Abstract
Multiple arthropod pests can affect the same crop in agricultural systems, requiring the integration of control methods. In the present study, the effects of residual exposure to four broad-spectrum insecticides/acaricides (azadiractin, abamectin, chlorfenapyr, and fenpyroximate) on immature (development and survival time) and adult females (longevity, fecundity, and fertility life table parameters) of the predatory mite Neoseiulus barkeri were evaluated. Additionally, the insecticides/acaricides were categorized according to their selectivity based on the classification proposed by the International Organization for Biological Control (IOBC) for assessing the susceptibility of arthropods in laboratory experiments. Method 004, proposed by the Insecticide Resistance Action Committee (IRAC), was adopted for the bioassays with predators exposed to insecticide-acaricide residues. Among the insecticides/acaricides studied, azadirachtin had minimal effects on immature and adult N. barkeri (all non-significant) and was considered harmless based on the classification of toxicity according to the standards/categories proposed by the IOBC. All other insecticides/acaricides affected immature and adult N. barkeri and were considered slightly harmful in terms of toxicity, according to the IOBC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Multiple arthropods can occur on the same crop in agricultural systems, making it difficult to manage harmful species, as the measures adopted to control one species can affect others that make up the community and lead to an outbreak of secondary pests (Gross and Rosenheim 2011). In these systems, both chemical and biological control, when used alone, are insufficient for the proper management of pest species (Bueno & Torres 2018). It is widely accepted that, for these systems, the best management option is the integration of control methods, which is only possible through the use of pesticides with low or no impact on biocontrol agents (Croft 1990).
Among biocontrol agents, predatory mites stand out not only because of their wide range of potential prey (including phytophagous mites and small insects such as whiteflies, fungus gnats, thrips, and even nematodes) (Gerson and Weintraub 2012; McMurtry et al. 2012) but also for their adoption in different crops (fruit, vegetable, and ornamental crops) (van Lenteren 2012; Knapp et al. 2018; van Lenteren et al. 2018). Recent selectivity studies have shown that xenobiotics used in agricultural systems, including those considered selective, have non-targeted (lethal or sublethal) effects on at least one phytoseiid species (Bergeron and Schmidt-Jeffris 2020; Schmidt-Jeffris et al. 2021). They also demonstrated that selectivity levels are not interchangeable between species (Bergeron and Schmidt-Jeffris 2020; Schmidt-Jeffris et al. 2021), highlighting the need to individually examine the main phytoseiid species in terms of the selective aspects of any and all xenobiotics used in agricultural systems.
One of the main commercial phytoseiid species is Neoseiulus barkeri (Hughes) (Acari: Phytoseiidae) (van Lenteren et al. 2018). It has been found in different parts of the world and it has been considered as an efficient predator (De Moraes et al., 2004; Demite et al. 2023). According to McMurtry et al. (2012), N. barkeri is a type-III generalist predator feeding on a wide range of prey species, including mites of different families, nematodes, and small insects such as thrips, whiteflies, and cochineals. Neoseiulus barkeri lives in a diverse habitat range, on leaves (pubescent or glabrous), in soil/litter, and in confined spaces on plants (mono- or dicotyledonous) (McMurtry et al., 2012; Demite et al. 2023). This predatory mite has been widely studied owing to its value as a biocontrol agent (Filgueiras et al. 2020a, b, c). It is commercialized in Europe, Latin America, and South America for the control of the broad mite Polyphagotarsonemus latus (Banks) (Acari: Tarsonemidae) (Fan and Petitt 1994), red palm mite, Raoiella indica Hirst (Acari: Tenuipalpidae) (Filgueiras et al. 2020a, b, c; Agrofit 2022), and onion thrips, Thrips tabaci L. (Thysanoptera: Thripidae) (van Lenteren et al. 2018). Despite its widespread use, its compatibility with insecticides and acaricides has been underexplored taking into account the great number of active ingredients. In addition, such studies are normally conducted by exposing only adults to toxic substances and ignoring the possible effects on immature individuals (see Zahid et al. 2017; Bashir et al. 2018). Adults are expected to be less susceptible than immature individuals owing to their greater weight (reduced dose per amount of body mass) and lower surface-to-volume ratio (reduced relative surface exposure). Given these assumptions, the International Organization for Biological Control (IOBC) recommends that its protocols include newly hatched larvae in insecticide/acaricide susceptibility bioassays (Hassan et al. 1985).
