Abstract
Life expectancy, and longevity have been increasing in recent years. However, this is, in most cases, accompanied by age-related diseases. Thus, it became essential to better understand the mechanisms inherent to aging, and to establish biomarkers that characterize this physiological process. Among all biomolecules, lipids appear to be a good target for the study of these biomarkers. In fact, some lipids have already been associated with age-related diseases. With the development of analytical techniques such as Mass Spectrometry, and Nuclear Magnetic Resonance, Lipidomics has been increasingly used to study pathological, and physiological states of an organism. Thus, the study of serum, and plasma lipidome in centenarians, and elderly individuals without age-related diseases can be a useful tool for the identification of aging biomarkers, and to understand physiological aging, and longevity. This review focus on the importance of lipids as biomarkers of aging, and summarize the changes in the lipidome that have been associated with aging, and longevity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The aging process has been extensively studied over the years. One of the best-known definitions for aging is that of Alex Comfort (1956), who described this process as “a progressive increase throughout life, or after a given stadium, in the likelihood that a given individual will die, during the next succeeding unit of time, from randomly distributed causes”. In 1991 Rose (1991), in the book Evolutionary Biology of Aging, suggests a more general definition, characterizing aging as "a persistent decline in the age-specific fitness components of an organism due to internal physiological deterioration resulting in a loss of adaptive response to stress, and an increasing probability of death". According to this definition, as a consequence of aging, some visible changes occur in the individual, such as loss of mobility, and agility, muscle weakness, loss of reproductive capacity, and immune functions (known as immunosenescence), which results in a greater susceptibility to diseases such as Type 2 Diabetes Mellitus (DM2), infections, cancer, and autoimmune, cardiovascular, and neurodegenerative diseases, all known as age-related diseases (Chen and Liao 2007; López-Otín et al. 2013; Graça et al. 2017). The deterioration of the body's functions associated with aging is caused either by internal factors, such as oxidative stress (Pierce et al. 2008; Richardson and Schadt 2014), and DNA replication errors (Hoeijmakers 2009), which cause damage to DNA, proteins, and lipids (López-Otín et al. 2013) or by environmental factors, such as diet, sedentary lifestyle, and exposure to pollutants, microorganisms, and ionizing radiation (Nigam et al. 2012).
We live in a world with an increasingly aging population, so the study of aging is crucial (Carvalho 2012; He et al. 2016; Department of Economic and Social Affairs 2019). The birth rate worldwide has decreased in recent years (Sander et al. 2015), and the average life expectancy, and longevity have increased, especially in more developed countries, so it is expected that in the coming years people older than 65 will be the biggest percentage of world’s population (He et al. 2016). As a consequence of the physiological decline inherent of aging, age-related diseases can appear, which reduce the healthy life span (Rose 1991; Chen and Liao 2007; López-Otín et al. 2013; Graça et al. 2017), and increase the burden of healthcare systems, and families (World Health Organization 2015). To improve the quality of life of the elderly, we can study models of healthy aging, such as centenarians or groups of elderly people without associated diseases (Woo 2017). Studying the mechanisms, and possible biomarkers of healthy (physiological) aging may allow to intervene in the future in a preventive way through anti-aging therapies, which will delay the onset of age-related diseases, and increase the healthy lifespan (Seals et al. 2016; Magalhaes et al. 2018).
The investigation of biomarkers of aging, whether physiological or pathological, has been particularly important over the past years. According to the definition by George Baker and Richard Sprott (1988), a biomarker of physiological aging is “a biological parameter of an organism that either alone or in some multivariate composite will, in the absence of disease, better predict functional capability at some late age than will chronological age”. The American Federation for the Investigation of Aging has defined the criteria to categorize a molecule or panel of molecules as biomarkers of physiological aging: they must predict the rate of aging, be able to monitor the basic mechanisms that influence the aging processes, and not just the effects of associated pathologies, must be able to be tested repeatedly, and easily, without adverse effects, and be able to be measured, for example, in blood tests, and it should apply to both animals, and humans so that it can be tested on animals before being validated on humans (Johnson 2006).
To date, some parameters have been described that can be considered biomarkers of biological age. These include markers of physiological function, such as lung function, cardiovascular function, bone health, body composition, glucose metabolism, and levels of lipoproteins. Some molecular biomarkers have also been described, including biomarkers of the endocrine system, immune system, and nervous system, and some molecular mechanisms, such as oxidative stress, and genomic instability (Graça et al. 2017). Despite the extensive list of potential biomarkers of aging, none of those fits the definition of biomarkers mentioned before, since these biomarkers are mainly used to distinguish healthy, and unhealthy elderly people, as well as to differentiate people at higher risk of developing age-related diseases (Vasto et al. 2010). As such, they can be considered biomarkers of pathological aging, however none of them is specific enough to be considered a biomarker of physiological aging (Lara et al. 2015). Among all biomolecules, lipids appear to be an ideal target in the search for biomarkers. Some lipid alterations have already been associated with age-related diseases, and it is also known that these biomolecules are involved in many physiological processes that suffer alterations with aging. It has also been described that the lipid profile changes with age (Gonzalez-Covarrubias 2013). Thus, the study of the lipidome of the elderly without age-related diseases may prove to be a useful tool for understanding physiological aging.
This work aims to describe the importance of lipids as biomarkers of aging and summarize the changes in the lipidome that have been associated with aging, and longevity. Hopefully, it will help the reader to perceive how lipidome can help to understand age by identifying altered lipid pathways associated with physiological aging.
Metabolomics, and lipidomics applied to the study of aging
Metabolomics is one of the -omics disciplines of systems biology that, together with genomics, transcriptomics, and proteomics, studies the different organizational levels of a biological system (Boccard et al. 2009). More specifically, metabolomics studies the metabolome, which is the set of all biomolecules (metabolites) that result from the genomic, and proteomic activity, and their concentrations, and interactions in complex matrices, such as cells, tissues, biofluids, or organisms (Nicholson et al. 1999; Boccard et al. 2009; Soltow et al. 2010; Vivanco et al. 2010; Johnson et al. 2018). These metabolites are key factors in biological processes, and cellular metabolism, and therefore are representative of the general physiological state, in normal or pathological conditions (Soltow et al. 2010; Barallobre-Barreiro et al. 2013; Johnson et al. 2018). Metabolites can have an endogenous origin if they are compounds resulting from processes of biosynthesis or catabolic reactions or of exogenous origin if they derive from the food (nutrients), exposure to pollutants, or products of the metabolization of drugs (Boccard et al. 2009). The human metabolome consists of thousands of low molecular weight compounds (< 1000 Da), which can be divided into more than 50 chemical classes, including carbohydrates, vitamins, amino acids, fatty acids (FA) or lipids (Boccard et al. 2009; Wishart et al. 2009).
About 80% of the human metabolome is represented by lipids, and the complete set of all biological lipids is called lipidome (Ejsing et al. 2009; Psychogios et al. 2011). The plasma lipidome consists of thousands of lipids, which perform different functions, and have different structures (Quehenberger et al. 2010). Lipidomics is one of the approaches of metabolomics, and studies the lipidome of a cell, tissue, or organism, by measuring lipids (Ejsing et al. 2009; Gonzalez-Covarrubias 2013). Lipidomics allows the search of biomarkers to assist in the diagnosis, therapy, and prognosis of age-related diseases, and longevity (Gonzalez-Covarrubias 2013).
The role of lipids in the human body
Lipids are a large group of biomolecules that, despite being chemically different, are all insoluble in water. LIPID MAPS initiative created a lipid classification system that divides lipids into eight main categories: FA, glycerolipids, glycerophospholipids, sphingolipids, sterols, prenol lipids, saccharolipids, and polyketides (Fahy et al. 2005; LIPID MAPS 2017). Lipids are essential to the human body, and perform several functions, ranging from energy storage to regulating fluidity of cell membranes (Nelson and Cox 2005), not to mention its role in regulation and signaling, insulation, and protection (Berg et al. 2004). Glycerolipids, in particular triacylglycerols (TG), are the organism's main source of energy storage (Nelson and Cox 2005). Most TG energy reserves are found in adipocytes, cells specialized in their storage, and synthesis, and that are related to insulation, and protection function of this biologic molecule. The energy yield of TG, that results from the oxidation of the FA, is much higher than of other biomolecules (Berg et al. 2004).
Lipids are key components in the signal transduction pathway with products of lipid metabolism serving as intracellular messengers, such as phosphatidylinositol (PI) which, when hydrolyzed, gives rise to diacylglycerol (DAG), the best studied lipid with this function (Hannun 1997; Nelson and Cox 2005). They can be used to synthesize hormones, such as prostaglandins, thromboxanes, leukotrienes, and steroid hormones (progesterone, testosterone, estrogen, and cortisol), which are derived from arachidonic acid (AA), and cholesterol, respectively (Nelson and Cox 2005). So, lipids may be involved in cellular signaling processes such as inflammation, survival, and responses to cellular stress (Hannun and Obeid 2008). Phospholipids are the main constituents of cell membranes (Shevchenko and Simons 2010; Muro et al. 2014). Membrane lipids constitute a large group of structurally different lipids (Klose et al. 2013). It is this enormous diversity that justifies the properties of cell membranes, and the various biological processes in which lipids are involved, such as trafficking across the membrane, the regulation of membrane protein composition, and cell architecture, and the formation of lipid rafts—specific sub-compartments in membranes that contribute to cell function (Lee 2004; Shevchenko and Simons 2010; Bigay and Antonny 2012). According to the KEGG database (last accessed September 12th 2020), there are several processes involved in lipid metabolism in humans, such as FA biosynthesis, degradation, and elongation; synthesis, and degradation of ketone bodies; steroids, bile acids, and steroid hormones biosynthesis; glycerolipids, glycerophospholipids, ether lipids, sphingolipids, AA, and linoleic acid metabolism, and unsaturated FA biosynthesis.
Lipidomics in the study of aging biomarkers
Metabolites may be possible biomarkers because they represent several intermediate metabolic pathways (Nicholson et al. 1999; Soltow et al. 2010), and through the identification of changes in metabolites, it is possible to understand the interactions between gene expression, enzyme activity, and metabolic reactions, and relate them to the phenotype (Nicholson et al. 2005; Soltow et al. 2010). In clinical practice, lipidomics are used to establish lipidic profiles that allow to predict the progression of several diseases. To improve the prognostic, and diagnostic power of these tests, the lipidic profiles can be combined with risk factors, such as demographic risk factors (ethnicity, geography, age, and sex); lifestyle (smoking, physical inactivity, and inadequate diet), or physiological risk factors (body mass index (BMI), DM2, dyslipidemia) (Rasmiena et al. 2013).
Lipidomics appears to be an even more important field in the search for new biomarkers of physiological, and pathological aging (Ishikawa et al. 2014; Meikle et al. 2014) because they are involved in biological processes that change with age, and because they have already been linked to longevity in several long-lived animal species (Gonzalez-Covarrubias 2013; Naudí et al. 2013), and since several diseases have deregulation of lipids in its onset, studying the lipidome, can allow to predict the risk of developing a disease in the future, understand the pathological mechanisms, and monitor the response to therapies, and identify possible biomarkers for the diagnosis, and prognosis of these diseases (Laaksonen et al. 2006; Ng et al. 2012; Rasmiena et al. 2013; Ishikawa et al. 2014; Meikle et al. 2014; Mielke et al. 2014).
