Abstract
Despite its rare occurrence, humans and animals have been prone to getting fast developing severe hypobaric hypoxia. Understanding the redox homeostasis related response of an aging heart to this type of hypoxia are crucially important, since the metabolism of myocardial tissue depends on the redox status of proteins. Rodents can tolerate hypoxic stress better than human subjects. This study was aimed at investigating the effects of fast developing severe hypobaric hypoxia on redox status biomarkers; such as, advanced oxidation protein products (AOPP), lipid hydroperoxides (LHPs), protein carbonyl groups (PCO), protein thiol groups (P-SH), and total thiol groups (T-SH) on the myocardial left ventricles of young and aged Wistar rats. The rats were gradually ascended and exposed to an 8000-meter hypobaric hypoxia. While AOPP levels showed no difference, the TSH and PSH concentrations decreased, and the PCO and LHP increased in both of the hypoxic groups than the controls. The TSH and PSH were lower, and AOPP, PCO and LHP were found to be higher in the elderly hypoxic groups than in the young ones. The significant outcome of the study represents that an 8000-meter hypobaric hypoxia could be considered as a severe hypoxic stress, but not life-treating for the rats and would affect both the young and aged left ventricles similarly in respect to impaired redox status. However, if the percentage increases are taken into consideration, it seems that the higher rate of protein oxidation occurs in young hearts; meanwhile aged hearts are more prone to T-SH oxidation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypobaric hypoxia is a deleterious condition that exposes cells to an inadequate oxygen supply due to the insufficient saturation of blood with oxygen at high altitudes. People gradually climb up high altitudes in order to ensure a proper acclimatization however; in some extreme cases there is no sufficient time to achieve adequate acclimatization. For example, helicopter and fighter pilots could fly up over 10,000 feet (~ 2500 meters) with a face mask for an oxygen supply (Thurber 2010) and aircrafts are able to fly at the extreme altitudes of 30,000–40,000 feet (~ 9000–12,000 meters) with the cabin pressurized to approximately 8000 feet (~ 2500 meters) (The Boeing Company 2016). In case of the occurrence of some technical malfunctions while flying over 10,000 feet, helicopter and fighter pilots may experience a sudden and extreme hypobaric hypoxia. Commercial aircrafts may lose their cabin pressure while ascending to higher altitudes after take-off during a flight without any indication of a major emergency situation, like the Helios aircraft accident in 2005 (Albright 2015). Not only human subjects but also rodents could experience this type of hypoxia during space flights (Chris 2014). Fast developing severe hypoxic hypoxia for human subjects would generally be life-threatening and may lead to the sudden death of passengers. In fact, an 8000 m altitude for humans has been described as the death zone (Parati et al. 2014, p. 29). Albeit, for rats this extreme altitude could be considered as a severe hypobaric hypoxic condition, and may not be considered as a life treating situation. Since rodents utilize behavioral thermoregulatory responses in order to lower their body temperature during the hypoxia (Gordon and Fogelson 1991), which leads to reduce their oxygen demand when the oxygen supply is limited (Dupré and Owen 1992). It appears that in the current literature of studies related to hypoxia the difference between humans’ and rats’ hypoxia tolerance had been ignored. In fact, the hypoxia-tolerance of both the species was considered to be similar in these studies. In the present study, we took into consideration this difference.
Human and animal subjects have a great potential to experience fast developing extreme hypoxia and the function of myocardial tissue depends on sustainable oxygen supply under this extreme condition (Clarke 2006; Virués-Ortega et al. 2006; Wilson et al. 2009). The contractile activity of the human heart increases in order to compensate for the oxygen deprivation at hypoxia (Zeng et al. 2014). Therefore, it is assumed that the left ventricle would be the most effected part of heart at hypoxia since the labor of mitochondria augments for ATP production and mitochondria turns out to be reactive oxygen species (ROS) producing the focal point at hypoxia (Magalhães et al. 2005; Murray 2009).
As it is in hypoxic exposure, throughout the aging process, excessive amounts of ROS are mainly derived from mitochondria with a progressively reduced efficacy of an antioxidant defense mechanism (Andriollo Sanchez et al. 2005). Moreover, the ageing process may lead to detrimental consequences for left ventricle functions (Hollingsworth et al. 2012). Therefore, it is expected that the left ventricles of elderly subjects are more prone to ROS mediated oxidative attacks during an acute severe hypobaric hypoxia condition.