In the present study, the effects of residual exposure to four insecticides/acaricides (azadiractin, abamectin, chlorfenapyr, and fenpyroximate) on immature (time of development and survival) and adult (longevity and reproductive parameters) N. barkeri were evaluated. The selected insecticides/acaricides are recommended and frequently used in agricultural systems against potential biological targets of the N. barkeri, in addition to having different modes of action (Van Leeuwen et al. 2015; De Rouck et al. 2023). Additionally, the insecticides/acaricides were classified according to their selectivity, following the classification proposed by the IOBC for evaluating the susceptibility of arthropods in laboratory experiments.
Methodology
Local and experimental conditions
The rearing and bioassays were carried out at the Laboratory of Mite and Insect Management (LAMAI) of the Federal University of Ceará (UFC) in an environment with controlled conditions and regulated to 25 ± 2 °C, 70 ± 10% RH, and 12 h of photophase.
Obtaining and rearing Neoseiulus barkeri
The N. barkeri population was collected from a sweet pepper crop in the municipality of Icapuí (Ceará, Brazil; 4°51´S, 37°21´W). Approximately 200 N. barkeri adults were used to establish the rearing units. These consisted of 7-L plastic pots (approximately 60 cm high and 25 cm in diameter) with two side holes (7 cm in diameter) closed with voile fabric to allow air to enter. A mixture of rice husks and vermiculite (3 L, in a 1:1 ratio) was added to each pot. Weekly, in each rearing unit, we added 500 mL of wheat bran infested with Tyrophagus putrescentiae (Schrank) (Acari: Acaridae), which served as food for the predators. The N. barkeri were reared for several generations (4 months) before they were used for the experiments.
To facilitate handling of the mites used in the experiments, 15 days before the experiments, a population of N. barkeri (ca. 100 females) was removed from the pots and kept in plastic trays (18 × 10 × 3.5 cm) containing wet polyethylene foam, and a black PVC plate (14 × 8 cm) surrounded by hydrophilic cotton moistened with distilled water was placed to prevent the mites from escaping. From these units, individuals of known age were obtained.
Pesticides and dosages
The products and their doses are listed in Table 1. The pesticides selected are registered by the Brazilian Ministry of Agriculture, Livestock and Food Supply and frequently used in agricultural systems against potential biological targets of N. barkeri in several crops (Agrofit 2022).
Bioassays with immature and adult Neoseiulus barkeri
Method 004 proposed by the Insecticide Resistance Action Committee (IRAC) (www.irac-online.org) was adopted for the bioassays. However, PVC discs were used to construct the experimental arenas instead of leaf discs. PVC discs (5 × 5 cm) were immersed for 5 s in 40 mL of the pesticides solution or distilled water (control). The PVC discs were then dried in the laboratory for 30 min. Discs were used to construct the experimental units. These consisted of Petri dishes (1.5 cm high, 9 cm diameter) containing polyethylene foam (1 cm thick, 8 cm diameter), superimposed by a filter paper disc (7 cm diameter), and PVC containing product residues or distilled water. In each unit, the edge of the PVC plate was covered with cotton moistened with distilled water to prevent mites from escaping. Tyrophagus putrescentiae individuals of different developmental stages were provided as food source. All bioassays were performed in triplicate (at a different time each one) with at least 10 experimental units per replicate to randomize effects related to uncontrollable procedures (that is, in the preparation of the solutions tested).
For the bioassay with immature N. barkeri, five recently emerged larvae (< 12-h-old) were transferred to the experimental units (previously treated with pesticides or distilled water) (50 individuals were evaluated per treatment). They were monitored every 12 h until they reached the adult stage or until the immature ones died. The parameters evaluated in this bioassay were immature survival and developmental time (larva–adult). These parameters were subjected to Kaplan-Meier survival analysis (general effect), followed by the log-rank test (paired comparisons) (Hosmer and Lemeshow 1999) using SAS software.
For the bioassay with N. barkeri adults, one newly emerged and pregnant female (ca. 7 days from eggs) was confined to the experimental units; 30 females were evaluated per treatment. Each experimental unit constituted a replicate. The females were monitored daily until death, and their longevity and fecundity were computed. The first 10 eggs of each female were isolated in new experimental units (residue-free) to determine the viability of the eggs and the sex ratio of the offspring, which was measured after mounting slides with the adult offspring. The longevity and fecundity of females were plotted as functions of time and then used to create fertility life tables, estimating the following parameters: net reproduction rate (R0), intrinsic population rate (rm), finite growth rate population (λ), and mean generation time (T). The errors related to each parameter were estimated using the bootstrap method (100,000 iterations). Subsequently, a paired bootstrap test was performed to compare the differences between treatments (Efron & Tibshirani 1993). Fertility life table parameters were calculated using TWOSEX-MSChart software (Chi 2020).