Several studies have shown that the composition of lipid cell membranes between vertebrates, invertebrates, and long-lived animals is different, and that the susceptibility of membrane lipids to peroxidation is inversely related to the longevity of the respective animal species (PAMPLONA et al. 2002; Hulbert et al. 2007; Pamplona 2008; Naudí et al. 2013). It is thought that the lipid profile of cell membranes has been optimized during evolution to be more resistant to oxidative stress. The evolution of aerobic life has resulted in the production of reactive oxygen species (ROS), leading to oxidative stress. For this reason, antioxidant defense systems emerged, and cell membranes underwent structural, and functional adaptations, which in turn determine the longevity of different organisms (Pamplona and Barja 2007; Pamplona 2008; Naudí et al. 2013). Jobson et al. (2010) tried to identify genetic targets of natural selection to increase longevity in mammals through a phylogenomic approach. They found that the genes involved in the lipid composition suffered an increased selective pressure in long-lived species, specifically genes involved in cellular lipid metabolism, like mitochondrial fatty acid synthase (FAS II) pathway genes Mecr, and Oxsm, Scd5 gene, and Elovl5 gene, all of them involved in FA elongation, and biosynthesis (Jobson et al. 2010). The plasma lipidome can serve as an improved resource to be associated with longevity, and is specific to each animal species (Jové et al. 2013; Bozek et al. 2017). Besides, some studies have associated mutations in genes related to lipid metabolism with increased lifespan in humans (Barzilai et al. 2001; Vaarhorst et al. 2011; Jové et al. 2017).
The analytical techniques most used in metabolomics studies are mass spectrometry (MS), and nuclear magnetic resonance (NMR) (Markley et al. 2017). MS-based technologies are the gold standard for metabolomics, and lipidomics studies (Boccard et al. 2009), and therefore have also been applied in the investigation of biomarkers of aging (Mishur and Rea 2012). MS allows the study metabolites using targeted or untargeted methodologies (Fiehn 2002; Boccard et al. 2009; Jové et al. 2016). Untargeted methods have been used to study changes in the lipidome associated with aging, and age-related diseases (Ishikawa et al. 2014; Meikle et al. 2014). Although not so widely used, NMR alone or coupled to MS can also be applied to metabolomics studies (Markley et al. 2017). Its main disadvantage is its lower sensitivity, when comparing to MS (Fan and Lane 2016). However, it has several advantages. With NMR, reproducible data, and accurate quantification of compounds are obtained, and it is possible to study different types of samples (cells, tissues). In vivo images can also be obtained, which allow studying the dynamics of metabolic pathways in tissues, and living organisms. NMR also allows to identify compounds with identical masses, and is more effective in determining structures of unknown compounds. This technique is also preferable for studying compounds that are difficult to ionize by MS (Fan and Lane 2016; Markley et al. 2017).
How the lipidome can help to understand disease: pathological aging
Due to the central role that lipids play in the body, changes in their metabolic pathways can lead to the development of diseases. Some of these diseases are hereditary, and occur due to deficiencies in enzymes that are involved in the metabolism of lipids, which leads to the deposition of these molecules, and their derivatives in cells. Some examples are Niemann-Pick, Fabry, and Gaucher diseases. Niemann-Pick disease develops due to the accumulation of cholesterol, and sphingomyelin in the tissues, due to a deficiency in the sphingomyelinase enzyme (Schuchman and Desnick 2017). Fabry's disease results from a deficiency in the enzyme α-galactosidase A, which leads to the accumulation of globotriaosylceramide in various tissues, and organs, including the skin, eyes, heart, kidneys, and vascular, and nervous systems (Yuasa et al. 2017). Gaucher's disease is due to a deficiency in the enzyme β-glucocerebrosidase, which leads to the accumulation of glucocerebroside in macrophages (Adar et al. 2016).
Several diseases can also arise with aging, such as atherosclerosis, DM2, arterial hypertension, dyslipidemia, cardiovascular, and neurodegenerative diseases (Belikov 2019). These pathologies are also associated with lipid changes, and disturbances in their metabolic pathways (Naudí et al. 2015; Hannun and Obeid 2018). Using metabolomics techniques, several authors have studied the plasma lipid profile characteristic of these pathologies (Kivipelto et al. 2002; Nelson et al. 2006; Bismuth et al. 2008; Li et al. 2009; Solomon et al. 2009; Graessler et al. 2009; Gall et al. 2010; Suhre et al. 2010; Hu et al. 2011; Spijkers et al. 2011; Han et al. 2011; Kulkarni et al. 2013; Whiley et al. 2014; González-Domínguez et al. 2014; Klavins et al. 2015; Marais 2015; Welty 2015; Hatano et al. 2015; Ramasamy 2016; Chan et al. 2017; LeWitt et al. 2017; Müller-Wieland et al. 2017; Taylor et al. 2017; Valaiyapathi et al. 2017; Zhang et al. 2017; Guedes et al. 2017; Staniszewska-slezak et al. 2018; Stoessel et al. 2018; Glaab et al. 2019; Hamid et al. 2019).
In hypertension, studies show differences in plasma levels of different lipid classes, between normotensive, and hypertensive individuals. In men, decreased plasma levels of phosphatidylethanolamine (PE) ethers, phosphatidylcholine (PC) ethers, in which AA is the most abundant FA moiety, are associated with pathogenesis of hypertension (Graessler et al. 2009). The increased plasma levels of ceramides in hypertensive individuals are observed, and this molecules could be associated to endothelial dysfunction, which contributes to hypertensive pathophysiology (Spijkers et al. 2011). Increased plasma levels of PC, and TG were observed in plasma samples from hypertensive individuals, and the lipid metabolism in this patients is different from that normotensive individuals (Hu et al. 2011). Some DAG species have been associated with high systolic, diastolic, and mean blood pressure values, and with a greater risk of normotensive individuals developing hypertension, what suggests an important role for the upregulation of the DAG axis in the pathogenesis of this disease (Kulkarni et al. 2013).
In DM2, some studies indicate that PC, lysophosphatidylcholines (LPC), and PC with polyunsaturated carbon chains are reduced, while PE were found at higher levels in plasma samples of diabetic individuals. This levels are associated with lower HDL, and total cholesterol levels, and higher TG levels in diabetic individuals (Suhre et al. 2010).Some free fatty acids (FFA) were found to be at increased levels in plasma of diabetic individuals (Li et al. 2009). The elevated levels of FFA, and lipids could be a factor for the development of DM2, and is associated with hyperinsulinemia, and insulin resistance (Shulman 20AD; Roden et al. 1996). It is described that there is a decrease in the concentration of lysophospholipids, and long-chain acylcarnitines with increased insulin resistance (Gall et al. 2010). Also, in diabetic mice there was an increase in plasma TG, and longer fatty acyl chains content, and this could be associated with dysregulated lipids metabolism in DM2 (Staniszewska-slezak et al. 2018).
Dyslipidemias consists of quantitative or qualitative changes in blood lipids (Ramasamy 2016). They can be divided into four different types: hypercholesterolemia, which corresponds to increased cholesterol levels (Taylor et al. 2017); hypertriglyceridemia, which corresponds to an increase in TG levels (Valaiyapathi et al. 2017); mixed dyslipidemia, which is a combination of the two previous types (Müller-Wieland et al. 2017), and the decrease in high-density lipoprotein (HDL) levels (Ramasamy 2016). There are other variants, such as hypobetalipoproteinemia, which is due to an increase in the number of low-density lipoprotein (LDL) receptors, and leads to a decrease in plasma cholesterol, abetalipoproteinemia, in which there is a deficiency in the secretion of lipoproteins containing apolipoprotein B (ApoB) (Welty 2015; Ramasamy 2016), and, finally, to dysbetalipoproteinemia or apolipoprotein E deficiency, in which an accumulation of TG-rich lipoproteins occurs (Marais 2015). Dyslipidemias are one of the main risk factors for atherosclerosis, and cardiovascular diseases (Ramasamy 2016). In atherosclerosis, Nelson et al. (2006) found that increased levels of plasma sphingomyelin (SM) are associated with subclinical disease, and severity, since plasma SM are involved in intermediate pathways between traditional risk factors, and subclinical atherosclerosis (Nelson et al. 2006). Also, the accumulation of ceramides seems to influence the development of atherosclerosis, since they are associated with the formation of foam cells, the aggregation of LDL, and the increase in ROS levels (Bismuth et al. 2008).
The neurodegenerative diseases most associated with the aging process are Alzheimer's disease (AD), and Parkinson's disease (PD) (Belikov 2019). In AD, higher levels of serum cholesterol during midlife have been associated with an increased risk of developing the disease, having a role in pathogenesis of the AD (Kivipelto et al. 2002), and greater cognitive impairment at older ages. This happens because the higher cholesterol levels in the midlife is associated with executive dysfunction, through increasing vascular pathology (Price et al. 2006). Other studies have shown that plasma levels of specific PC species are decreased, and the levels of two specific LPC species are increased in AD patients (Whiley et al. 2014; Klavins et al. 2015). Also, the ratio PC/LPC can differentiate between healthy individuals, individuals with mild cognitive impairment, and AD patients (Klavins et al. 2015). These results can be explained by aberrant activity of the enzyme phospholipase A2, what catalyze the cleavage of phospholipids in FA, and LPC. The overactivation of this enzyme is responsible for the abnormal metabolism of brain phospholipids, and changes in cell membranes (Farooqui et al. 2004). Moreover, β-amyloid42 peptides can increase the phospholipase A2 activity, and promotes alterations in physical properties of cell membrane (Hicks et al. 2008). González-Domínguez et al. (2014) found that the serum levels of PC with polyunsaturated fatty acids (PUFA), PE, LPC, lysophosphatidylethanolamines (LPE), and plasmalogens, plasmenylethanolamine, and plasmenylcholine, are decreased in these patients, whereas levels of certain PC species with saturated fatty acids (SFA), are increased. Once again, these observed lipid changes may be associated with overactivation of the phospholipase A2 enzyme (Farooqui et al. 2004). In the case of PCs, the overactivation of this enzyme may be related to the dysregulation of biosynthesis, and remodeling of the acyl chains, which will change the profile of FA (Farooqui et al. 1997). The imbalance between saturated FA, and unsaturated FA can also contribute to the alterations in cell membranes (González-Domínguez et al. 2014). The PE alterations are could be related with oxidative stress associated with AD, since they are rich in oxidable FA, and the brain is very susceptible to reactive oxygen species (Migliore et al. 2005; González-Domínguez et al. 2014). The decrease in plasmalogens may be associated with the dysfunction of peroxisomes (Kou et al. 2011), and with the increased activity of the enzyme plasmalogen-selective phospholipase A2 in the brain of AD patients (Farooqui 2010). In addition, plasmalogens are mainly constituted by polyunsaturated carbon chains, which makes them more susceptible to reactive oxygen species, and oxidative stress (Guan et al. 1999; González-Domínguez et al. 2014). In the brain of AD patients it has been described an impairment in metabolism of lysophospholipids, which results from the increased activity of the enzymes lysophospholipid acyltransferase, which recycles lysophospholipids into phospholipids, and lysophospholipase, which converts lysophospholipids in lysophosphatidic acid (Ross et al. 1998; Umemura et al. 2006). Lysophospholipids so can be considered intermediates in the neuronal metabolic pathways involved in the pathophysiology of AD (Frisardi et al. 2011). The levels of SM are decreased, especially those with long aliphatic chains in their composition, and the levels of ceramides are increased in these patients. The changes observed in the two lipid species seem to be involved in the inflammatory stress associated with neuronal apoptosis, and in the AD pathogenesis (Han et al. 2011).