Proteins are the main targets of ROS (Du and Gebicki 2004) due to their high concentrations in cells and are able to eliminate 50–75% of the total ROS (Kalousová et al. 2005). The protein oxidation among the other type of macromolecular oxidations has been known to play a primary role in the aging process (Çakatay et al. 2001, 2003; Stadtman and Levine 2000), and also in the hypoxia condition (Magalhães et al. 2005). Oxidatively modified proteins interfere with cellular metabolic functions either by the loss of catalytic and structural integrity or by the disruption of regulatory pathways in cardiac tissue (Cebe et al. 2014; Simsek et al. 2019). Among the various oxidative products of proteins, PCO formation is one of the well-recognized modifications of ROS- mediated protein oxidation. The determination of the groups’ PCO levels has been accepted as a sensitive evaluation for the assessment of the extent of oxidative protein damage in myocardia tissue (Cebe et al. 2014; Simsek et al. 2019). As for being a sensitive marker of ROS mediated protein oxidation, which measures the PCO levels in the left ventricle would provide us with an idea about the effects of fast-developing severe hypoxia. The –SH moieties of cysteine residues in myocardial proteins are highly vulnerable to an ROS attack by various mechanisms, which form disulfide bonds and thiol radicals (Cebe et al. 2014; Simsek et al. 2019). Furthermore, intra- or inter-protein cross-linked oxidation products can be formed by the oxidation of –SH groups (Cebe et al. 2014; Simsek et al. 2019). –SH groups of proteins form a fraction of both non-enzymatic antioxidant systems and free radical hazard biomarkers. T-SH fraction comprises both P-SH and Np-SH (non-protein –SH) fractions (Cebe et al. 2014). The most abundant fraction of Np-SH also includes a reduced form of glutathione (GSH) (Cebe et al. 2014; Çakatay et al. 2003; Dringen et al. 2000). It was reported that GSH transforms into its oxidized form by mitochondrial ROS at hypoxia (Mansfield et al. 2004) and T-SH level decreases during hypoxia (Dalle-Donne et al. 2003). Moreover, the presence of strong negative correlations among P-SH, T-SH and PCO was reported (Çakatay et al. 2000). The assessment of –SH groups of myocardial proteins in the left ventricles of old and young rats would be a reliable marker of the severity of ROS induced oxidative protein damage and the efficiency of non-enzymatic antioxidant systems after severe hypobaric hypoxia. AOPPs are formed mainly as a result of the action of neutrophil-derived chlorinated compounds, which leads to the formation of dityrosine containing cross-linked protein products. AOPPs are considered to be a reliable marker to estimate the degree of oxidant-mediated protein damage (Alderman et al. 2002; Çakatay et al. 2003). It was reported that plasma levels of AOPP after hypoxic exposure at 5700-meter and 6300-meter altitudes show no significant difference (Devi et al. 2007) in rats, but after hypoxic exercise, it was shown that it tends to increase (Pialoux et al. 2006).
LHPs, which are initial-products of oxidized lipids, have detrimental oxidative effects on myocardial cells via highly toxic hydroxyl radicals. LHPs may also react with transition metals such as iron or copper to generate reactive aldehydes that oxidatively damage cellular membranes (Stadtman 2004). LHPs are useful biomarkers of cardiac lipid peroxidation since they are the primary products of ROS (Cebe et al. 2014; Simsek et al. 2019). Thiobarbituric acid reactive substances (TBARS) and Malondialdehyde (MDA) are widely used conventional markers of oxidized lipids and are reported to be higher in elderly people (Matés et al. 2002). It was reported that LHP levels were found to be elevated in cardiac muscle, blood (Devi et al. 2007) and skeletal muscle (Çakatay et al. 2003) of old male and/or female rats.
Today, not only pilots or passengers but also experimental animals are more likely to develop a severe hypobaric hypoxia condition during flights or spaceflights. Rats are expected to tolerate an extreme hypoxia like 8000-meter altitude, which is known as the death zone for a human. The cardiomyocytes of aged rats are more likely to be vulnerable to oxidative protein damage than their younger counterparts at fast developing severe hypobaric hypoxia, since the formation of ROS mediated oxidative injury increases in the myocardial ageing process. Therefore, it is extremely important to understand the redox status of aged myocardial left ventricles at fast developing severe hypobaric hypoxia. Furthermore, to the best of our knowledge, there has been no study related to reveal the extent of oxidative protein damage in the left ventricles of young and aged rats exposed to fast-developing severe hypobaric hypoxia. Therefore, the aim of the study was to investigate changes in the levels of various ROS mediated proteins and lipid oxidation products in myocardial tissue of the left ventricles of aged and young rats during the condition of fast developing severe hypobaric hypoxia.
Materials and methods
Chemicals
All reagents used were of analytical grade. Deionized water was used for the preparation of reagents. Reagents were stored at + 4 °C and were equilibrated at room temperature for 30 min prior to use.
Apparatus
All centrifugation procedures were performed with a Z 323 K cooled centrifuge (Hermle, Germany). PCO, T-SH, AOPP, and LHP concentrations were assessed with Spectra max plus 384 (Molecular Devices., USA) Spectrophotometer.
Experimental animals and procedures
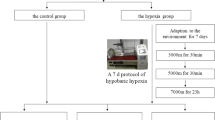
Adult male young (4 months, 200–300 grams) and old (24 months, 300–450 grams) Wistar Albino rats were supplied by the Experimental Animal Production and Research Center, Başkent University, Ankara, Turkey. The young and old rats were housed in conventional wire-mesh cages, two rats per cage, in a temperature regulated room at 21 ± 1 °C, humidity at 45–50% and 12-h light–dark cycles at Ankara University Medical Faculty, Department of Physiology Animal Care and Research Center. All animals were given ad libitum free access to natural food and water by drinking bottle throughout the housing period and the course of the experiment. They were fed with a standard laboratory diet. Interventions concerning experimental animals were performed according to ‘‘Principles of Laboratory Animal Care’’ (NIH publication No. 85-23, revised 1985).