Classification of the selectivity of pesticides according to IOBC
Pesticides were classified according to their selectivity, according to the classification proposed by the IOBC to evaluate the susceptibility of arthropods in experiments conducted in the laboratory (Sterk et al. 1999). This classification was based on the cumulative effect of the product on the target organism, and the cumulative effect (E) was calculated according to the formula proposed by Vogt et al. (1992): E = 100% - (100% - M%) × R1 × R2, where M% = adult corrected mortality, R1 = ratio of the average number of daily eggs between treatments and the control, and R2 = average egg viability ratio between treatments and the control. The data for calculating the cumulative effect were obtained in the bioassay with N. barkeri adults, considering only the first 10 days of evaluation. Once the cumulative effect was known, it was categorized as follows: 1, harmless (E < 30%); 2, slightly harmful (30 < E < 79%); 3, moderately harmful (80 < E < 99%); 4, harmful (E > 99%).
Results
Only abamectin and chlofenapyr affected the survival of the immature N. barkeri (Fig. 1; χ2 = 983.3, d.f. = 4, P < 0.0001). The percentage of immature individuals that reached the adult stage when exposed to abamectin and chlofenapyr residues was approximately 90 and 35%, respectively (Fig. 1). All immature individuals that were not exposed to residues (control) or those exposed to azadirachtin and fenpyroximate residues reached the adult stage (100% larva–adult viability). The developmental time (larva to adult) of N. barkeri was affected only by exposure to fenpyroximate residues (Fig. 1; χ2 = 196.3, d.f. = 4, P = 0.0006). The exposure to fenpyroximate residues reduced the developmental time of N. barkeri. Approximately 80% of the immature individuals exposed to fenpyroximate residues reached the adult stage in 4 days; this percentage was 100% on the following day (5th day). Adults were detected on day 4 in the other treatments as well; however, these accounted for < 40% of the immature individuals, most of which reached the adult stage between days 5 and 6.
Cumulative percentage of Neoseiulus barkeri adults as a function of their development time (larvae to adult) when exposed or not to residues of the insecticides/acaricides abamectin, azadirachtin, fenpyroximate, and chlorfenapyr. Curves followed by the same letter do not differ from each other in terms of the cumulative percentage of adults, whereas curves marked with an asterisk differ from the others in terms of development time (pairwise comparisons using log-rank test)
The exposure to azadirachtin residues did not affect the survival curve (χ2 = 19.41, d.f. = 4, P = 0.0007) or the mean survival time of N. barkeri females (χ2 = 18.21, d.f. = 4, P = 0.0011); the other products affected both parameters (Fig. 2A,B). Females not exposed to pesticide residues survived for an average of 37.95 days and a maximum of 87 days, with the first mortality event observed at 14 days (Fig. 2A,B). Exposure to pesticides residues promoted mortality events before the first 10 days of evaluation, with an average survival of 21.7, 26.6, 22.7, and 12.0 days and a maximum survival of 70, 70, 39, and 77 days for abamectin, azadirachtin, chlorfenapyr, and fenpyroximate, respectively.
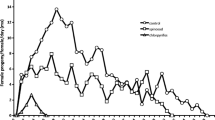
Regardless of exposure to pesticide residues, N. barkeri females showed oviposition peaks at the beginning of the oviposition period (before day 10), which were close to 3.0 eggs/female (Fig. 2C). The oviposition periods were 38, 41, 51, 28, and 34 days for females not exposed or exposed to abamectin, azadirachtin, chlorfenapyr, and fenpyroximate residues, respectively. The average number of eggs per female per day was also not affected by exposure to pesticide residues (χ2 = 3.60, d.f. = 4, P = 0.46; Fig. 2D).
Survival curves (A), mean (± SE) survival time (B), average number of eggs per female as a function of oviposition period (C), and mean (± SE) number of eggs per female per day (D) of Neoseiulus barkeri females either exposed or not to residues of the insecticides/acaricides abamectin, azadirachtin, fenpyroximate, and chlorfenapyr. Means within a panel marked with the same letter do not differ from each other through the bootstrap test
The exposure of N. barkeri females to azadirachtin residues did not alter any of the fertility life table parameters; all other products altered (reduced) at least one of the fertility life table parameters (Fig. 3A–D). The exposure of N. barkeri females to abamectin and chlorfenapyr residues resulted in a reduction in the net reproduction rate, intrinsic rate of population growth, and finite rate of population growth (Fig. 3A–C). The exposure of N. barkeri females to fenpyroximate residues reduced only the net reproduction rate and mean generation time (Fig. 3A,D).
