registro/fichas/pdf/Lista_Sustancias_activas_aceptadas_excluidas.pdf. Cited 10 Feb 2016 (in Spanish)
MAGRAMA (Ministry of Agriculture, Food and environment) (2016c) Official phytosanitary products entry. http://www.magrama.gob.es/es/agricultura/temas/sanidad-vegetal/productos-fitosanitarios/registro/menu.asp. Cited 10 Feb 2016 (in Spanish)
Nadimi A, Kamali K, Arbabi M, Abdoli F (2011) Study on persistence tests of miticides abamectin and fenproximate to predatory mite Phytoseiulus persimilis (Acarina: Phytoseiidae). Afr J Agric Res 6(2):338–342
OJEC (Official Journal of the European Community) (2008) Commision regulation (EC) No. 404/2008 of 6 May 2208 amending Annex II to Council Regulation (EEC) No. 2092/91 on organic production of agricultural products as concerns the authorization of spinosad, potassium bicarbonate and copper octanoate and the use of ethylene. OJEC 120:8–10
OJEU (Official Journal of the European Union) (2009) Directive 2009/128/EC of the European Parliament and of the Council of 21 October 2009, establishing a framework for Community action to achieve the sustainable use of pesticides. OJEU 309:71–86
Oliveira MRV, Henneberry TJ, Anderson P (2001) History, current status, and collaborative research projects for Bemisia tabaci. Crop Prot 20:709–723
Rahman T, Spafforda H, Broughton S (2011) Compatibility of spinosad with predaceous mites (Acari) used to control Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae). Pest Manag Sci 67:993–1003
Robledo Camacho A, van der Blom J, Sánchez Martínez JA, Torres Giménez S (2009) Control biológico en invernaderos hortícolas. Coexphal-FAECA, Almería (in Spanish)
Roditakis E, Fytrou N, Staurakaki M, Vontas J, Tsagkarakou A (2014) Activity of flonicamid on the sweet potato whitely Bemisia tabaci (Homoptera: Aleyrodidae) and its natural enemies. Pest Manag Sci 70:1460–1467
Rodrigues JR, Miranda NRC, Rosas JDF, Maciel CM, Torres LM (2002) Side-effects of fifteen insecticides on predatory mites (Acari: Phytoseiidae) in apple orchards. IOBC/WPRS Bull 25(11):53–61
Roubos CR, Rodriguez-Saona C, Rufus I (2014) Mitigating the effects of insecticides on arthropod biological control at field and landscape scales. Biol Control 75:28–38
Shipp JL, Wang K, Binns MR (2000) Economic injury levels for western flower thrips (Thysanoptera: Thripidae) on greenhouse cucumber. J Econ Entomol 93(6):1732–1740
Smagghe G, Gomez LE, Dhadialla TS (2013) The bisacylhydrazine insecticides for selective pest control. Adv Insect Physiol 43:163–249
Sparks T, Watson G, Loso M, Geng C, Babcock J, Thomas J (2013) Sulfoxaflor and the sulfoximine insecticides: chemistry, mode of action and basis for efficacy on resistant insects. Pestic Biochem Physiol 107:1–7
Sterk G, Heuts F, Merck N, Bock J (2003) Sensitivity of non-target arthropods and beneficial fungal species to chemical and biological plant protection products: results of laboratory and semi-field trials. In: Proceedings of the first international symposium on biological control of arthropods, USDA forest service, Honolulu, USA, pp 306–313
STSC (1987) Statgraphics user’s guide, Version 5.1. Graphic software system. STSC, Rockville, USA
Tirello P, Pozzebon A, Duso C (2013) The effect of insecticides on the non-target predatory mite Kampimodromus aberrans: laboratory studies. Chemosphere 93:1139–1144
Tohnishi M, Nakao H, Furuya T, Seo A, Kodama H, Tsubata K, Fujioka S, Kodama H, Hirooka T, Nishimatsu T (2005) Flubendiamide, a novel insecticide highly active against lepidopterous insect pests. J Pestic Sci 30(4):354–360
van de Veire M, Tirry L (2003) Side effects of pesticides on four species of beneficials used in IPM in glasshouse vegetable crops: “worst case” laboratory tests. IOBC/WPRS Bull 26(5):41–50
van de Veire M, Cornelis W, Tirry L (2001) Development of a laboratory test method to determine the duration of pesticide-effects on predatory mites. IOBC/WPRS Bull 24(4):61–66
van de Veire M, Viñuela E, Bernardo U, Tirry L, Adan A, Viggiani G (2004) Duration of the toxicity of abamectin and spinosad on the parasitic wasp Encarsia formosa Gahan in Northern and Southern Europe. IOBC/WPRS Bull 27(6):21–30
Wanumen AC, Carvalho GA, Medina P, Viñuela E, Adán A (2016) Residual acute toxicity of some modern insecticides toward two mirid predators of tomato pests. J Econ Entomol 109(3):1079–1085
Acknowledgements
This study was supported by the Spanish Ministry of Science and Innovation (Projects AGL2010-22196-C02-02 and AGL2013-47603-C2-1-R to E. Viñuela and P. Medina and PhD grant to Mª M. Fernández). E. Viñuela, P. del Estal and P. Medina are members of the Associate Unit IVAS (CSIC-UPM): Control of insect vectors of viruses in horticultural sustainable systems.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Marta Montserrat.
Rights and permissions
About this article
Cite this article
Fernández, M.M., Medina, P., Wanumen, A. et al. Compatibility of sulfoxaflor and other modern pesticides with adults of the predatory mite Amblyseius swirskii. Residual contact and persistence studies. BioControl 62, 197–208 (2017). https://doi.org/10.1007/s10526-017-9784-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-017-9784-1