In PD, some authors have found elevated levels of ganglioside GM3 (Chan et al. 2017), and ganglioside-NANA-3 (Zhang et al. 2017) in the plasma of patients. This two members of gangliosides family are precursors for the complex gangliosides of a- and b- series in the brain (Proia 2004; Blanz and Saftig 2016). Those gangliosides are metabolized by enzyme β-glucosidase (GCase), and the reduced GCase activity in the nervous system (Murphy et al. 2014) has been associated with PD (Papagiannakis et al. 2015; Kim et al. 2016), maybe because of the alteration in lipid metabolism by GCase (Chan et al. 2017). In addition, GM3 can regulate α-synuclein-induced channel formation through its binding to the protein's ganglioside-binding domain (GBD), thus being able to play a protective role in the pathogenesis of PD (Di Pasquale et al. 2010). The GM3 also can accelerate α-synuclein aggregation (Grey et al. 2015). The serum levels of some PUFA (eicosapentaenoate) appear to be decreased, what could be associated with α-synuclein patological aggregation (Assayag et al. 2007; Hatano et al. 2015). Plasma levels of some SFA (dodecanoic acid, and hexadecanoic acid) appear to be increased in these patients, which indicates that there is a greater breakdown of lipids, which may be associated with diet or the disease itself (Glaab et al. 2019). In addition, the accumulation of fatty acids may represent changes in their oxidation, which may be involved in the pathophysiology of PD (Ruipérez et al. 2010). Plasma levels of some N-acyl-phosphatidylethanolamines (NAPE) were decreased in patients with PD, and this panel of NAPEs allows to distinguish between women with, and without the disease. These decreased levels may be associated with the transfer of NAPEs from the plasma to the CNS (Hamid et al. 2019). Other authors have observed an increase in the levels of NAPEs in the brain of mice with PD (Basit et al. 2016). The levels of various FA, glycerophospholipids, and sphingolipids are altered in these patients: it has been observed an increase in plasma levels of some PC species, and platelet-activating factors, and a decrease of some PC, PE, and SM species in PD patients (Stoessel et al. 2018). These results indicate that PD is associated with changes in the glycerophospholipids, and sphingolipids metabolism (Kori et al. 2016). In another study, an increase in serum levels of LPE, lysophosphatidylserine (LPS), monohexosylceramide, and some plasmalogens, and SM species, and a decrease in serum levels of dihydrosphingomyelin, phosphatidic acid (PA), and some PE, PC, plasmalogens, and NAPE species were observed in patients’ carriers of the GBA mutation. The accumulation of lipids like monohexosylceramide in carriers of GBA mutation could be associated with disease development (Guedes et al. 2017). In addition, decreased levels of PE can lead to endoplasmic reticulum stress, and the accumulation of α-synuclein, and consequently, cell death (Wang et al. 2014). Some medium- and long-chain FA in plasma have a positive correlation with the disease, being predictive of its progression (LeWitt et al. 2017). In a study of rats treated with rotenone, an mitochondrial toxin, the authors observed that there were major changes in brain, and systemic fatty acid metabolism (Tyurina et al. 2015).
The study of the lipid profile of age-related diseases may, in the future, help to better understand them, and assist health professionals in their diagnosis, and treatment. In the same way, the study of the lipid profile of healthy elderly people can allow us to understand the differences in the metabolic pathways in physiological aging (Gonzalez-Covarrubias 2013).
Lipid changes in physiological and healthy aging
Throughout aging, there are changes in several physiological processes, which translates into changes in the lipidome. Therefore, the scientific community has joined efforts to understand which of these alterations can characterize the aging process, trying to assess which lipid classes suffered alterations over the years, in plasma, and serum samples, mainly using MS, and NMR-based approaches (Lawton et al. 2008; Lee et al. 2009; Yu et al. 2012; Collino et al. 2013; Gonzalez-covarrubias et al. 2013; Ishikawa et al. 2014; Montoliu et al. 2014; Mielke et al. 2015; Jové et al. 2016, 2017; Darst et al. 2019; Pradas et al. 2019; Wong et al. 2019).
Concerning to FA, different studies present different results. Collino et al. (2013) observed that the levels of 11,12- dihydroxy-eicosatrienoic acid (11,12-DiHETrE), 9-hydroxy-octadecadienoic acid (9-HODE), and 9-oxo-octadecadienoic acid (9-oxo-HODE) decreased in centenarians, on the opposite to the levels of 15-hydroxy-eicosatetraenoic acid (15-HETE), and leukotriene E4 (LTE4), which were found to increase, when compared to adults, and elderly groups. Other authors verified that the levels of several FA, and their derivatives, like AA (Lee et al. 2009), resolvin D6, hydroxyl FA, prostaglandin (Jové et al. 2016), and oxidized FA (oxFA) (in women) (Ishikawa et al. 2014) decrease with age, in plasma, and serum samples, and the levels of acylcarnitines (Yu et al. 2012; Darst et al. 2019), oxFA (in men) (Ishikawa et al. 2014), β-hydroxybutyrate (Lawton et al. 2008), long-chain FA, and others FA (Lawton et al. 2008; Darst et al. 2019) increase. Jové et al. (2017) show that increased levels of SFA, and longer carbon chains, and decreased levels of PUFA are associated with extreme longevity. The authors realized that the FA C16:0, and C22:1n-9 appear to be promising biomarkers of extreme longevity. Gonzalez-Covarrubias et al. (2013) found that the lipidome associated with longevity is characterized by a high monounsaturated fatty acids (MUFA)/PUFA ratio.
Regarding glycerolipids, Gonzalez-Covarrubias et al. (2013) found that there are decreased levels of long-chain TG in descendants of nonagenarians, similar to Montoliu et al. (2014), that observed a decrease in long-chain TG, and DAG, but also an increase in very long-chain TG in centenarians. Finally, Jové et al. found that the levels of DAG, and TG were at increased levels in groups of adults, and centenarians (Jové et al. 2017), and that the levels of monoacylglycerol 22:1, and DAG 33:2 were decreased with age (Jové et al. 2016). We can conclude that the differences observed in the levels of TG, and DAG between the studies, may be due to the increase or decrease in the levels of specific glycerolipid species.
Phospholipids are one of the classes that experience different changes during aging, either increased or decreased levels, depending on the species. For example, PC ethers have been associated with longevity, being found at high levels in the female descendants of nonagenarians (Gonzalez-covarrubias et al. 2013). In centenarians, polyunsaturated PC ethers were found at increased levels, while saturated PC ethers were found at decreased levels (Montoliu et al. 2014). Pradas et al. (2019) found that there was a general decrease in the levels of ether lipids in the plasma of centenarians comparatively with adults, and no differences comparatively to elderly. Also, they found that lipids with alkyl bonds were reduced in groups of elderly individuals, and centenarians compared to adults, and lipids with alkenyl bonds (plasmalogens) were reduced in centenarians compared to adults, and elderly subjects. These authors observed that there is a specific signature of ether lipids in the plasma of centenarians, with a significant increase of 15 ether lipids derived from PC with a predominant presence of the alkyl form, consisting of shorter carbon chains, and less content of double bonds; a significant decrease in 6 ether lipids derived from PE with a predominant presence of the alkenyl form, consisting of longer carbon chains, and greater content of double bonds. This specific lipid signature can mean greater resistance to lipid peroxidation in the centenarians, and consequently, to lower levels of oxidative stress, which is known to be related to exceptional longevity in various organisms, including humans (Pamplona and Barja 2007; Pradas et al. 2019). However, Collino et al. (2013) described a decrease in PC ethers in centenarians. In general, the levels of PC species appear to be increased in centenarians (Collino et al. 2013; Montoliu et al. 2014), and elderly men, and women (Ishikawa et al. 2014). Levels of diacyl phosphatidylcholine increased with age in women (Yu et al. 2012), and LPC levels decrease with age (Collino et al. 2013). Concerning the remaining phospholipids, Gonzalez-Covarrubias et al. (2013) observed that the levels of PE 38:6 were found to be decreased in female descendants of nonagenarians. Jové et al. (2017) found that the levels of specific species of LPC, LPE, lysophosphatidylglycerol (LPG), PA, PE, phosphatidylglycerol (PG), and phosphatidylserine (PS) were increased in young adults, and centenarians, while other specific species of LPE, lysophosphatidylinositol (LPI), PA, PG, and PS decreased. The variability in the results presented by the different studies for the levels of phospholipids is notorious. This difference may be related to the specific species of phospholipids that are analyzed, which may vary between studies. In addition, there is a vast amount of structurally different phospholipids that perform different body functions, integrating mechanisms that change with aging. Thus, it is expected that the levels of the various phospholipids will vary depending on the changes in the different pathways in which they are involved.
In general, there seems to be an increase in the levels of ceramides, and dihydroceramides with age (Ishikawa et al. 2014; Mielke et al. 2015). However, Jové et al. (2017) found decreased levels of ceramide d39:0, glucosylceramides, and ganglioside GM3 in adults, and centenarians. Regarding SM, they seem to increase in healthy aging (Yu et al. 2012; Collino et al. 2013), and are found at high levels in centenarians (Montoliu et al. 2014), and descendants of nonagenarians (Gonzalez-covarrubias et al. 2013). Darst et al. (2019) reported an increase in 24 sphingolipids with age. To the best of our knowledge, only Collino et al. (2013) described decreased levels of SM in centenarians.
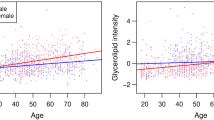
Regarding sterols, during the aging process it appears to be an increase in cholesterol, and cholesteryl esters levels (Lawton et al. 2008; Ishikawa et al. 2014; Jové et al. 2017). Nevertheless, Lawton et al. (2008) found that dehydroepiandrosterone sulfate (DHEA-S) levels decreased with age. Also, Darst et al. (2019) concluded that the 11-ketoethiocololone glucuronide, an androgenic steroid, and cortisol increased with age but the levels of 29 other sterols decreased with age. The levels of 7β-hydroxycholesterol, and 27-hydroxycolesterol were also found to decrease with age, after the age of 50 (Lee et al. 2009). Wong et al. (2019) realized that, in APOE ε3 homozygous individuals, there is a generalized, and significantly decrease in plasma levels of all lipid classes, except for DAG species, especially after 95 years of age. However, all lipid classes were negatively affected by age. This decrease is independent of sex, and BMI. The authors also describe a significant positive interaction of age with sex for species of ceramide, DAG, TG, PC, PE, and cholesteryl esters 20:X, and a more marked negative effect of age on men than on women. Tables 1, and 2 show the summary of the described lipid changes in serum, and plasma, respectively, in healthy aging, and longevity, divided by lipid classes.