Mean (± SE) net reproduction rate (offspring/individual) (A), intrinsic growth rate (day− 1) (B), finite population growth rate (day− 1) (C), and mean generation time (days) (D) of Neoseiulus barkeri either exposed or not to residues of abamectin, azadirachtin, fenpyroximate, or chlorfenapyr. Means within a panel marked with the same letter do not differ from each other through the bootstrap test
Considering the cumulative effects of the evaluated pesticides and the toxicity classification according to the standards/categories proposed by the IOBC, azadirachtin was the only product considered harmless to N. barkeri, with a cumulative effect of < 30%. The other products were considered to be slightly harmful to N. barkeri, with cumulative effects ranging from 30 to 79% (Table 2).
Discussion
Among the insecticides/acaricides studied, azadirachtin had minimal effects on immature and adult N. barkeri (all non-significant) and was considered harmless based on the classification of toxicity according to the standards/categories proposed by the IOBC. All other insecticides/acaricides affected immature and adult N. barkeri and were considered slightly harmful in terms of toxicity, according to the IOBC.
Azadirachtin is the most successful botanical insecticide/acaricide in agricultural systems worldwide, especially in organic systems (Isman and Grieneisen 2014; Campos et al. 2019). Despite the high frequency of azadirachtin use, its safety for non-target arthropods has been questioned, especially considering its sublethal effects (Qi et al. 2001; Cordeiro et al. 2010; Lima et al. 2015a). In the present study, bioassays were conducted with immature and adult N. barkeri to evaluate both lethal effects (survival of immature and newly emerged females) and sub-lethal effects (immature individual development time and reproductive parameters of newly emerged females); none of the parameters analyzed were altered by exposure to azadirachtin residues. These results suggest that azadirachtin at the dose used here is harmless to N. barkeri, which is also the IOBC classification in terms of its toxicity. Similar results have been observed for other predatory mites such as Euseius gallicus Kreiter and Tixier (Put et al. 2016), Neoseiulus baraki (Athias-Henriot) (both Acari: Phytoseiidae) (Lima et al. 2016), Stratiolaelaps scimitus (Womersley), and Cosmolaelaps brevistilis (Karg) (both Acari: Laelapidae) (Duarte et al. 2020). On the other hand, there are species of predatory mites that are sensitive to azadirachtin, such as Iphiseiodes zuluagai Denmark and Muma (Acari: Phytoseiidae), which shows high mortality (ca. 90%) after exposure to azadirachtin (Mourão et al. 2004). Such differences in the susceptibility of predatory mites to azadirachtin may be explained by both the intrinsic characteristics of the product (formulation, doses, quantity of active ingredients, or part of the plant used to obtain the extract) and the intrinsic characteristics of the predator species (age, size, eating habits, cuticular and/or enzymatic composition) (Bueno et al. 2017; Vidal and Kreiter 1995; Lima et al. 2015b; Bergeron and Schmidt-Jeffris 2020; Schmidt-Jeffris et al. 2021).
Abamectin, chlorfenapyr, and fenpyroximate affected both immature and adult N. barkeri and were considered slightly harmful in terms of their toxicity according to the classification proposed by the IOBC. Such products are considered to have a broad spectrum of action and are used against insects and mites in a wide variety of plants (Yu 2008; Bergeron and Schmidt-Jeffris 2020; Zhao et al. 2017; Agrofit 2022); therefore, effects on N. barkeri were expected. In immature N. barkeri, abamectin and chlorfenapyr reduced survival without altering the development time; the opposite was observed for fenpyroximate. The reduced survival of immature phytoseiids when exposed to abamectin, chlorfenapyr, or fenpyroximate residues has already been reported in previous studies involving species such as Neoseiulus fallacis (Garman), Neoseiulus californicus McGregor, and Phytoseiulus persimilis Athias-Henriot. In some cases, none of the immature phytoseiids reached adulthood (100% reduction) (Kim & Yoo 2002; Bergeron and Schmidt-Jeffris 2020). However, the reduction in development time after exposure to toxic residues is not mentioned in relation to predatory mites, perhaps because this parameter has not been considered in previous studies or because of its non-alteration. In the present study, we observed that the exposure of immature N. barkeri to fenpyroximate residues resulted in a shorter time required to reach the adult stage. A similar result was recently observed by Bozhgani et al. (2018) for immature N. californicus individuals when exposed to spirotetramat, especially due to the reduced egg incubation time, and to a lesser extent for protonymphs and deutonymphs. In the present study, the development of immature N. barkeri was calculated from neonatal larvae; therefore, it is possible that the observed reduction was underestimated.