The old and young rats were divided into two groups each as the control and hypobaric hypoxic groups (six animals for each of the four groups). The animals were exposed to hypobaric hypoxia corresponding to an altitude of 8000 meters (~ 26,247 feet) for 6 h in a hypoxic chamber after gradual adaptation over a period of 40–45 min. After simulating 1600 meters of altitude, the rats were kept for 5 min at every ascending 800 meters until reaching the simulated 8000 meters. The re-pressurization process was applied in a similar manner until descent to Ankara’s altitude level (938 meters above sea level). Both the depressurization and the re-pressurization processes were modified according to the Helios accident (Air-Accident-Investigation-Aviation-Safety-Board 2006), and the studies of Magalhães et al. (2005) and Udayabanu et al. (2008). The chamber’s temperature was maintained at 21 ± 1 °C during the experiment. The control group was also kept in the hypobaric chamber without any hypoxic treatment in order to experience the stress of the hypobaric chamber prior to being euthanized. The specially designed hypobaric chamber used in the research simulated an atmospheric pressure of 37.8 kPa (284 mm Hg), which is equivalent to an altitude of 8000 meters (Höpfl et al. 2003).
At the end of the experiment, the rats were put to sleep by intramuscular injection of ketamine hydrochloride (Ketasol 10%, Richter-Pharma, Wels, Austria) and xylasine (Alfazyne 2%, Alfasan, Woerden, The Netherlands). This study was approved by Hacettepe University Animal Welfare Committee (07.11.2006, 2006/65).
Preparation of tissue samples
The extracted hearts of the rats were excised immediately and immersed in physiological saline. The major heart vessels, valves and atria were trimmed away and the left ventricles were cut open and rinsed free of blood. The left ventricles were snap frozen in liquid nitrogen and kept at − 80 °C until the day of assay. The left ventricles (50–80 mg) were diluted to a 1:21 ratio with an ice-cold homogenizing buffer (KH2PO4-K2HPO4, 100 mM, pH 7.4), and then homogenized with a homogenizator X250D (CAD, Germany). The homogenate was centrifuged at 2800×g for 10 min with a Z 323 K cooled centrifuge (Hermle, Germany). Various analytic determinations were performed in the supernatant fraction.
Analytical methods
Determination of protein carbonyl groups
PCO concentrations were assessed spectrophotometrically using Reznick and Packer’s method (Reznick and Packer 1994). PCO groups react with 2,4-dinitrophenylhydrazine (DNPH) to generate chromophoric dinitrophenylhydrazones. The supernatant fraction was transferred to a plastic tube, left for 15 min at room temperature, and then a streptomycin sulfate solution (10%, w/v) was added to a final concentration of 1% to precipitate any extracted DNA which could react with DNPH and contribute to the carbonyl level. The DNPH was dissolved in HC, accompanied by blanks in HCl alone. After the DNPH reaction, the proteins were precipitated with an equal volume of 20% (w/v) trichloroacetic acid (TCA) and washed three times with 4 ml of an ethanol/ethyl acetate mixture (1:1). Washings were achieved by a mechanical disruption of pellets in the washing solution using a small spatula, and re-pelleting by centrifugation at 6000×g for 5 min. Finally, the precipitates were dissolved in 6 mol/l guanidine-HCl solution and the absorbance was measured at 360 nm, using the molar extinction coefficient of DNPH of ε = 22,000 L mol−1 cm−1.
Determination of thiol groups
Myocardial T-SH and Np-SH concentrations were analyzed by using 5,5-dithiobis (2-nitrobenzoic acid) (DTNB) reagent (Sedlak and Lindsay 1968). Some modifications were realized from the previously described T-SH method in order to apply small volumes of samples. Aliquots of 20 µl of tissue homogenates were mixed in 1.5 ml test tubes with 400 µl of 0.2 M Tris buffer, pH 8.2, and 20 µl of 0.0 l M DTNB for the determination of T-SH groups. Np-SH samples were assayed in the following way: Aliquots of 20 µl of the homogenates were mixed in 400 µl of 50% TCA. The tubes were shaken intermittently for 10 min and centrifuged at 3000×g for 15 min. Supernatant fractions were assayed for T-SH. The absorbance values were read at 412-nm against a reagent blank with no homogenate. The value of molar extinction coefficient of thiol groups at wavelength 412 nm is ε = 13,100 L mol−1 cm−1. The protein bound thiol groups (P-SH) were calculated by subtracting the Np-SH from T-SH (Sedlak and Lindsay 1968).
Determination of advanced protein oxidation product groups
Spectrophotometric determination of myocardial AOPP levels was performed by volumetric modification of Hanasand’s method (Hanasand et al. 2012). Samples were prepared in the following manner: 10 µl of supernatant, 40 µl of PBS, and 200 µl citric acid solution (20 mmol/L) were mixed in a microplate. One minute later, 10 µl of 1.16 M potassium iodide was added to each of the microplate wells. The absorbance of the reaction mixture was immediately read at 340 nm against a blank. The absorbance of chloramine-T standards was run in duplicate in order to increase the precision of the assay. The related absorbance was linear within the range of 0–100 µmol/L. AOPP concentration was expressed as micromoles per liter of chloramine-T equivalents. All readings were performed within 2 min after a potassium iodide addition, for avoiding an uncontrollable color development which leads to a possible deviation from the chloramine standards curve. The AOPP-bovine serum albumin (BSA) positive control and untreated BSA were prepared in vitro and tested according to the AOPP assay protocol (Zeng et al. 2014).