Possible effect of dietary habits in lipid profile during aging
Dietary habits change throughout life. Therefore, it is expected that the basic diet of older individuals will be different from the diet of individuals of other ages (Yannakoulia et al. 2018; Kehoe et al. 2019). Changes in dietary habits in the elderly may be related to several factors, such as loss of appetite due to decreased sense of smell and taste, the interference of drugs with food intake, absorption and metabolism, the deterioration of oral health that requires the choice of some foods over others and social and psychological changes that can modify the way people eat (Ship 1999; Amarya et al. 2015; Pilgrim et al. 2015). Dietary habits thus influence the nutritional status of individuals, and in the elderly this can translate into malnutrition states (Zhu et al. 2010; Wysokiński et al. 2015). For example, several micronutrients, such as vitamins D, B2 and B12, folate and calcium are at levels below those recommended for the elderly (Kehoe et al. 2019). There are no significant changes in protein and carbohydrate intake with age (Yannakoulia et al. 2018), however there is an increase in salt (Cappuccio et al. 2015; Moreira et al. 2018) and sugar intake (Sluik et al. 2016; Ruiz et al. 2017).
In the elderly, there is a gradual decrease in the total intake of lipids (Anderson et al. 2011). More specifically, there is an increase in the intake of SFA (which should be as low as possible) and a decrease in the intake of MUFA and PUFA (Ter Borg et al. 2015). With regard to PUFAs, there appears to be a deficit in the intake of ω3-PUFA, such as α-linoleic acid, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in the elderly (Carrière et al. 2007). This specific group of PUFA is extremely important as it has numerous benefits. ω3-PUFA are associated with the maintenance of bone health and muscle tone, inhibit TG synthesis in the liver, decrease the inflammatory process and decrease the risk of cognitive performance decline associated with aging (Uauy and Valenzuela 2000; Farina et al. 2011; Kesse-Guyot et al. 2011; Smith et al. 2011; Úbeda et al. 2012). EPA and especially DHA further reduce the risk of cardiovascular and neurodegenerative diseases (Freund-Levi et al. 2006; Gebauer et al. 2006). Currently, there are already several dietary supplements in order to increase the levels of ω3-PUFA (mainly EPA and DHA) (Plourde and Cunnane 2007). Often, the ω3-PUFA used in supplements are derived from fish oils (Uauy and Valenzuela 2000; Hulbert et al. 2014).
It would make sense that changes in dietary lipid intake associated with aging would influence the plasma or serum lipidome. However, studies that referred to changes in the lipid profile induced by the diet in the elderly are missing. Abbott et al. (2012) tried to understand the relationship between the FA ingested in the diet and the phospholipids of the cell membranes and the TG of the adipose tissue and plasma. The authors concluded that diet SFA, MUFA and PUFA do not significantly influence membrane lipids. On the other hand, the composition of TG in adipose tissue and plasma appeared to be influenced in accordance with the dietary FA. It should be noted that some of the authors tried to minimize the influence of the diet on the lipidomic results by controlling the timing of sample acquisition and in some of the studies, blood samples were taken in the morning after donors fasted overnight (8h-14h) (Yu et al. 2012; Collino et al. 2013; Ishikawa et al. 2014; Montoliu et al. 2014; Jové et al. 2016; Darst et al. 2019; Pradas et al. 2019).
Altered lipidic pathways associated with physiological aging
Centenarians are recognized as representing the ideal physiological aging model, and therefore, are widely studied (Franceschi and Bonafè 2003; Franceschi et al. 2007). Although the causes of centenarians' longevity are not yet completely understood, it is believed that it may be due to a lower incidence of serious diseases (Barzilai et al. 2003; Andersen et al. 2012; Kheirbek et al. 2017), and the increased activity of protective mechanisms (Andersen et al. 2012). Although they also show signs of inflammation with aging, these individuals do not appear to suffer from its negative consequences. This is because there seems to be a balance between pro- and anti-inflammatory factors, which causes a slower, limited, and balanced increase in inflammation levels with age, compared to elderly non-centenarians (Franceschi and Bonafè 2003; Montoliu et al. 2014). As oxidative stress, and inflammation regulate a series of metabolic processes, it is possible to infer that the study of changes in the metabolome in these individuals allows to understand which are the processes inherent to physiological aging (Montoliu et al. 2014).
The role of different lipid classes in cell senescence
Sphingolipids
It has been reported that senescent cells have increased levels of glycerophosphocholine, reinforcing the importance of phospholipid metabolism in cellular senescence (Gey and Seeger 2013). Choline is associated to the synthesis of SM, and PC, from which DAG, and ceramides are produced (Tang et al. 2013; Montoliu et al. 2014). Sphingolipids appear to play an important role in healthy aging (Jové et al. 2017). SM can be constituents of cell membranes, and be associated with cholesterol, also forming lipid rafts, essential for the formation of the cell membrane, transport, and metabolism processes (Ito et al. 2000). In neuronal cells, they can even promote signal transduction (Yu et al. 2012). Also, some sphingolipids species, such as ceramides, are involved in cell signaling (Maceyka and Spiegel 2014), mediating processes such as cell senescence, and differentiation, autophagy, and apoptosis (Hannun and Obeid 2008, 2011). Oxidative stress accelerates the degradation of SM in ceramides. SMAses activity increases with age, which leads to a greater degradation of SM in ceramides (Smith et al. 2006; Smith and Schuchman 2008).
Phospholipids
As previously mentioned, changes in membrane's phospholipids will change its fluidity, which will interfere with the function of membrane proteins, and the transport of substances across the membrane (Shevchenko and Simons 2010). One of the compartments of cell membrane that suffer alterations with age is lipid rafts/caveolae, that include G protein-coupled receptor (Barnett-Norris et al. 2005; Naru et al. 2008). Caveolin-1, a component of caveolae, and whose levels increase with aging, induces changes in signal transduction, and in cell morphology associated with senescence (Park et al. 2000; Park 2005). Also, the G protein is sensible to alterations in the lipid composition of the cell membrane (Alemany et al. 2007), and senescence leads to changes in cellular properties, such as cell morphology, and loss of homeostasis (Naru et al. 2008). In senescent cells, there is a greater uptake of specific PC species stearic acid (C18:0), and AA (C20:4), and long-chain FA, so the expenditure of specific phospholipids may be related to membrane changes associated with cell senescence, therefore the importance of depicting the lipidome in chemical detail (Naru et al. 2008; Engelmann and Wiedmann 2010). The increase in uptake of PC species with long-chain FA can also be associated with the development of some age-associated diseases, such as cardiovascular diseases (Engelmann and Wiedmann 2010). These senescent-related changes can be associated with an increase in the surface area during senescence (Naru et al. 2008). Phospholipids, PC, and PE are the main components of cell membranes. It has also been previously described that the PC/PE ratio is an modulator of membrane integrity, since the alterations in PC levels could change the characteristics of lipid bilayer, and consequently their fluidity (Li et al. 2006). Also, PC/PE ratio could be associated with inflammation. The decrease in PC/PE ratio results in a change of membrane potential that allows the flow of ions, and ion radicals what is associated with the beginning of inflammation. The loss of membrane integrity also allows the influx of extracellular components, such as cytokines (Kmiec 2001; Li et al. 2006). It was proven that, in centenarians, and long-lived individuals, the lipid composition of erythrocyte membrane allows a higher membrane fluidity, and a decreased susceptibility to a peroxidation (Caprari et al. 1999; Rabini et al. 2002; Puca et al. 2008).
FA
Some studies report an inverse relationship between the content of double bonds of membrane lipids, and longevity in mammals (Pamplona et al. 1999).The susceptibility to lipid peroxidation increases exponentially with the increase in the number of FA double bonds (Hulbert et al. 2007). Decreased levels of PUFA are associated with less lipid peroxidation, and consequently, less oxidative damage (PAMPLONA et al. 2002).
Ether lipids
Plasmalogens are components of the cell membranes, and exist mainly in cardiac cells, and neurons. They perform several functions: participate in the fusion of membranes, in the signal transduction mediated by G protein, in the transport of cholesterol, and assist in the control of vesicular function, and the function of membrane proteins. Also, they are involved in maintaining some properties of the membranes, such as their fluidity, and the stability of the lipid raft microdomains. They may also be involved in cell signaling, giving rise to second messengers (Wallner and Schmitz 2011; Braverman and Moser 2012; Dean and Lodhi 2018). Plasmalogens could have an antioxidant effect, being able to protect other unsaturated membrane lipids from oxidation, since due to their vinyl ether bond, they are more susceptible to oxidation by reactive species (Goldfine 2010; Wallner and Schmitz 2011; Braverman and Moser 2012; Dean and Lodhi 2018; Pradas et al. 2019). Moreover, studies suggests that plasmalogens could protect unsaturated lipids from membranes from oxygen singlet oxidation (Broniec et al. 2011), and that plasmalogens can scavenging some ROS (Morand et al. 1988; Maeba et al. 2002; Skaff et al. 2008; Wu et al. 2019).
The plasma/serum lipidome, and aging
Sphingolipids
An increase in the concentration of SM may indicate a decrease in the risk of developing age-related diseases (Gonzalez-covarrubias et al. 2013). This is especially relevant in women, where higher levels of SM are associated with a lower risk of developing AD (Mas et al. 2012). Elevated levels of ceramides have been reported to regulate processes such as cell senescence, diabetes, insulin resistance, inflammation, neurodegenerative disorders, and atherosclerosis (Samad et al. 2006; Bismuth et al. 2008; Arana et al. 2010; Han et al. 2011; Spijkers et al. 2011; Guedes et al. 2017). Increased levels of SM, and decreased levels of ceramides can indicate greater protection against oxidative stress, and greater inclusion of SM in cell membranes, mechanisms that can be associated with healthy aging (Ichi et al. 2009; Corre et al. 2010; Gonzalez-covarrubias et al. 2013).
Phospholipids
The observed increase in the levels of PC, and PE in centenarians emphasizes that, in these individuals, there is greater conservation of cell membranes (Montoliu et al. 2014). PE is also a modulator of inflammation, and apoptosis (Gibellini and Smith 2010): PE 38:6, which is highly polyunsaturated, carries pro-inflammatory precursor molecules, such as those involved in the AA lipid pathway, from which eicosanoids are synthesized (Maskrey et al. 2007; Gonzalez-covarrubias et al. 2013). Decreased levels of this specific PE may suggest increased protection against lipid peroxidation, and the synthesis of pro-inflammatory molecules (Gonzalez-covarrubias et al. 2013). Another important lipid class for the inflammatory process is PI, from which AA is derived (Leslie 2015). Centenarians benefit from a perfect balance between pro- and anti-inflammatory eicosanoids, which translates into decreased inflammation due to their unique ability to modulate the AA metabolic pathway. This modulation can be proven with the increase in the levels of PI, and most of the PE species already reported (Montoliu et al. 2014). Also, changes in plasma phospholipid levels have been linked to impaired memory in elderly individuals (Mapstone et al. 2014).