Residues of abamectin, chlorfenapyr, and fenpyroximate also affected the survival and longevity of N. barkeri females but not their average daily fecundity. In arthropods, changes in reproductive parameters are usually explained by a physiological trade-off between fecundity and longevity (Guedes et al. 2016, 2017). In this way, only part of the energy obtained through food is converted into eggs, whereas the rest is used to maintain vital activities of the organism, such as metabolic costs and respiration (Baumgärtner et al. 1987). The production of eggs by predatory mites requires a lot of food, not only because of the number of eggs produced, but also because of the amount of energy invested per egg (Sabelis 1985). The lack or reduction of energy investment can prolong the longevity of mites, as has been observed in N. californicus (Gotoh et al. 2006). In the present study, N. barkeri females did not show changes in the average daily fecundity after exposure to the products; however, their longevity was reduced, causing them to have lower total fecundity. This fact certainly contributed to the reduction of fertility life table parameters, especially R0, rm, and λ. A reduction in the life table parameters and fertility of predators due to exposure to toxic residues has been frequently observed in several phytoseiid species (Hamedi et al. 2011; Park et al. 2011; Alinejad et al. 2014, 2020; Lima et al. 2016). The average generation time of predatory mites after toxin exposure typically increases this parameter (Hamedi et al. 2011; Park et al. 2011; Alinejad et al. 2014, 2020; Lima et al. 2016). In the present study, exposure to fenpyroximate reduced the mean generation time. This reduction is probably a consequence of a reduction in the immature development time. However, this is an issue that deserves further investigation.
The present study demonstrated that azadirachtin is harmless to immature and adult N. barkeri, and that abamectin, chlorfenapyr, and fenpyroximate are slightly harmful to immature and adult N. barkeri. These results reinforce the findings of a recent meta-analysis using just over 150 articles, revealing that immature and adult phytoseiids do not differ in terms of their sensitivity to toxicants (Schmidt-Jeffris et al. 2021). It is possible that the insecticides tested in this study are compatible with the predatory mite N. barkeri. However, laboratory experiments may not reproduce the effects of the repeated application of pesticides in a growing season, which commonly occurs in several agricultural systems (Duso et al. 2020). Thus, field experiments are also recommended, they are considered more realistic for a variety of reasons, such as non-perfect coverage (and the presence of refugia), the ability of phytoseiids to leave pesticide-treated areas and return, the decay of residues in the field (due to UV and other environmental factors) (Duso et al. 2020). In addition, the extent to which pesticides induce negative impacts on predatory mites depends on the exposure method and duration as well as the concentration and pesticide group (Ghazy et al. 2016).
References
Abbott WS (1925) A method of computing the effectiveness of an insecticide. J Econ Entomol 18:265–267
Agrofit (2022) Sistema de agrotóxicos Fitossanitários do Ministério da Agricultura, Pecuária e Abastecimento, http://www.agricultura.gov.br. Acessed in: 22 december 2022
Alinejad M, Kheradmand K, Fathipour Y (2014) Sublethal effects of fenazaquin on life table parameters of the predatory mite Amblyseius Swirskii (Acari: Phytoseiidae). Exp Appl Acarol 64:361–373. https://doi.org/10.1007/s10493-014-9830-y
Alinejad M, Kheradmand K, Fathipour Y (2020) Demographic analysis of sublethal effects of propargite on Amblyseius Swirskii (Acari: Phytoseiidae): advantages of using age-stage, two sex life table in ecotoxicological studies. Syst Appl Acarol 25:906–917. https://doi.org/10.11158/saa.25.5.11