Determination of lipid hydroperoxides
The ferrous oxidation with xylenol orange, version 2 (FOX2) colorimetric method (Wolff 1994) was used to assess lipid peroxidation in the left ventricle homogenates. The recipe for measuring LHP is as follows: 100 mM xylenol orange, 250 mM ammonium ferrous sulfate, 90% methanol (HPLC grade), 4 mM butylated hydroxytoluene, 25 mM H2SO4 were used in the assay. 50 μL of the sample were added to 950 mL (in a 1-milliliter micro centrifuge vial), vortexed, and incubated at room temperature for 30 min. The absorbance value was read at 560 nm after the removal by centrifugation of any flocculated material. The signal was read against a standard curve prepared from H2O2.
Determination of protein concentrations
Protein concentrations in the heart left ventricle were determined according to the Bradford Method (Bradford 1976).
Statistics
The SPSS 25.0 program was used for data analysis. PCO, AOPP, P-SH, T-SH and LHP levels of the left ventricle of the heart were compared using both the Kruskal–Wallis non-parametric and ANOVA (analysis of variance) parametric test. Since all data were normally distributed, the results of the ANOVA test were given. Student ‘t’ tests were also used to determine which group created the difference. Descriptive statistics were given as mean ± SEM. Correlation coefficients were calculated using the Pearson’s correlation. The percentage increases of ROS markers were also calculated with the following formula of “[(the data of hypoxic animals—the data of control animals)/the data of control animals]*100″ for the myocardial left ventricles in both the aged and young rats after hypoxic exposure. When not specified, p < 0.05 was considered significant.
Results
Throughout the experimental protocol all experimental animals survived and we did not lose any rats before the scarification process. Rats preferred to keep motionless in the hypoxic chamber during the hypoxic exposure period. They were observed as responsive to external stimulation and in a conscious state at the end of re-pressurization.
Specific redox status biomarkers were used to determine the extent of oxidative damage in myocardial tissue of the young and aged rat groups after fast-developing severe hypobaric hypoxia. In both the young and aged hypobaric hypoxic groups, the PCO and LHP were higher than their respective control groups (p < 0.001 for each). The PCO and LHP of the aged hypobaric hypoxic groups were also higher than those of the young hypobaric hypoxic groups (p < 0.001 for each). The AOPP levels in the both aged and young hypobaric hypoxic groups were higher than those of their respective control groups (p < 0.001 for each). Moreover, the AOPP was found to be high in the aged hypobaric hypoxic group relative to the young hypobaric hypoxic group (p < 0.001). The T-SH and P-SH levels in the young and aged hypobaric hypoxic groups were found to be lower in relation to their respective control groups (p < 0.001 for all groups). The T-SH and P-SH of the aged hypobaric hypoxic group were lower than those of the young hypobaric hypoxic group (p < 0.05 for each) (see Table 1). A high negative correlation was observed between P-SH and PCO (Figs. 1 and 2).
The results of the percentage increases of the ROS markers were contrary to the expected outcomes of the statistical analysis of the ROS markers (Table 2). The highest percentage increases of PCO, LHP and AOPP were calculated in the myocardial left ventricles of the young rats and the lowest percentage decreases in T-SH and P-SH were observed in those of the aged rats after hypoxic exposure.
Discussion
Both human subjects and experimental animals may experience fast developing severe hypobaric hypoxia even within 30 min of the takeoff, during flight or spaceflight. The 8000-meter altitude above the sea level was known as a death zone for humans (Parati et al. 2014, p. 29), but it seems that this type of hypoxia could be considered as a severe hypoxic stress condition but not fatal for the rodents. The formation of ROS in aging heart tissue augments due to mitochondrial impairment at hypoxia (Magalhães et al. 2005; Solaini and Harris 2005). The aging heart with its impaired redox homeostasis is considered as one of the most affected organs from hypoxia (Gianni et al. 2004; Magalhães et al. 2005; Virués-Ortega et al. 2006; Wilson et al. 2009) and the left ventricle is the most effected part of heart from hypoxia due its main responsibility related to pumping blood throughout the circulatory system. Considering that the ageing effect on the systolic and diastolic functions of the left ventricle (Carrick-Ranson et al. 2012) could worsen with age, the current study was therefore aimed to assess the levels of protein and lipid oxidation biomarkers at the myocardial tissue of the left ventricles of the elderly rats exposed to fast-developing severe hypobaric hypoxia. Despite this interest, no one to the best of our knowledge has studied this issue.
Previous works have failed to address the redox status in aging and hypoxia therefore we believe that our study provides the original findings for current literature. At a fast developing severe hypoxic condition, PCO, AOPP and LHP levels of myocardial left ventricles were found to be significantly higher, while T-SH and P-SH concentrations were determined to be lower in the left ventricles of both the aged and young hypobaric hypoxic rat groups than in their respective controls. On comparing the elderly and young hypoxic groups, it was found that PCO, AOPP and LHP levels were elevated, and the concentrations of T-SH and P-SH were found to be lower in the left ventricles of the elderly rats.
Both the biological aging process and the hypoxic condition lead to high levels of PCO formation. PCO concentrations on the blood plasma of elderly individuals (Badr-el-din et al. 2010; Çakatay et al. 2008), and on the skeletal muscle of aged rats and humans were found to be higher (Çakatay et al. 2003; Gianni et al. 2004). The PCO levels of rats at hypobaric hypoxia were reported to be increased in the red quadriceps (Radák et al. 1997), and brain (Dewi et al. 2018). Our experimental results regarding myocardial PCO levels of both aged and young rats were essentially the same as that reported by others (Çakatay et al. 200; Gianni et al. 2004; Çakatay et al. 2008; Badr-el-din et al. 2010). Besides, as was expected the elderly subjects have been represented to have a higher rate of PCO formation with respect to younger subjects at a severe hypoxic stress condition.