Glycerolipids
Regarding TG, which may be associated with TG-rich lipoproteins, it is known that high levels of these molecules are related to an increased risk of developing some pathologies, such as atherosclerosis (Ramasamy 2016; Valaiyapathi et al. 2017). TG with long carbon chains, and highly unsaturated preferentially suffer peroxidation (Rhee et al. 2011). Since TG with long carbon chains undergo β-oxidation in mitochondria, and peroxisomes, and the metabolic capacity of these organelles decreases with age. The attenuation of the increase of unsaturated TG species may mean that, in physiological aging, there is a more efficient β-oxidation process (Gonzalez-covarrubias et al. 2013). Their levels increase significantly in women after menopause (Sugiyama and Agellon 2012). This increase is believed to be related to the decrease in estrogen secretion (Bjørnerem et al. 2004) since this hormone regulates lipoprotein metabolism (Knopp et al. 1994). Some TG species (with a higher number of carbons, and double bond content) have also been associated with a decreased risk of developing DM2 (Rhee et al. 2011).
FA
It is described in the literature that in adult individuals, and centenarians, there is a lower number of double bonds in FA than in the elderly. Therefore, in centenarians, lipids are not so prone to undergo lipid peroxidation, and this occurs at a similar level to that of young adults. So FAs such as C16:0, and C22:1n-9 appear to be promising biomarkers of extreme longevity (Jové et al. 2017; Pradas et al. 2019). Also, the ratio MUFA/PUFA is considered a biomarker of longevity (Caprari et al. 1999), and the conversion of polyunsaturated lipids to monounsaturated can be a hallmark of physiological aging (Gonzalez-covarrubias et al. 2013).
Ether lipids
Some studies show that plasmalogens are negatively related to several diseases in which there is an increase in oxidative stress, including age-related diseases, such as obesity, pre-diabetes, and DM2, cardiovascular diseases, cancer, and AD (Wallner and Schmitz 2011; Braverman and Moser 2012; Huynh et al. 2017; Meikle and Summers 2017; Dean and Lodhi 2018). Other studies indicate that there is a decrease in the levels of plasmalogens with age (Maeba et al. 2007, 2008). According to Pradas et al. (2019) in the centenarians there is an increase in the levels of PC ethers with alkyl groups, with shorter carbon chains, and lower number of double bonds, and a decrease in the levels of PE ethers with alkenyl groups, with longer carbon chains, and greater number of double bonds. The susceptibility of lipids to peroxidation increases as a function of the content of double bonds in carbon chains. Given this composition pattern, it can be concluded that the lipid ethers of the centenarians have a greater resistance to lipid peroxidation. The profile of ether lipids observed in centenarians indicate a physiological adaptation to an inherently low oxidative stress, which can be considered an optimized mechanism of exceptional longevity. This adaptation may be due to differences in the maintenance, and catabolism of poly and monounsaturated lipid species, and plasmalogens biosynthesis (Meikle and Summers 2017).
Conclusion
In a world in which life expectancy is increasing, it is necessary to understand the biochemical mechanisms that are related to aging. The use of metabolomic techniques has allowed the study of biochemical changes associated with various diseases, and physiological processes, such as aging. The development of these techniques, and the appearance of increasingly sophisticated devices allow for a better, and detailed analyses of the human metabolome in particular lipidome. It is possible to use plasma, serum, urine to search for new biomarkers, with the advantages of they are easily accessible since they are collected almost non-invasively.
During aging, there are disturbances in the metabolic pathways of the main biomolecules in the body. However, in physiological aging, these disorders are not so pronounced as in pathological aging, especially what concerns lipid metabolism. Based on that, one can say that the lipid signature varies with aging, and that in centenarians, descendants of centenarians, and elderly without age-related diseases, there are pronounced changes in the lipid profile. Table 3 summarizes putative biomarkers of longevity as well as putative biomarkers of healthy aging. In fact, healthy aging can be assessed by measuring molecules, in particular lipids, in biological fluids. Increases in PC ether 32:1, SM 24:1, SM 16:0, sterols and glycerolipids and decreased levels of and LPC 18:2, LPC 20:4 and sphingolipids are found in healthy aged individuals. These variations can be considered predators of healthy aging and reflects changes in metabolic lipidic pathways upon age and reflects in increased antioxidant capacity, and lower levels of lipid peroxidation, and inflammation.
Lipidomic methods of analysis are becoming more accessible, easier to implement and more sensitive. Thus, it is possible to identify well-known lipidic biomarkers in individuals in order to establish their molecular age. Lipidomic era makes possible to further investigate new biomarkers that characterize longevity, and that allow the development of therapies that may increase healthy life span, acting preventively avoid or slow the development of age-related diseases.
References
Abbott SK, Else PL, Atkins TA, Hulbert AJ (2012) Fatty acid composition of membrane bilayers: importance of diet polyunsaturated fat balance. Biochim Biophys Acta 1818:1309–1317. https://doi.org/10.1016/j.bbamem.2012.01.011
Adar T, Ilan Y, Elstein D, Zimran A (2016) Liver involvement in Gaucher disease—review and clinical approach. Blood Cells Mol Dis 68:66–73
Alemany R, Perona JS, Sánchez-Dominguez JM et al (2007) G protein-coupled receptor systems and their lipid environment in health disorders during aging. Biochim Biophys Acta 1768:964–975. https://doi.org/10.1016/j.bbamem.2006.09.024
Amarya S, Singh K, Sabharwal M (2015) Changes during aging and their association with malnutrition. J Clin Gerontol Geriatr 6:78–84. https://doi.org/10.1016/j.jcgg.2015.05.003
Andersen SL, Sebastiani P, Dworkis DA et al (2012) Health span approximates life span among many supercentenarians: compression of morbidity at the approximate limit of life span. J Gerontol 67:395–405
Anderson AL, Harris TB, Tylavsky FA et al (2011) Dietary patterns and survival of older adults. J Am Diet Assoc 111:84–91. https://doi.org/10.1016/j.jada.2010.10.012
Arana L, Gangoiti P, Ouro A et al (2010) Ceramide and ceramide 1-phosphate in health and disease. Lipids Health Dis 9:1–12
Assayag K, Yakunin E, Loeb V et al (2007) Polyunsaturated fatty acids induce alpha-synuclein- related pathogenic changes in neuronal cells. Am J Pathol 171:2000–2011
Barallobre-Barreiro J, Chung Y-L, Mayr M (2013) Proteomics and metabolomics for mechanistic insights and biomarker discovery in cardiovascular disease. Rev Española Cardiol (English Ed) 66:657–661
Barnett-Norris J, Lynch D, Reggio PH (2005) Lipids, lipid rafts and caveolae: their importance for GPCR signaling and their centrality to the endocannabinoid system. Life Sci 77:1625–1639. https://doi.org/10.1016/j.lfs.2005.05.040
Barzilai N, Gabriely I, Gabriely M et al (2001) Offspring of centenarians have a favorable lipid profile. J Am Geriatr Soc 49:76–79
Barzilai N, Atzmon G, Schechter C et al (2003) Unique lipoprotein phenotype and genotype associated with exceptional longevity. J Am Med Assoc 290:2030–2040
Basit A, Pontis S, Piomelli D, Armirotti A (2016) Ion mobility mass spectrometry enhances low-abundance species detection in untargeted lipidomics. Metabolomics 12:50. https://doi.org/10.1007/s11306-016-0971-3
Belikov AV (2019) Age-related diseases as vicious cycles. Ageing Res Rev 49:11–26
Berg JM, Tymoczko JL, Stryer L (2004) Bioquímica. Quinta. W. H Freeman and Company, Rio de Janeiro
Bigay J, Antonny B (2012) Curvature, Lipid packing, and electrostatics of membrane organelles: defining cellular territories in determining specificity. Dev Cell 23:886–895
Bismuth J, Lin P, Yao Q, Chen C (2008) Ceramide: a common pathway for atherosclerosis? Atherosclerosis 196:497–504
Bjørnerem Å, Straume B, Midtby M et al (2004) Endogenous sex hormones in relation to age, sex, lifestyle factors, and chronic diseases in a general population: the Tromsø study. J Clin Endocrinol Metab 89:6039–6047
Blanz J, Saftig P (2016) Parkinson’s disease: acid-glucocerebrosidase activity and alpha-synuclein clearance. J Neurochem 139:198–215. https://doi.org/10.1111/jnc.13517
Boccard J, Veuthey JL, Rudaz S (2009) Knowledge discovery in metabolomics: an overview of MS data handling. J Sep Sci 33:290–304
Bozek K, Khrameeva EE, Reznick J et al (2017) Lipidome determinants of maximal lifespan in mammals. Sci Rep 7:1–10
Braverman NE, Moser AB (2012) Functions of plasmalogen lipids in health and disease. Biochim Biophys Acta 1822:1442–1452
Broniec A, Klosinski R, Pawlak A et al (2011) Interactions of plasmalogens and their diacyl analogs with singlet oxygen in selected model systems. Free Radic Biol Med 50:892–898. https://doi.org/10.1016/j.freeradbiomed.2011.01.002.Interaction
Cappuccio FP, Ji C, Donfrancesco C et al (2015) Geographic and socioeconomic variation of sodium and potassium intake in Italy: results from the MINISAL-GIRCSI programme. BMJ Open 5:e007467. https://doi.org/10.1136/bmjopen-2014-007467
Caprari P, Scuteri A, Salvati AM et al (1999) Aging and red blood cell membrane: a study of centenarians. Exp Gerontol 34:47–57
Carrière I, Delcourt C, Lacroux A et al (2007) Nutrient intake in an elderly population in southern France (POLANUT): deficiency in some vitamins, minerals and ω-3 PUFA. Int J Vit Nutr Res 77:57–65. https://doi.org/10.1024/0300-9831.77.1.57
Carvalho A (2012) Censos 2011. Resultados DEFINITIVOS - Portugal, 15th edn. Lisboa
Chan RB, Perotte AJ, Zhou B et al (2017) Elevated GM3 plasma concentration in idiopathic Parkinson ’ s disease: a lipidomic analysis. PLoS ONE 12:1–13
Chen Y-J, Liao H-F (2007) NK/NKT cells and aging. Int J Gerontol 1:65–76
Collino S, Montoliu I, Martin J et al (2013) Metabolic signatures of extreme longevity in Northern Italian centenarians reveal a complex remodeling of lipids, amino acids, and gut microbiota metabolism. PLoS ONE 8:1–12