Bashir MH, Zahid M, Khan MA, Shahid M, Khan AK, Amrao L (2018) Pesticides toxicity for Neoseiulus barkeri (Acari: Phytoseiidae) and non-target organisms. Pak J Agric Sci 55:63–71. https://doi.org/10.21162/PAKJAS/18.5277
Baumgärtner J, Bieri M, Delucchi V (1987) Growth and development of immature life stages of Propylaea 14-punctata L. and Coccinella 7-punctata L. [Col.: Coccinellidae] simulated by the metabolic pool model. Entomophaga 32:415–423. https://doi.org/10.1007/BF02372451
Bergeron PE, Schmidt-Jeffris RA (2020) Not all predators are equal: miticide non-target effects and differential selectivity. Pest Manag Sci 76:2170–2179. https://doi.org/10.1002/ps.5754
Bozhgani NSS, Talebi AA (2018) The effects of spirotetramat on the demographic parameters of Neoseiulus californicus (Phytoseiidae). Syst Appl Acarol 23:1952–1964. https://doi.org/10.11158/saa.23.10.7
Bueno A, de F, Carvalho GA, Santos AC et al (2017) dos, Pesticide selectivity to natural enemies: challenges and constraints for research and field recommendation. Cienc Rural 47:e20160829. https://doi.org/10.1590/0103-8478cr20160829
Campos EVR, Proença PLF, Oliveira JL et al (2019) Use of botanical insecticides for sustainable agriculture: future perspectives. Ecol Indic 105:483–495. https://doi.org/10.1016/j.ecolind.2018.04.038
Chi H (2020) TWOSEX-MSChart: a computer program for the age-stage, two-sex life table analysis. Available online at: http://140.120.197.173/ecology/
Cordeiro EMG, Corrêa AS, Venzon M, Guedes RNC (2010) Insecticide survival and behavioral avoidance in the lacewings Chrysoperla externa and Ceraeochrysa cubana. Chemosphere 81:1352–1357. https://doi.org/10.1016/j.chemosphere.2010.08.021
Croft BA (1990) Arthropod biological control agents and pesticides. John Wiley and Sons Inc
De Moraes GJ, McMurtry JA, Denmark HA, Campos CB (2004) A revised catalog of the mite family Phytoseiidae. Zootaxa 434(1):1–494. https://doi.org/10.11646/zootaxa.434.1.1
De Rouck S, Inak E, Dermauw W, Van Leeuwen T (2023) A review of the molecular mechanisms of acaricide resistance in mites and ticks. Insect Biochem Mol Bio 196:103981. https://doi.org/10.1016/j.ibmb.2023.103981
Demite PR, Moraes GJ, McMurtry JA, Denmark HA, Castilho RC (2023) Phytoseiidae database. www.lea.esalq.usp.br/phytoseiidae. Accessed 22 Oct 2023
Duarte A, da Bastos Pazini F, Duarte J JLP, et al (2020) Compatibility of pesticides used in strawberry crops with predatory mites Stratiolaelaps Scimitus (Womersley) and Cosmolaelaps Brevistilis (Karg). Ecotoxicology 29:148–155. https://doi.org/10.1007/s10646-020-02164-w
Duso C, Van Leeuwen T, Pozzebon A (2020) Improving the compatibility of pesticides and predatory mites: recent findings on physiological and ecological selectivity. Curr Opin Insect Sci 39:63–68. https://doi.org/10.1016/j.cois.2020.03.005
Fan Y, Petitt FL (1994) Biological control of broad mite, Polyphagotarsonemus Latus (banks), by Neoseiulus Barkeri Hughes on pepper. Biol Control 4:390–395. https://doi.org/10.1006/bcon.1994.1049
Filgueiras RMC, Mendes JA, Sousa Neto EP, Monteiro NV, Melo JWS (2020a) Neoseiulus Barkeri Hughes (Acari: Phytoseiidae) as a potential control agent for Raoiella indica Hirst (Acari: Tenuipalpidae). Syst Appl Acarol 25:593–606. https://doi.org/10.11158/saa.25.4.1
Filgueiras RMC, Mendes JA, Da Silva FWB, Sousa Neto EP, Melo JWS (2020b) Prey stage preference and functional and numerical responses of Neoseiulus Barkeri Hughes (Acari: Phytoseiidae) to eggs of Raoiella Indica Hirst (Acari: Tenuipalpidae). Syst Appl Acarol 25:1147–1157. https://doi.org/10.11158/saa.25.6.16
Filgueiras RMC, Silva BW, Sousa Neto EP, Mendes JA, Melo JWS (2020c) Can the prey species Raoiella indica Hirst (Acari: Tenuipalpidae) support the development and reproduction of Neoseiulus Barkeri Hughes (Acari: Phytoseiidae). Syst Appl Acarol 25:1485–1494. https://doi.org/10.11158/saa.25.8.10
Gerson U, Weintraub PG (2012) Mites (Acari) as a factor in greenhouse management. Annu Rev Entomol 57:229–247. https://doi.org/10.1146/annurev-ento-120710-100639