AOPP levels were reported to be increased in human plasma after exposure to hypoxia (Pialoux et al. 2006). However, the four-month old rats’ plasma levels of AOPP at 5700 meters and 6300 meters’ altitude had no significant difference from their own control groups (Devi et al. 2007). This finding provides an additional support for rats having a better tolerance to hypoxia. In contradiction to the findings of Devi et al. (2007), in our work, the AOPP levels of the myocardial tissue of the left ventricles of both old and young rats were found to be higher than those of their own control groups, since in the current study the hypoxic stress altitude was 8000 meters. Among the elderly people, the near death individuals’ plasma AOPP levels were found to be higher than those of the other elderly people (Silva et al. 2014). Furthermore, it was observed that the plasma AOPP levels of elderly people were found to be higher than those of the younger ones at a normoxic condition (Çakatay et al. 2008). To the best of our knowledge, there is no study related to the AOPP levels of aged myocardium at hypoxia. Our findings substantiate the previous findings of Çakatay et al. (2008) at a normoxic condition; the AOPP levels of aged myocardial tissue of the left ventricle were found to be higher those of the young myocardial left ventricle after a fast developing severe hypobaric hypoxia.
In the light of the previous reports in the literature, the myocardial LHPs levels of elderly rats have been investigated in either hypoxic or normoxic conditions. As far as we know this is the first experimental study trying to clarify the myocardial redox status of aged rats at severe hypoxic exposure. It was previously reported that a high level of LHPs had been found in rat lungs (Smita et al. 2015) and in plasma at altitudes of 5700 meters and 6300 meters (Devi et al. 2007), as well as in human plasma during hypobaric hypoxia (Bailey et al. 2000), while the LHP of skeletal muscle illustrated no increase at hypoxic exposure (Radák et al. 1997; Bailey et al. 2000). A high level of MDA was reported in the blood plasma, liver and brain of aged rats (Badr-el-din et al. 2010). The LHP levels were found to be higher in elderly human plasma and the skeletal muscle tissue of aged rats (Çakatay et al. 2008, 2003). In the current study, the statistical findings related to the LHPs levels were found to be higher at the left ventricles of aged and young rats than in those of their respective control groups. Moreover, the LHPs of the normoxic elderly control rats was statistically higher than those of the normoxic young controls. This higher baseline level, would be a reason why elderly rats had higher LHPs levels in their myocardial tissue of their left ventricles than the young animals at a severe hypobaric hypoxic condition. Impaired redox homeostasis depending on the aging process could be considered as additive effect of ROS inducing damage on lipids for elderly subjects after severe hypoxic exposure.
P-SH and T-SH are commonly used oxidative status biomarkers, they are also crucial components of the non-enzymatic antioxidant mechanism in aging cells (Çakatay et al. 2008; Cebe et al. 2014). Since the thiol (–SH) group in the side chain of the cysteine is sensitive to the impaired redox status (Eaton 2006; Eaton et al. 2002), the lower myocardial thiol concentration indicates the highest ROS mediated oxidative damage. In this study, the high negative correlations were observed between myocardial P-SH and PCO levels in both elderly and young rats after exposure to severe hypoxic stress. Thiol groups might act as a free radical scavenger in the myocardial tissue of the left ventricles of the rats and a higher rate of ROS formation may lead to higher rate of myocardial protein oxidation at severe hypoxia. Therefore, being an elderly individual at this hypoxic condition could be a disadvantage with respect to a higher rate of myocardial thiol group oxidation. Moreover, consistent with the current literature, the myocardial P-SH and T-SH levels of both groups of rats were found to be lower than their own control groups, while both the P-SH and T-SH levels of the aged rats were statistically lower than those of the young rats after exposure to severe hypobaric hypoxia, which might be explained by the higher level of thiol oxidation in the elderly. Çakatay et al. (2008) and Yıldırım et al. (2017), reported that the plasma levels of T-SH groups in elderly individuals had been lower than that of their young counterparts (Çakatay et al. 2008; Yıldırım et al. 2017). Unlike the aforementioned research involving the plasma of human participants with this redox biomarker, we found no significant differences between the myocardial T-SH levels of aged and young control animals before hypoxic stress was applied. Our current results provide further evidence that thiol groups in an aging myocardial left ventricle may play a cardio protective role from ROS induced oxidative damage at a fast developing severe hypobaric hypoxia.
The myocardial levels of oxidative damage biomarkers after fast developing severe hypobaric hypoxic condition were higher in aged animals, which may be considered as an additive effect of being elder. Therefore, we wondered whether or not elevated level of oxidative damage biomarkers in aged rats are because of the effect of being aged rather than the effect of severe hypoxia. The percentage increase in the calculated ratio of PCO, AOPP and LHP levels at the left ventricles of young rats were higher than those of aged rats while the aged rats had a greater tendency to decrease in ratios of T-SH and P-SH compared to young rats at a fast developing severe hypoxic exposure. Furthermore, the baseline levels of control groups’ PCO, AOPP and LHP were higher in aged myocardia than those of young myocardia. Similarly, Badr-el-din et al. (2010) reported that levels of PCO groups of aged animals was higher than those of young animals in the plasma (27.2%), liver (31.6%) and brain (31.6%) (Badr-el-din et al. 2010). Moreover, the normoxic control groups’ myocardial T-SH and P-SH levels of elderly rats were lower than those of young rats, though statistically there were no differences between the thiol levels of the aged and young controls animals. The percentage increase in calculated ratio in the current study emphasizes that more myocardial proteins and lipids might have been oxidized in the left ventricles of young rats than in the aged rats during fast-developing severe hypobaric hypoxia and that the thiol groups primarily act as scavengers of ROS in elderly rats.