Comfort A (1956) The biology of senescence. Rinehart, New York
Corre I, Niaudet C, Paris F (2010) Plasma membrane signaling induced by ionizing radiation. Mutat Res 704:61–67
Darst BF, Koscik RL, Hogan KJ et al (2019) Longitudinal plasma metabolomics of aging and sex. Aging (Albany NY) 11:1262–1282
Dean JM, Lodhi IJ (2018) Structural and functional roles of ether lipids. Protein Cell 9:196–206
Di Pasquale E, Fantini J, Chahinian H et al (2010) Altered ion channel formation by the Parkinson’s-disease-linked E46K mutant of α-synuclein is corrected by GM3 but not by GM1 gangliosides. J Mol Biol 397:202–218. https://doi.org/10.1016/j.jmb.2010.01.046
Ejsing CS, Sampaio JL, Surendranath V et al (2009) Global analysis of the yeast lipidome by quantitative shotgun mass spectrometry. Proc Natl Acad Sci USA 106:2136–2141
Engelmann B, Wiedmann MKH (2010) Cellular phospholipid uptake: flexible paths to coregulate the functions of intracellular lipids. Biochim Biophys Acta 1801:609–616
Fahy E, Subramaniam S, Brown HA et al (2005) A comprehensive classification system for lipids. J Lipid Res 46:839–861
Fan TW-M, Lane AN (2016) Applications of NMR spectroscopy to systems biochemistry. Prog Nucl Magn Reson Spectrosc 92:18–53
Farina EK, Kiel DP, Roubenoff R et al (2011) Protective effects of fish intake and interactive effects of long-chain polyunsaturated fatty acid intakes on hip bone mineral density in older adults: the Framingham Osteoporosis Study. Am J Clin Nutr 93:1142–1151. https://doi.org/10.3945/ajcn.110.005926
Farooqui AA (2010) Studies on plasmalogen-selective phospholipase A2 in brain. Mol Neurobiol 41:267–273. https://doi.org/10.1007/s12035-009-8091-y
Farooqui AA, Yang HC, Horrocks L (1997) Involvement of phospholipase A2 in neurodegeneration. Neurochem Int 30:517–522. https://doi.org/10.1016/S0197-0186(96)00122-2
Farooqui AA, Ong WY, Horrocks LA (2004) Biochemical aspects of neurodegeneration in human brain: involvement of neural membrane phospholipids and phospholipases A2. Neurochem Res 29:1961–1977. https://doi.org/10.1007/s11064-004-6871-3
Fiehn O (2002) Metabolomics - The link between genotypes and phenotypes. Plant Mol Biol 48:155–171
Franceschi C, Bonafè M (2003) Centenarians as a model for healthy aging. Biochem Soc Trans 31:457–461
Franceschi C, Capri M, Monti D et al (2007) Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev 128:92–105
Freund-Levi Y, Eriksdotter-Jönhagen M, Cederholm T et al (2006) ω-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study—a randomized double-blind trial. Arch Neurol 63:1402–1408. https://doi.org/10.1001/archneur.63.10.1402
Frisardi V, Panza F, Seripa D et al (2011) Glycerophospholipids and glycerophospholipid-derived lipid mediators: a complex meshwork in Alzheimer’s disease pathology. Prog Lipid Res 50:313–330. https://doi.org/10.1016/j.plipres.2011.06.001
Gall WE, Beebe K, Lawton KA et al (2010) Αlpha-hydroxybutyrate is an early biomarker of insulin resistance and glucose intolerance in a nondiabetic population. PLoS ONE 5:e10883
Gebauer SK, Psota TL, Harris WS, Kris-Etherton PM (2006) n-3 Fatty acid dietary recommendations and food sources to achieve essentiality and cardiovascular benefits. Am J Clin Nutr 83:1526S-1535S. https://doi.org/10.1093/ajcn/83.6.1526s
Gey C, Seeger K (2013) Metabolic changes during cellular senescence investigated by proton NMR-spectroscopy. Mech Ageing Dev 134:130–138
Gibellini F, Smith TK (2010) The Kennedy pathway—de novo synthesis of phosphatidylethanolamine and phosphatidylcholine. IUBMB Life 62:414–428
Glaab E, Trezzi JP, Greuel A et al (2019) Integrative analysis of blood metabolomics and PET brain neuroimaging data for Parkinson’s disease. Neurobiol Dis 124:555–562
Goldfine H (2010) The appearance, disappearance and reappearance of plasmalogens in evolution. Prog Lipid Res 49:493–498
Gonzalez-covarrubias V, Beekman M, Uh H et al (2013) Lipidomics of familial longevity. Aging Cell 12:426–434
Gonzalez-Covarrubias V (2013) Lipidomics in longevity and healthy aging. Biogerontology 14:663–672
González-Domínguez R, García-Barrera T, Gómez-Ariza JL (2014) Combination of metabolomic and phospholipid-profiling approaches for the study of Alzheimer’s disease. J Proteomics 104:37–47
Graça A, Magalhães S, Nunes A (2017) Biological predictors of aging and potential of FTIR to study age-related diseases and aging metabolic fingerprint. Curr Metab 5(2):119–137
Graessler J, Schwudke D, Schwarz PEH et al (2009) Top-down lipidomics reveals ether lipid deficiency in blood plasma of hypertensive patients. PLoS ONE 4:e6261
Grey M, Dunning CJ, Ricardo G et al (2015) Acceleration of α-synuclein aggregation by exosomes. J Biol Chem 290:2969–2982. https://doi.org/10.1074/jbc.M114.585703
Guan Z, Wang Y, Cairns NJ et al (1999) Decrease and structural modifications of phosphatidylethanolamine plasmalogen in the brain with Alzheimer disease. J Neuropathol Exp Neurol 58:740–747. https://doi.org/10.1097/00005072-199907000-00008
Guedes LC, Chan RB, Gomes MA et al (2017) Serum lipid alterations in GBA-associated Parkinson’s disease. Park Relat Disord 44:58–65
Hamid Z, Basit A, Pontis S et al (2019) Gender specific decrease of a set of circulating N-acylphosphatidyl ethanolamines (NAPEs) in the plasma of Parkinson’s disease patients. Metabolomics 15:1–9
Han X, Rozen S, Boyle SH et al (2011) Metabolomics in early Alzheimer ’ s disease: identification of altered plasma sphingolipidome using shotgun lipidomics. PLoS ONE 6:e21643
Hannun YA (1997) Chapter 21: lipids as second messengers. Princ Med Biol 7:487–513. https://doi.org/10.1016/S1569-2582(97)80129-8
Hannun YA, Obeid LM (2008) Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol 9:139–150
Hannun YA, Obeid LM (2011) Many ceramides. J Biol Chem 286:27855–27862
Hannun YA, Obeid LM (2018) Sphingolipids and their metabolism in physiology and disease. Nat Rev Mol Cell Biol 19:175–191
Hatano T, Saiki S, Okuzumi A et al (2015) Identification of novel biomarkers for Parkinson’s disease by Metabolomic technologies. J Neurol Neurosurg Psychiatry 87:295–301
He W, Goodkind D, Kowal P (2016) An aging world: 2015 International Population Reports. Aging (Albany NY) 165:P95/09-1
Hicks JB, Lai Y, Sheng W et al (2008) Amyloid-β peptide induces temporal membrane biphasic changes in astrocytes through cytosolic phospholipase A2. Biochim Biophys Acta 1778:2512–2519. https://doi.org/10.1016/j.bbamem.2008.07.027
Hoeijmakers JH (2009) DNA damage, aging, and cancer. N Engl J Med 361:1475–1485
Hu C, Kong H, Qu F et al (2011) Application of plasma lipidomics in studying the response of patients with essential hypertension to antihypertensive drug therapy. Mol Biosyst 7:3271–3279
Hulbert AJ, Pamplona R, Buffenstein R, Buttemer WA (2007) Life and death: metabolic rate, membrane composition, and life span of animals. Physiol Rev 87:1175–1213
Hulbert AJ, Kelly MA, Abbott SK (2014) Polyunsaturated fats, membrane lipids and animal longevity. J Comp Physiol B Biochem Syst Environ Physiol 184:149–166. https://doi.org/10.1007/s00360-013-0786-8
Huynh K, Martins RN, Meikle PJ (2017) Lipidomic profiles in diabetes and dementia. J Alzheimer’s Dis 59:433–444
Ichi I, Kamikawa C, Nakagawa T et al (2009) Neutral sphingomyelinase-induced ceramide accumulation by oxidative stress during carbon tetrachloride intoxication. Toxicology 261:33–40
Ishikawa M, Maekawa K, Saito K et al (2014) Plasma and serum lipidomics of healthy white adults shows characteristic profiles by subjects’ gender and age. PLoS ONE 9:1–12
Ito J, Nagayasu Y, Yokoyama S (2000) Cholesterol–sphingomyelin interaction in membrane and apolipoprotein-mediated cellular cholesterol efflux. J Lipid Res 41:894–904
Jobson RW, Nabholz B, Galtier N (2010) An evolutionary genome scan for longevity-related natural selection in mammals. Mol Biol Evol 27:840–847
Johnson TE (2006) Recent results: biomarkers of aging. Exp Gerontol 41:1243–1246. https://doi.org/10.1016/j.exger.2006.09.006
Johnson LC, Martens CR, Santos-Parker JR et al (2018) Amino acid and lipid associated plasma metabolomic patterns are related to healthspan indicators with ageing. Clin Sci 132:1765–1777
Jové M, Naudí A, Aledo JC et al (2013) Plasma long-chain free fatty acids predict mammalian longevity. Sci Rep 3:1–8
Jové M, Maté I, Naudí A et al (2016) Human aging is a metabolome-related matter of gender. J Gerontol 71:578–585
Jové M, Naudí A, Gambini J et al (2017) A stress-resistant lipidomic signature confers extreme longevity to humans. J Gerontol 72:30–37
Kehoe L, Walton J, Flynn A (2019) Nutritional challenges for older adults in Europe: current status and future directions. Proc Nutr Soc 78:221–233. https://doi.org/10.1017/S0029665118002744
Kesse-Guyot E, Péneau S, Ferry M et al (2011) Thirteen-year prospective study between fish consumption, long-chain N-3 fatty acids intakes and cognitive function. J Nutr Heal Aging 15:115–120. https://doi.org/10.1007/s12603-011-0023-7
Kheirbek RE, Fokar A, Shara N et al (2017) Characteristics and incidence of chronic illness in community- dwelling predominantly male U.S Veteran Centenarians. J Am Geriatr Soc 65:2100–2106
Kim HJ, Jeon B, Song J et al (2016) Leukocyte glucocerebrosidase and β-hexosaminidase activity in sporadic and genetic Parkinson disease. Park Relat Disord 23:99–101. https://doi.org/10.1016/j.parkreldis.2015.12.002
Kivipelto M, Helkala E, Laakso MP et al (2002) Apolipoprotein E epsilon 4 allele, elevated midlife total cholesterol level, and high midlife systolic blood pressure are independent risk factors for late-life Alzheimer disease. Ann Intern Med 137:149–155. https://doi.org/10.7326/0003-4819-137-3-200208060-00006
Klavins K, Koal T, Dallmann G et al (2015) The ratio of phosphatidylcholines to lysophosphatidylcholines in plasma differentiates healthy controls from patients with Alzheimer ’ s disease and mild cognitive impairment. Alzheimer’s Dement Diagn Assess Dis Monit 1:295–302
Klose C, Surma MA, Simons K (2013) Organellar lipidomics—background and perspectives. Curr Opin Cell Biol 25:406–413
Kmiec Z (2001) Cooperation of liver cells in health and disease. Adv Anat Embryol Cell Biol 161:1–151. https://doi.org/10.1007/978-3-642-56553-3