Ghazy NA, Osakabe M, Negm MW, Schausberger P, Gotoh T, Amano H (2016) Phytoseiid mites under environmental stress. Biol Control 96:120–134. https://doi.org/10.1016/j.biocontrol.2016.02.017
Gotoh T, Tsuchiya A, Kitashima Y (2006) Influence of prey on developmental performance, reproduction and prey consumption of Neoseiulus californicus (Acari: Phytoseiidae). Exp Appl Acarol 40:189–204. https://doi.org/10.1007/s10493-006-9032-3
Gross K, Rosenheim JA (2011) Quantifying secondary pest outbreaks in cotton and their monetary cost with causal-inference statistics. Ecol Appl 21:2770–2780. https://doi.org/10.1890/11-0118.1
Guedes RNC, Smagghe G, Stark JD, Desneux N (2016) Pesticide-Induced stress in Arthropod Pests for Optimized Integrated Pest Management Programs. Annu Rev Entomol 61:43–62. https://doi.org/10.1146/annurev-ento-010715-023646
Guedes RNC, Walse SS, Throne JE (2017) Sublethal exposure, insecticide resistance, and community stress. Curr Opin Insect Sci 21:47–53. https://doi.org/10.1016/j.cois.2017.04.010
Hamedi N, Fathipour Y, Saber M (2011) Sublethal effects of abamectin on the biological performance of the predatory mite, Phytoseius plumifer (Acari: Phytoseiidae). Exp Appl Acarol 53:29–40
Hassan SA, Bigler F, Blaisinger P et al (1985) Standard methods to test the side-effects of pesticides on natural enemies of insects and mites developed by the IOBC/WPRS Working Group ‘Pesticides and beneficial organisms’. EPPO Bull 15:214–255. https://doi.org/10.1111/j.1365-2338.1985.tb00224.x
Hosmer DW, Lemeshow S (1999) Applied survival analysis: time-to-event. Wiley-Interscience
IRAC (2022) Insecticide Resistance Action Committee, www.irac-online.org. Acessed in 15 october 2022
Isman MB, Grieneisen ML (2014) Botanical insecticide research: many publications, limited useful data. Trends Plant Sci 19:140–145. https://doi.org/10.1016/j.tplants.2013.11.005
Kim SS, Yoo SS (2002) Comparative toxicity of some acaricides to the predatory mite, Phytoseiulus persimilis and the twospotted spider mite. Tetranychus Urticae BioControl 47:563–573. https://doi.org/10.1023/A:1016585607728
Knapp M, Van Houten Y, Van Ball, Groot T (2018) Use of predatory mites in commercial biocontrol: current status and future prospects. Acarologia 58:72–82. https://doi.org/10.24349/acarologia/20184275
Lima DB, Melo JWS, Gondim MGC et al (2015a) Acaricide-impaired functional predation response of the phytoseiid mite Neoseiulus Baraki to the coconut mite Aceria Guerreronis. Ecotoxicology 24:1124–1130. https://doi.org/10.1007/s10646-015-1459-z
Lima DB, Melo JWS, Guedes NMP et al (2015b) Bioinsecticide-predator interactions: Azadirachtin behavioral and reproductive impairment of the coconut mite predator Neoseiulus Baraki. PLoS ONE 10(2):e0118343. https://doi.org/10.1371/journal.pone.0118343
Lima DB, Melo JWS, Gondim MGC et al (2016) Population-level effects of abamectin, azadirachtin and fenpyroximate on the predatory mite Neoseiulus Baraki. Exp Appl Acarol 70:165–177. https://doi.org/10.1007/s10493-016-0074-x
McMurtry JA, Moraes GJ, Sourassou NF (2012) Revision of the lifestyles of phytoseiid mites (Acari: Phytoseiidae) and implications for biological control strategies. Syst Appl Acarol 18: 297–320. https://doi.org/10.11158/saa.18.4.1
Mourão SA, Silva JCT, Guedes RNC, Venzon M, Jham GN, Oliveira CL, Zanuncio JC (2004) Seletividade De extratos de nim (Azadirachta indica A. Juss.) Ao ácaro Predador Iphiseiodes Zuluagai (Denmark & Muma) (Acari: Phytoseiidae). Neotrop Entomol 33:613–617. https://doi.org/10.1590/S1519-566X2004000500011
Park JJ, Kim M, Lee JH et al (2011) Sublethal effects of fenpyroximate and pyridaben on two predatory mite species, Neoseiulus Womersleyi and Phytoseiulus Persimilis (Acari, Phytoseiidae). Exp Appl Acarol 54:243–259. https://doi.org/10.1007/s10493-011-9435-7
Put K, Bollens T, Wäckers F, Pekas A (2016) Non-target effects of commonly used plant protection products in roses on the predatory mite Euseius Gallicus Kreiter & Tixier (Acari: Phytoseiidae). Pest Manag Sci 72:1373–1380. https://doi.org/10.1002/ps.4162