Aging myocardial tissue is more vulnerable to an ROS induced oxidative attack at a fast developing severe hypobaric hypoxia. Besides, an ROS induced impaired redox homeostasis in the myocardial tissue of left ventricles of aged rats were greater than in those of the young controls. It seems that the logical reason for the elderly animals having a high level of ROS induced oxidative damage may have been related to the impaired redox nature of ageing itself rather than the effect of hypoxic exposure because the percentage increase of ROS hazard biomarkers were found to be lower in the myocardial tissue of the elderly animals. Thiol groups were also found to be lower in myocardial tissue of hypoxic aged rats than in the heart tissue of hypoxic young rats. Being part of the non-enzymatic antioxidant defense system, thiol groups in elderly rats may play a primary role for protecting the myocardial left ventricle from further ROS hazards in hypoxic exposure. Besides, the higher percentage increase in numbers of myocardial left ventricular ROS markers in young rats at severe hypoxia can be explained by several reasons. First, the young rats have a fast mitochondrial metabolism in their heart tissues; second, the myocardia have a slow antioxidant turnover; third, myocardial tissue is one of the highest oxygen consumers in the body.
Conclusion
The experimental findings of the study lead to us to think that the myocardial tissue of the left ventricles of young rats are more vulnerable to oxidative hazards than those of the aged rats, which have higher baseline levels of ROS hazards due to ageing itself and thiol groups in aged myocardial left ventricles may have a primary role in protecting heart tissue from ROS damage at a fast-developing severe hypoxic exposure. Besides, this study’s findings emphasis that the rats, being heterothermic, have better tolerance to fast developing severe hypobaric hypoxia at '8000 meters' which was determined as the death zone for human beings.
References
Air Accident Investigation Aviation Safety Board A (2006) Aircraft accident report helios airways flight HCY522 BOEING 737–31S at Grammatiko, Hellas on 14 August 2005. 11 - 2006. https://www.academia.edu/29376872/HELIOS_Airways_Flight_HCY522_Boeing_737-31S_A_Technical_Report. Accessed 25 Feb 2019
Albright J (2015) Recognizing and preventing slow-onset hypoxia. Bus Commer Aviat (BCA) 12:63–66. https://aviationweek.com/business-businessaviation/recognizing-and-preventing-slow-onset-hypoxia. Accessed 14 Mar 2019
Alderman C, Shah S, Foreman J, Chain B, Katz D (2002) The role of advanced oxidation protein products in regulation of dendritic cell function. Free Radic Biol Med 32(5):377–385. https://doi.org/10.1016/S0891-5849(01)00735-3
Andriollo Sanchez M, Hininger-Favier I, Meunier N, Venneria E, O’Connor J, Maiani G, Coudray C, Roussel A (2005) Age-related oxidative stress and antioxidant parameters in middle-aged and older. European subjects: the ZENITH study. Eur J Clin Nutr 59(2):58–62. https://doi.org/10.1038/sj.ejcn.1602300
Badr-el-din N, Noaman E, Fattah S, Ghoneum M (2010) Reversal of age-associated oxidative stress in rats by MRN-100, a hydro-ferrate fluid. In Vivo 24(4):525–534
Bailey D, Castell L, Newsholme E, Davies B (2000) Continuous and intermittent exposure to the hypoxia of altitude: implications for glutamine metabolism and exercise performance. Br J Sports Med 34:210–212. https://doi.org/10.1136/bjsm.34.3.210
Bradford M (1976) A rapid sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Çakatay U, Telci A, Yilmaz İ, Akçay T, Sivas A (2000) Yaşlanmanın plazma oksidatif protein hasarına etkisi. Cerrahpaşa Tıp Derg 31:220–223
Çakatay U, Telci A, Kayalı R, Tekeli F, Akçay T, Sivas A (2001) Relation of oxidative protein damage and nitrotyrosine levels in the aging rat brain. Exp Gerontol 36(2):221–229. https://doi.org/10.1016/s0531-5565(00)00197-2
Çakatay U, Telci A, Kayalı R, Tekeli F, Akçay T, Sivas A (2003) Relation of aging with oxidative protein damage parameters in the rat skeletal muscle. Clin Biochem 36(1):51–55. https://doi.org/10.1016/s0009-9120(02)00407-1
Çakatay U, Kayali R, Uzun H (2008) Relation of plasma protein oxidation parameters and paraoxonase activity in the ageing population. Clin Exp Med 8(1):51–57. https://doi.org/10.1007/s10238-008-0156-0
Carrick-Ranson G, Hastings JL, Bhella PS, Shibata S, Fujimoto N, Palmer MD, Boyd K, Levine BD (2012) Effect of healthy aging on left ventricular relaxation and diastolic suction. Am J Physiol Heart Circ Physiol 303(3):H315–H322. https://doi.org/10.1152/ajpheart.00142.2012