Knopp RH, Zhu X, Bonet B (1994) Effects of estrogens on lipoprotein metabolism and cardiovascular disease in women Robert. Atherosclerosis 110:S83–S91
Kori M, Aydln B, Unal S et al (2016) Metabolic biomarkers and neurodegeneration: a pathway enrichment analysis of Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis. OMICS J Integr Biol 20:645–661. https://doi.org/10.1089/omi.2016.0106
Kou J, Kovacs GG, Höftberger R et al (2011) Peroxisomal alterations in Alzheimer’s disease. Acta Neuropathol 122:271–283. https://doi.org/10.1007/s00401-011-0836-9
Kulkarni H, Meikle PJ, Mamtani M et al (2013) Plasma lipidomic profile signature of hypertension in mexican american families: specific role of diacylglycerols. Hypertension 62:621–626
Laaksonen R, Katajamaa M, Päivaä H et al (2006) A systems biology strategy reveals biological pathways and plasma biomarker candidates for potentially toxic statin-induced changes in muscle. PLoS ONE 1:1–9
Lara J, Cooper R, Nissan J et al (2015) A proposed panel of biomarkers of healthy ageing. BMC Med 13:1–8. https://doi.org/10.1186/s12916-015-0470-9
Lawton KA, Berger A, Mitchell M et al (2008) Analysis of the adult human plasma metabolome. Pharmacogenomics 9:383–397
Lee AG (2004) How lipids affect the activities of integral membrane proteins. Biochim Biophys Acta 1666:62–87
Lee J, Seet RCS, Huang SH et al (2009) Different patterns of oxidized lipid products in plasma and urine of dengue fever, stroke, and parkinson ’ s disease patients: cautions in the use of biomarkers of oxidative stress. Antioxid Redox Signal 11:407–420
Leslie CC (2015) Cytosolic phospholipase A2: physiological function and role in disease. J Lipid Res 56:1386
LeWitt PA, Li J, Lu M et al (2017) Metabolomic biomarkers as strong correlates of Parkinson disease progression. Neurology 88:862–869
Li Z, Agellon LB, Allen TM et al (2006) The ratio of phosphatidylcholine to phosphatidylethanolamine influences membrane integrity and steatohepatitis. Cell Metab 5:321–331
Li X, Xu Z, Lu X et al (2009) Comprehensive two-dimensional gas chromatography/time-of-flight mass spectrometry for metabonomics: biomarker discovery for diabetes mellitus. Anal Chim Acta 633:257–262
LIPID MAPS (2017) Lipid Classification System
López-Otín C, Blasco MA, Partridge L et al (2013) The hallmarks of aging. Cell. https://doi.org/10.1016/j.cell.2013.05.039
Maceyka M, Spiegel S (2014) Sphingolipid metabolites in inflammatory disease. Nature 510:58–67
Maeba R, Sawada Y, Shimasaki H et al (2002) Ethanolamine plasmalogens protect cholesterol-rich liposomal membranes from oxidation caused by free radicals. Chem Phys Lipids 120:145–151. https://doi.org/10.1016/S0009-3084(02)00101-9
Maeba R, Maeda T, Kinoshita M et al (2007) Plasmalogens in human serum positively correlate with high-density lipoprotein and decrease with aging. J Atheroscler Thromb 14:12–18
Maeba R, Hara H, Ishikawa H et al (2008) Myo-inositol treatment increases serum plasmalogens and decreases small dense LDL, particularly in hyperlipidemic subjects with metabolic syndrome. J Nutr Sci Vitaminol (Tokyo) 54:196–202
Magalhaes S, Goodfellow BJ, Nunes A (2018) Aging and proteins: what does proteostasis have to do with age? Curr Mol Med 18:178–189. https://doi.org/10.2174/1566524018666180907162955
Mapstone M, Cheema AK, Fiandaca MS et al (2014) Plasma phospholipids identify antecedent memory impairment in older adults. Nat Med 20:415–418. https://doi.org/10.1038/nm.3466
Marais D (2015) Dysbetalipoproteinemia: an extreme disorder of remnant metabolism. Curr Opin Lipidol 26:292–297
Markley JL, Brüschweiler R, Edison AS et al (2017) The future of NMR-based metabolomics. Curr Opin Biotechnol 43:34–40
Mas E, Croft KD, Zahra P et al (2012) Resolvins D1, D2, and other mediators of self-limited resolution of inflammation in human blood following n-3 fatty acid supplementation. Clin Chem 58:1476–1484
Maskrey BH, Bermu A, Morgan AH et al (2007) Activated platelets and monocytes generate four hydroxyphosphatidylethanolamines via lipoxygenase. J Biol Chem 282:20151–20163
Meikle PJ, Summers SA (2017) Sphingolipids and phospholipids in insulin resistance and related metabolic disorders. Nat Rev Endocrinol 13:79–91
Meikle PJ, Wong G, Barlow CK, Kingwell BA (2014) Lipidomics: Potential role in risk prediction and therapeutic monitoring for diabetes and cardiovascular disease. Pharmacol Ther 143:12–23
Mielke MM, Haughey NJ, Bandaru VVR et al (2014) CSF sphingolipids, β-amyloid, and tau in adults at risk for Alzheimer’s disease. Neurobiol Aging 35:2486–2494
Mielke MM, Venkata V, Bandaru R et al (2015) Demographic and clinical variables affecting mid- to late-life trajectories of plasma ceramide and dihydroceramide species. Aging Cell 14:1014–1023
Migliore L, Fontana I, Colognato R et al (2005) Searching for the role and the most suitable biomarkers of oxidative stress in Alzheimer’s disease and in other neurodegenerative diseases. Neurobiol Aging 26:587–595. https://doi.org/10.1016/j.neurobiolaging.2004.10.002
Mishur RJ, Rea SL (2012) Applications of mass spectrometry to metabolomics and metabonomics: detection of biomarkers of aging and of age-related diseases. Mass Spectrom Rev 31:70–95
Montoliu I, Scherer M, Beguelin F et al (2014) Serum profiling of healthy aging identifies phospho - and sphingolipid species as markers of human longevity. Aging (Albany NY) 6:9–25
Morand OH, Zoeller RA, Raetz CRH (1988) Disappearance of plasmalogens from membranes of animal cells subjected to photosensitized oxidation. J Biol Chem 263:11597–11606. https://doi.org/10.1016/s0021-9258(18)38001-3
Moreira P, Sousa AS, Guerra RS et al (2018) Sodium and potassium urinary excretion and their ratio in the elderly: results from the nutrition UP 65 study. Food Nutr Res. https://doi.org/10.29219/fnr.v62.1288
Müller-Wieland D, Leiter LA, Cariou B et al (2017) Design and rationale of the ODYSSEY DM-DYSLIPIDEMIA trial: Lipid-lowering efficacy and safety of alirocumab in individuals with type 2 diabetes and mixed dyslipidaemia at high cardiovascular risk. Cardiovasc Diabetol 16:1–10
Muro E, Atilla-gokcumen GE, Eggert US, Bement W (2014) Lipids in cell biology: how can we understand them better? Mol Bio Cell 25:1819–1823
Murphy KE, Gysbers AM, Abbott SK et al (2014) Reduced glucocerebrosidase is associated with increased α-synuclein in sporadic Parkinson’s disease. Brain 137:834–848. https://doi.org/10.1093/brain/awt367
Naru E, Takanezawa Y, Kobayashi M et al (2008) Increased levels of a particular phosphatidylcholine species in senescent human dermal fibroblasts in vitro. Hum Cell 21:70–78
Naudí A, Jové M, Ayala V et al (2013) Membrane lipid unsaturation as physiological adaptation to animal longevity. Front Physiol 4:1–13
Naudí A, Cabré R, Jové M et al (2015) Lipidomics of human brain aging and Alzheimer’s disease pathology. Int Rev Neurobiol 122:1–57
Nelson DL, Cox MM (2005) Chapter 10: lipids. In: Nelson DL, Cox MM (eds) Lehninger: principles of biochemistry, 4th edn. WH Freeman, New York
Nelson JC, Jiang X, Tabas I et al (2006) Plasma Sphingomyelin and subclinical atherosclerosis: findings from the multi-ethnic study of atherosclerosis. Am J Epidemiol 163:903–912
Ng TW, Khan AA, Meikle PJ (2012) Investigating the pathogenesis and risk of Type 2 diabetes: clinical applications of metabolomics. Clin Lipidol 7:641–659
Nicholson JK, Lindon JC, Holmes E (1999) “Metabonomics”: understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica 29:1181–1189
Nicholson JK, Holmes E, Wilson ID (2005) Gut microorganisms, mammalian metabolism and personalized health care. Nat Rev Microbiol 3:431–438
Nigam Y, Knight J, Bhattacharya S, Bayer A (2012) Physiological changes associated with aging and immobility. J Aging Res 2012:1–2. https://doi.org/10.1155/2012/468469
Pamplona R (2008) Membrane phospholipids, lipoxidative damage and molecular integrity: a causal role in aging and longevity. Biochim Biophys Acta 1777:1249–1262
Pamplona R, Barja G (2007) Highly resistant macromolecular components and low rate of generation of endogenous damage: two key traits of longevity. Ageing Res Rev 6:189–210. https://doi.org/10.1016/j.arr.2007.06.002
Pamplona R, Portero-Otín M, Ruiz C et al (1999) Double bond content of phospholipids and lipid peroxidation negatively correlate with maximum longevity in the heart of mammals. Mech Ageing Dev 112:169–183
Pamplona R, Barja G, Portero-Otín M (2002) Membrane fatty acid unsaturation, protection against oxidative stress, and maximum life span: a homeoviscous-longevity adaptation? Ann NY Acad Sci 959:475–490
Papagiannakis N, Xilouri M, Koros C et al (2015) Lysosomal alterations in peripheral blood mononuclear cells of Parkinson’s disease patients. Mov Disord 30:1830–1834. https://doi.org/10.1002/mds.26433
Park SC (2005) New molecular target for modulation of aging process. Antioxid Redox Signal 8:620–627. https://doi.org/10.1089/ars.2006.8.620
Park WY, Park JS, Cho KA et al (2000) Up-regulation of caveolin attenuates epidermal growth factor signaling in senescent cells. J Biol Chem 275:20847–20852. https://doi.org/10.1074/jbc.M908162199
Pierce A, Mirzaei H, Muller F et al (2008) GAPDH is conformationally and functionally altered in association with oxidative stress in mouse models of amyotrophic lateral sclerosis. J Mol Biol 382:1195–1210
Pilgrim AL, Robinson SM, Sayer AA, Roberts HC (2015) An overview of appetite decline in older people. Nurs Older People 27:29–35. https://doi.org/10.7748/nop.27.5.29.e697
Plourde M, Cunnane SC (2007) Extremely limited synthesis of long chain polyunsaturates in adults: implications for their dietary essentiality and use as supplements. Appl Physiol Nutr Metab 32:619–634. https://doi.org/10.1139/H07-034
Pradas I, Jové M, Huynh K et al (2019) Exceptional human longevity is associated with a specific plasma phenotype of ether lipids. Redox Biol 21:1–9
Price JF, McDowell S, Whiteman MC et al (2006) Ankle brachial index as a predictor of cognitive impairment in the general population: ten-year follow-up of the Edinburgh Artery Study. J Am Geriatr Soc 54:763–769. https://doi.org/10.1111/j.1532-5415.2006.00702.x
Proia RL (2004) Gangliosides help stabilize the brain. Nat Genet 36:1147–1148. https://doi.org/10.1038/ng1104-1147
Psychogios N, Hau DD, Peng J et al (2011) The human serum metabolome. PLoS ONE 6:1–23