Qi B, Gordon G, Gimme W (2001) Effects of neem-fed prey on the predacious insects Harmonia conformis (Boisduval) (Coleoptera: Coccinellidae) and Mallada signatus (Schneider) (Neuroptera: Chrysopidae). Biol Control 22:185–190. https://doi.org/10.1006/bcon.2001.0965
Sabelis MW (1985) Life history: reproduction. In: Helle W, Sabelis MW (eds) Spider mites: their biology, natural enemies and control, vol 1B. Elsevier, Amsterdam, pp 73–82
Schmidt-Jeffris RA, Beers EH, Sater C (2021) Meta-analysis and review of pesticide non-target effects on phytoseiids, key biological control agents. Pest Manag Sci 77:4848–4862. https://doi.org/10.1002/ps.6531
Sterk G, Hassan SA, Baillod M, Bakker F, Bigler F, Blümel S, Bogenschütz H, Boller E, Bromand B, Brun J, Calis JNM, Coremans-Pelseneer J, Duso C, Garrido A, Grove A, Heimbach U, Hokkanen H, Jacas J, Lewis G, Moreth L, Polgar L, Roversti L, Samsoe-Petersen L, Sauphanor B, Shaub L, Stäubli A, Tuset JJ, Vainio A, Van De Veire M, Viggiani G, Viñuela E, Vogt H (1999) Results of the seventh joint pesticide testing programme carried out by the IOBC/WPRS-Working Group ‘Pesticides and Beneficial Organisms’. BioControl 44:99–117. https://doi.org/10.1023/A:1009959009802
Tibshirani RJ, Efron B (1993) An introduction to the bootstrap. Monographs on statistics and applied probability. Chapman & Hall, London
Torres JB, de Bueno A F (2018) Conservation biological control using selective insecticides – A valuable tool for IPM. Biol Control 126:53–64. https://doi.org/10.1016/j.biocontrol.2018.07.012
Van Leeuwen T, Tirry L, Yamamoto A, Nauen R, Dermauw W (2015) The economic importance of acaricides in the control of phytophagous mites and an update on recent acaricide mode of action research. Pestic Biochem Physiol 121:12–21. https://doi.org/10.1016/J.PESTBP.2014.12.009
van Lenteren JC (2012) The state of commercial augmentative biological control: plenty of natural enemies, but a frustrating lack of uptake. Biocontrol 57:1–20. https://doi.org/10.1007/s10526-011-9395-1
van Lenteren JC, Bolckmans K, Köhl J et al (2018) Biological control using invertebrates and microorganisms: plenty of new opportunities. Biocontrol 63:39–59. https://doi.org/10.1007/s10526-017-9801-4
Vidal C, Kreiter S (1995) Resistance to a range of insecticides in the Predaceous Mite Typhlodromus pyri (Acari: Phytoseiidae): inheritance and physiological mechanisms. J Econ Entomol 88:1097–1105. https://doi.org/10.1093/jee/88.5.1097
Vogt H, Rumpf S, Wetzel C, Hassan SA (1992) A field method for testing effects of pesticides on larvae of the green lacewing Chrysoperla carnea Steph. (Neuroptera, Chrysopidae). IOBC/WPRS Bull 15:176–182
Yu SJ (2008) The toxicology and biochemistry of insecticides. CRC Press. https://doi.org/10.1201/9781420059762
Zahid M, Bashir MH, Khan BS, Shahid M (2017) Toxicity of Some Selected Pesticides against Neoseiulus barkeri (Acari: Phytoseiidae) Under Laboratory Conditions. Pak J Zool 49:163–170. https://doi.org/10.17582/journal.pjz/2017.49.1.163.170
Zhao Y, Wang Q, Wang Y et al (2017) Chlorfenapyr, a potent alternative insecticide of phoxim to control Bradysia odoriphaga (Diptera: Sciaridae). J Agric Food Chem 65:5908–5915. https://doi.org/10.1021/acs.jafc.7b02098
Acknowledgements
We would like to acknowledge CNPq (National Council for Scientific and Technological Development) for financial support of the present research (310356/2020-2). The manuscript is part of MCM MSc Dissertation.
Author information
Authors and Affiliations
Contributions
MCM, RMCF and JWSM conceived and designed the experiments. MCM, FWBS and RMCF conducted the experiments. MCM, DBL and JWSM analyzed the data. MCM, RMCF, DBL and JWSM wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Matos, M.C., Silva, F.W., Filgueiras, R.M. et al. Compatibility of pesticides with the predatory mite Neoseiulus barkeri. Exp Appl Acarol 92, 27–39 (2024). https://doi.org/10.1007/s10493-023-00865-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-023-00865-5