Cebe T, Yanar K, Atukeren P, Ozan T, Kuruç AI, Kunbaz A, Sitar ME, Mengi M, Aydın MŞ, Eşrefoğlu M, Aydın S, Çakatay U (2014) A comprehensive study of myocardial redox homeostasisin naturally and mimetically aged rats. AGE 36(6):1–14. https://doi.org/10.1007/s11357-014-9728-y
Chris B (Writer) (2014) SpaceX's CRS-4 Dragon completes Tuesday arrival at ISS: NASA spaceflight.com. https://www.nasaspaceflight.com/2014/09/crs-4-dragon-tuesday-iss-arrival/. Accessed 20 Mar 2019
Clarke C (2006) Acute mountain sickness: medical problems associated with acute and subacute exposure to hypobaric hypoxia. Postgrad Med J 82(973):748–753. https://doi.org/10.1136/pgmj.2006.047662
Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R (2003) Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta 329(1–2):23–38. https://doi.org/10.1016/s0009-8981(03)00003-2
Devi S, Vani R, Subramanyam MV, Reddy S, Jeevaratnam K (2007) Intermittent hypobaric hypoxia-induced oxidative stress in rat erythrocytes: protective effects of vitamin E, vitamin C, and carnitine. Cell Biochem Funct 25(2):221–231. https://doi.org/10.1002/cbf.1344
Dewi S, Mulyawan W, Wanandi SI, Sadikin M (2018) The effect of intermittent hypobaric hypoxia on oxidative stress status and antioxidant enzymes activity in rat brain. Acta Biochim Indones 1(2):46–51
Dringen R, Gutterer JM, Hirrlinger J (2000) Glutathione metabolism in brain metabolic interaction between astrocytes and neurons in the defense against reactive oxygen species. Eur J Biochem 267(16):4912–4916. https://doi.org/10.1046/j.1432-1327.2000.01597.x
Du J, Gebicki J (2004) Proteins are major initial targets of hydroxyl free radicals. Int J Biochem Cell Biol 36(11):2334–2343. https://doi.org/10.1016/j.biocel.2004.05.012
Dupré R, Owen T (1992) Behavioral thermoregulation by hypoxic rats. J Exp Zool 262(2):230–235. https://doi.org/10.1002/jez.1402620213
Eaton P (2006) Protein thiol oxidation in health and disease: techniques for measuring disulfides and related modifications in complex protein mixtures. Free Radic Biol Med 40(11):1889–1899. https://doi.org/10.1016/j.freeradbiomed.2005.12.037
Eaton P, Wright N, Hearse D, Shattock M (2002) Glyceraldehyde phosphate dehydrogenase oxidation during cardiac ischemia and reperfusion. J Mol Cell Cardiol 34(11):1549–1560. https://doi.org/10.1006/jmcc.2002.2108
Gianni P, Jan K, Douglas M, Stuarta P, Tarnopolsky M (2004) Oxidative stress and the mitochondrial theory of aging in human skeletal muscle. Exp Gerontol 39(9):1391–1400. https://doi.org/10.1016/j.exger.2004.06.002
Gordon CJ, Fogelson L (1991) Comparative effects of hypoxia on behavioral thermoregulation in rats, hamsters, and mice. Am J Physiol 260(1 Pt 2):R120–R125. https://doi.org/10.1152/ajpregu.1991.260.1.R120
Hanasand M, Omdal R, Norheim K, Gøransson L, Brede C, Jonsson G (2012) Improved detection of advanced oxidation protein products in plasma. Clin Chim Acta 413(9–10):901–906. https://doi.org/10.1016/j.cca.2012.01.038
Hollingsworth KG, Blamire AM, Keavney BD, Macgowan GA (2012) Left ventricular torsion, energetics, and diastolic function in normal human aging. Am J Physiol Heart Circ Physiol 302(4):H885–H892. https://doi.org/10.1152/ajpheart.00985.2011
Höpfl G, Ogunshola O, Gassmann M (2003) Hypoxia and high altitude. The molecular response. Adv Exp Med Biol 543:89–115. https://doi.org/10.1007/978-1-4419-8997-0_7
Kalousová M, Zima T, Tesař V, Dusilová-Sulková S, Škrha J (2005) Advanced glycoxidation end products in chronic diseases-clinical chemistry and genetic background. Mutat Res, Fundam Mol Mech Mutagen 579(1–2):37–46. https://doi.org/10.1016/j.mrfmmm.2005.03.024
Magalhães J, Ascensão A, Soares J, Ferreira R, Neuparth M, Marques F, Duarte J (2005) Acute and severe hypobaric hypoxia increases oxidative stress and impairs mitochondrial function in mouse skeletal muscle. J Appl Physiol 99(4):1247–1253. https://doi.org/10.1152/japplphysiol.01324.2004
Mansfield KD, Simon MC, Keith B (2004) Hypoxic reduction in cellular glutathione levels requires mitochondrial reactive oxygen species. J Appl Physiol 97(4):1358–1366. https://doi.org/10.1152/japplphysiol.00449.2004
Matés J, Pérez-Gómez C, Castro INd, Asenjo M, Márquez J (2002) Glutamine and its relationship with intracellular redox status, oxidative stress and cell proliferation/death. Int J Biochem Cell Biol 34(5):439–458. https://doi.org/10.1016/s1357-2725(01)00143-1
Murray A (2009) Metabolic adaptation of skeletal muscle to high altitude hypoxia: how new technologies could resolve the controversies. Genome Med 1(12):117–126. https://doi.org/10.1186/gm117