Puca AA, Andrew P, Novelli V et al (2008) Fatty acid profile of erythrocyte membranes as possible biomarker of longevity. Rejuvenation Res 11:63–72
Quehenberger O, Armando AM, Brown AH et al (2010) Lipidomics reveals a remarkable diversity of lipids in human plasma. J Lipid Res 51:3299–3305
Rabini RA, Moretti N, Staffolani R et al (2002) Reduced susceptibility to peroxidation of erythrocyte plasma membranes from centenarians. Exp Gerontol 37:657–663
Ramasamy I (2016) Update on the molecular biology of dyslipidemias. Clin Chim Acta 454:143–185
Rasmiena AA, Ng TW, Meikle PJ (2013) Metabolomics and ischaemic heart disease. Clin Sci 124:289–306
Rhee EP, Cheng S, Larson MG et al (2011) Lipid profiling identifies a triacylglycerol signature of insulin resistance and improves diabetes prediction in humans. J Clin Invest 121:1402–1411
Richardson AG, Schadt EE (2014) The role of macromolecular damage in aging and age-related disease. J Gerontol 69:S28–S32
Roden M, Price TB, Perseghin G et al (1996) Mechanism of free fatty acid–induced insulin resistance in humans. J Clin Invest 97:2859–2865
Rose RM (1991) Evolutionary biology of aging. Oxford University Press, Oxford
Ross BM, Moszczynska A, Erlich J, Kish SJ (1998) Phospholipid-metabolizing enzymes in Alzheimer’s disease: increased lysophospholipid acyltransferase activity and decreased phospholipase A2 activity. J Neurochem 70:786–793. https://doi.org/10.1046/j.1471-4159.1998.70020786.x
Ruipérez V, Darios F, Davletov B (2010) Alpha-synuclein, lipids and Parkinson’s disease. Prog Lipid Res 49:420–428. https://doi.org/10.1016/j.plipres.2010.05.004
Ruiz E, Rodriguez P, Valero T et al (2017) Dietary intake of individual (Free and intrinsic) sugars and food sources in the Spanish population: findings from the ANIBES study. Nutrients 9:275. https://doi.org/10.3390/nu9030275
Samad F, Hester KD, Yang G et al (2006) Altered adipose and plasma sphingolipid metabolism in obesity: a potential mechanism for cardiovascular and metabolic risk. Diabetes 55:2579–2587. https://doi.org/10.2337/db06-0330
Sander M, Oxlund B, Jespersen A et al (2015) The challenges of human population ageing. Age Ageing 44:185–187. https://doi.org/10.1093/ageing/afu189
Schuchman EH, Desnick RJ (2017) Types A and B Niemann-pick disease. Mol Genet Metab 120:27–33
Seals DR, Justice JN, Larocca TJ (2016) Physiological geroscience: targeting function to increase healthspan and achieve optimal longevity. J Physiol 594:2001–2024
Shevchenko A, Simons K (2010) Lipidomics: coming to grips with lipid diversity. Nat Rev Mol Cell Biol 11:593–598
Ship JA (1999) The influence of aging on oral health and consequences for taste and smell. Physiol Behav 66:209–215. https://doi.org/10.1016/S0031-9384(98)00267-4
Shulman GI (2000) Cellular mechanisms of insulin resistance. J Clin Invest 106:171–176
Skaff O, Pattison DI, Davies MJ (2008) The vinyl ether linkages of plasmalogens are favored targets for myeloperoxidase-derived oxidants: a kinetic study. Biochemistry 47:8237–8245. https://doi.org/10.1021/bi800786q
Sluik D, van Lee L, Engelen AI, Feskens EJM (2016) Total, free, and added sugar consumption and adherence to guidelines: the Dutch National Food Consumption Survey 2007–2010. Nutrients 8:70. https://doi.org/10.3390/nu8020070
Smith EL, Schuchman EH (2008) The unexpected role of acid sphingomyelinase in cell death and the pathophysiology of common diseases. FASEB J 22:3419–3431
Smith AR, Visioli F, Frei B, Hagen TM (2006) Age-related changes in endothelial nitric oxide synthase phosphorylation and nitric oxide dependent vasodilation: evidence for a novel mechanism involving sphingomyelinase and ceramide-activated phosphatase 2A. Aging Cell 5:391–400
Smith GI, Atherton P, Reeds DN et al (2011) Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: a randomized controlled trial. Am J Clin Nutr 93:402–412. https://doi.org/10.3945/ajcn.110.005611.INTRODUCTION
Solomon A, Kareholt I, Ngandu T et al (2009) Serum total cholesterol, statins and cognition in non-demented elderly. Neurobiol Aging 30:1006–1009
Soltow QA, Jones DP, Promislow DEL (2010) A network perspective on metabolism and aging. Integr Comp Biol 50:844–854
Spijkers JA, Van Den ARFP, Janssen BJA et al (2011) Hypertension is associated with marked alterations in sphingolipid biology: a potential role for ceramide. PLoS ONE 6:1–9
Sprott RL (1988) Biomarkers of aging. Exp Gerontol 23:223–239
Staniszewska-slezak E, Wiercigroch E, Fedorowicz A et al (2018) A possible FTIR-based plasma fingerprint of ACE-I induced reversal of endothelial dysfunction in diabetic mice. J Biophoton 11:1–11
Stoessel D, Schulte C, Teixeira dos Santos MC et al (2018) Promising metabolite profiles in the plasma and CSF of early clinical Parkinson’s disease. Front Aging Neurosci 10:1–14
Sugiyama MG, Agellon LB (2012) Sex differences in lipid metabolism and metabolic disease risk. Biochem Cell Biol 90:124–141
Suhre K, Meisinger C, Döring A et al (2010) Metabolic footprint of diabetes: a multiplatform metabolomics study in an epidemiological setting. PLoS ONE 5:e13953
Tang WHW, Wang Z, Levison BS et al (2013) Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med 368:1575–1584
Taylor B, Cheema A, Soslowsky L (2017) Tendon pathology in hypercholesterolemia and familial hypercholesterolemia. Curr Rheumatol Rep 19:17–22
Ter Borg S, Verlaan S, Mijnarends DM et al (2015) Macronutrient intake and inadequacies of community-dwelling older adults, a systematic review. Ann Nutr Metab 66:242–255. https://doi.org/10.1159/000435862
Tyurina YY, Polimova AM, Maciel E et al (2015) LC/MS analysis of cardiolipins in substantia nigra and plasma of rotenone-treated rats: implication for mitochondrial dysfunction in Parkinson’s disease. Free Radic Res 49:681–691. https://doi.org/10.3109/10715762.2015.1005085
Uauy R, Valenzuela A (2000) Marine oils: The health benefits of n-3 fatty acids. Nutrition 16:680–684. https://doi.org/10.1016/S0899-9007(00)00326-9
Úbeda N, Achón M, Varela-Moreiras G (2012) Omega 3 fatty acids in the elderly. Br J Nutr 107:S137–S151. https://doi.org/10.1017/S0007114512001535
Umemura K, Yamashita N, Yu X et al (2006) Autotaxin expression is enhanced in frontal cortex of Alzheimer-type dementia patients. Neurosci Lett 400:97–100. https://doi.org/10.1016/j.neulet.2006.02.008
Department of Economic and Social Affairs (2019) World population prospects 2019: highlights. In: United Nations
Vaarhorst AAM, Beekman M, Suchiman EHD et al (2011) Lipid metabolism in long-lived families: the Leiden Longevity Study. Age (Omaha) 33:219–227
Valaiyapathi B, Sunil B, Ashraf AP (2017) Approach to Hypertriglyceridemia in the pediatric population. Pediatr Rev 38:424–434
Vasto S, Scapagnini G, Bulati M et al (2010) Biomarkes of aging. Front Biosci S2:72. https://doi.org/10.2741/s72
Vivanco F, Barderas MG, Laborde CM et al (2010) Metabolomic profiling for identification of novel potential biomarkers in cardiovascular diseases. J Biomed Biotechnol 2011:1–9
Wallner S, Schmitz G (2011) Plasmalogens the neglected regulatory and scavenging lipid species. Chem Phys Lipids 164:573–589
Wang S, Zhang S, Liou LC et al (2014) Phosphatidylethanolamine deficiency disrupts α-synuclein homeostasis in yeast and worm models of Parkinson disease. Proc Natl Acad Sci USA 111:E3976–E3985. https://doi.org/10.1073/pnas.1411694111
Welty FK (2015) Hypobetalipoproteinemia and Abetalipoproteinemia. Curr Opin Lipidol 25:161–168
Whiley L, Sen A, Heaton J et al (2014) Evidence of altered phosphatidylcholine metabolism in Alzheimer’s disease. Neurobiol Aging 35:271–278
Wishart DS, Knox C, Guo AC et al (2009) HMDB: a knowledgebase for the human metabolome. Nucleic Acids Res 37:603–610
Wong MWK, Braidy N, Pickford R et al (2019) Plasma lipidome variation during the second half of the human lifespan is associated with age and sex but minimally with BMI. PLoS ONE 14:1–22
Woo J (2017) Designing Fit for Purpose Health and Social Services for Ageing Populations. Int J Environ Res Public Health 14:457. https://doi.org/10.3390/ijerph14050457
World Health Organization (2015) World report on ageing and health. World Health Organization, Geneva
Wu Y, Chen Z, Darwish WS et al (2019) Choline and ethanolamine plasmalogens prevent lead-induced cytotoxicity and lipid oxidation in HepG2 cells. J Agric Food Chem 67:7716–7725. https://doi.org/10.1021/acs.jafc.9b02485
Wysokiński A, Sobów T, Kłoszewska I, Kostka T (2015) Mechanisms of the anorexia of aging—a review. Age (Omaha) 37:9821. https://doi.org/10.1007/s11357-015-9821-x
Yannakoulia M, Mamalaki E, Anastasiou CA et al (2018) Eating habits and behaviors of older people: where are we now and where should we go? Maturitas 114:14–21. https://doi.org/10.1016/j.maturitas.2018.05.001
Yu Z, Zhai G, Singmann P et al (2012) Human serum metabolic profiles are age dependent. Aging Cell 11:960–967
Yuasa T, Takenaka T, Higuchi K et al (2017) Fabry disease. J Echocardiogr 15:151–157
Zhang J, Zhang X, Wang L, Yang C (2017) High performance liquid chromatography-mass spectrometry (LC-MS) based quantitative lipidomics study of ganglioside-NANA-3 plasma to establish its association with Parkinson’s disease patients. Med Sci Monit 23:5345–5353
Zhu K, Devine A, Suleska A et al (2010) Adequacy and change in nutrient and food intakes with aging in a seven-year cohort study in elderly women. J Nutr Heal Aging 14:723–729. https://doi.org/10.1007/s12603-010-0324-2
Funding
This work was supported by projects UID/BIM/04501/2013 and P2020-PTDC/DTP-PIC/5587/2014, co-funded by Fundação para a Ciência e Tecnologia (FCT) I.P., PIDDAC and by European Regional Development Fund (FEDER), POCI-01-0145-FEDER007628 and the Integrated Programme of SR&TD “pAGE” (Reference CENTRO-01-0145-FEDER-000003), co-funded by Centro 2020 program, Portugal 2020, European Union, through the European Regional Development Fund. SM is supported by FCT through the individual PhD Grant SFRH/BD/131820/2017.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declared that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Almeida, I., Magalhães, S. & Nunes, A. Lipids: biomarkers of healthy aging. Biogerontology 22, 273–295 (2021). https://doi.org/10.1007/s10522-021-09921-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10522-021-09921-2