Parati G, Giuliano A, Bilo G, Torlasco C (2014) Highcare projects: high altitude cardiovascular research. OGM - Scientific Publishing, Services & Communication, Milano. http://www.cairimini.it/wp-content/uploads/2016/05/Highcare_book.-11-yrs-of-high-altitude-research.pdf. Accessed 22 Mar 2019
Pialoux V, Mounier R, Ponsot E, Rock E, Mazur A, Dufour S, Richard R, Richalet J, Coudert J, Fellmann N (2006) Effects of exercise and training in hypoxia on antioxidant/pro-oxidant balance. Eur J Clin Nutr 60(12):1345–1354. https://doi.org/10.1038/sj.ejcn.1602462
Radák Z, Asano K, Lee K, Ohno H, Nakamura A, Nakamoto H, Goto S (1997) High altitude training ıncreases reactive carbonyl derivatives but not lipid peroxidation in skeletal muscle of rats. Free Radic Biol Med 22(6):1109–1114. https://doi.org/10.1016/s0891-5849(96)00350-4
Reznick AZ, Packer L (1994) [38] Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods Enzymol 233:357–363. https://doi.org/10.1016/S0076-6879(94)33041-7
Sedlak J, Lindsay R (1968) Estimation of total, protein bound, and non-protein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem 25(1):192–205. https://doi.org/10.1016/0003-2697(68)90092-4
Silva TDO, Jung IE, Moresco RN, Barbisan F, Ribeiro EE, Ribeiro EA, Motta K, Britto E, Tasch E, Bochi G, Duarte MMMF, Oliveira ARD, Marcon M, Beló C, Montagner GFDS, Cruz D (2014) Association between advanced oxidation protein products and 5-year mortality risk among amazon riparian elderly population. Free Radic Res 49(2):204–209. https://doi.org/10.3109/10715762.2014.992895
Simsek B, Yanar K, Kansu A, Belce A, Aydin S, Çakatay U (2019) Caloric restriction improves the redox homeostasisin the aging male rat heart even when started inmiddle-adulthood and when the body weight is stable. Biogerontology 20:127–140. https://doi.org/10.1007/s10522-018-9781-5
Smita K, Qadar-Pasha M, Jain S (2015) Oxidative stress and histopathological evaluation of rat lung tissue during hypobaric hypoxia. J Proteom Bioinform 8(6):108–115. https://doi.org/10.4172/jpb.1000358
Solaini G, Harris D (2005) Biochemical dysfunction in heart mitochondria exposed to ischaemia and reperfusion. Biochem J 1(390):377–394. https://doi.org/10.1042/BJ20042006
Stadtman E (2004) Role of oxidant species in aging. Curr Med Chem 11(9):1105–1112. https://doi.org/10.2174/0929867043365341
Stadtman E, Levine R (2000) Protein oxidation. Ann N Y Acad Sci 899:191–208
The Boeing Company (2016) Boing 737-800 flight crew operation manual. The Boeing Company, Seattle Washington. http://www.737flightsimulator.co.uk/737info/B737OM.pdf. Accessed 15 Feb 2019
Thurber M (Producer) (2010) Study: pilots ıgnore oxygen regulations. Business Aviation. https://www.ainonline.com/aviation-news/aviation-international-news/2010-01-26. Accessed 13 Feb 2019
Udayabanu M, Kumaran D, Nair RU, Srinivas P, Bhagat N, Aneja R, Katyal A (2008) Nitric oxide associated with iNOS expression inhibits acetylcholinesterase activity and induces memory impairment during acute hypobaric hypoxia. Brain Res 1230:138–149. https://doi.org/10.1016/j.brainres.2008.06.081
Virués-Ortega J, Garrido E, Javierre O, Kloezeman K (2006) Human behavior and development under high-altitude conditions. Dev Sci 9(4):400–410. https://doi.org/10.1111/j.1467-7687.2006.00505.x
Wilson M, Newman H, Imray C (2009) The cerebral effects of ascent to high altitudes. Lancet Neurol 8(2):175–791. https://doi.org/10.1016/s1474-4422(09)70014-6
Wolff S (1994) Ferrous ion oxidation in presence of ferric ion indicator xylenol orange for measurement of hydroperoxides. Methods Enzymol 233:182–189. https://doi.org/10.1016/s0076-6879(94)33021-2
Yıldırım E, İpek E, Bavunoğlu I, Yıldırım N, Cengiz M, Yavuzer S, Yavuzer H, Erman H, Uzun H (2017) The impact of protein oxidation on sustained and white coat hypertension. Anatol J Cardiol 17(3):210–216
Zeng J, Zhong Z, Li X, Wu Q, Zheng S, Zhou J, Ye W, Xie F, Wu X, Huang Z, Chen J (2014) Advanced oxidation protein products accelerate bone deterioration in aged rats. Exp Gerontol 50:64–71. https://doi.org/10.1016/j.exger.2013.11.014
Acknowledgements
The authors thank Prof. Dr. Metin Baştuğ and Prof. Dr. Hakan Fıçıcılar of the Physiology Department from Ankara University, Faculty of Medicine, for their contribution and technical support. The authors also thank Rudolf Peter Jelen from Çankaya University, Department of Foreign Languages, English Preparatory Unit, for his editorial contributions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ağaşcıoğlu, E., Çolak, R., Demirel, H. et al. Impaired redox homeostasis in the heart left ventricles of aged rats experiencing fast-developing severe hypobaric hypoxia. Biogerontology 20, 711–722 (2019). https://doi.org/10.1007/s10522-019-09826-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10522-019-09826-1