Abstract
The application of herbal medicine is one of the world’s most ancient approaches to treating diseases. This study aimed to examine the immunomodulatory effect of Radix Scutellariae water extract (RSWE) on the Macrobrachium rosenbergii immunity by reverse gavage feeding (RGF). In this study, M. rosenbergii was administered RSWE by RGF to stimulate its immunity. Furthermore, total hemocyte count (THC), phenoloxidase (PO), respiratory burst (RB), transglutaminase (TG), and lysozyme activity in hemocytes were evaluated to determine the immunological responses. Meanwhile, midgut and hepatopancreas were isolated to observe immune-related genes (LGPB, PE, and proPO). The study employed four treatments (0, 1, 5, and 10 µg of RWSE), and each treatment consisted of 3 replications. All data were analyzed using SPSS 17.0 (one-way ANOVA and Duncan test) to evaluate the differences among treatments. The results showed that the 5 µg RSWE was the optimum dosage to enhance the immune responses and induce the expression of immune-related genes in the hepatopancreas and midgut of M. rosenbergii. Finally, the RGF method can be used to investigate the immunomodulatory effect of RSWE on prawn, and the immune response associated with each dose can be compared precisely.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The giant freshwater prawn, Macrobrachium rosenbergii, is a commercially important freshwater crustacean species (Lalrinsanga et al. 2014; Nguyen et al. 2019). However, the rapid development of prawn industry has promoted the incidence of infectious diseases, mainly caused by viruses and bacteria (Chen et al. 2001a; Hsu et al. 2005; Suanyuk and Dangwetngam 2014). Lactococcus garvieae has proven to be one of the significant coccus pathogens for prawns, in which L. garvieae infection in M. rosenbergii causes cumulative mortality of around 30 to 75% (Chen et al. 2001b; Chen and Wang 2001). Several strategies have been implemented to deal with bacterial infections (Serrano 2005). However, antibiotics have adverse biosafety and biosecurity implications (Guo et al. 2011; Lakshmi et al. 2013; Sørum et al. 2006). Therefore, a solution is needed to solve the problem of antibiotics, such as plant-based medicines recently used as therapeutic alternatives (Disch et al. 2017; Valladão et al. 2015).
In aquaculture, medicinal herbs have been reported to promote growth, improve immunity, and possess antimicrobial properties (Direkbusarakom et al. 1998; Jian and Wu 2004; Sivaram et al. 2004; Yahaya et al. 2015). Radix Scutellariae is the dried root of the medicinal herb Scutellariae baicalensis Georgi. In addition, it shows various therapeutic effects (e.g., anti-inflammation, anti-cancer, and anti-virus-related diseases), and it has been proved that flavonoids are the most abundant constituents and stimulate these therapeutic effects. The major bioactive flavones in Radix Scutellariae existing are aglycones (baicalein, wogonin, oroxylin A) and glycosides (baicalin, wogonoside, oroxylin A-7-glucuronide) (Li et al. 2011). Meanwhile, the main bioactive component in the water extract of Radix Scutellaria (WERS) is baicalin (Yan et al. 2022). Noticing the promising potential of Radix Scutellariae, several studies have been conducted to evaluate the effect of Radix Scutellariae supplementation on aquatic animals, such as olive flounder (Cho et al. 2013) and tilapia (Yin et al. 2006). However, to the best of our knowledge, no studies have considered the effects of Radix Scutellariae on prawns.
Injection, immersion, and oral administrations are the most common delivery routes of plant extracts in aquaculture (Reverter et al. 2014). However, as previously reviewed, each method has advantages and disadvantages (Assefa and Abunna 2018; Galindo-Villegas and Hosokawa 2004). In the present study, through the prawn’s anus, WERS was directly introduced to the region of the anterior midgut connecting with the hepatopancreas for stimulating immune responses. This method is called reverse gavage feeding (RGF) (Aranguren and Lightner 2009; Tran et al. 2013; Xie et al. 2017). Delivering WERS to the midgut directly at least partially avoids the first-pass metabolism effect and the possibility of WERS-food interactions when applied WERS incorporation in the feed. In addition, the RGF method is less traumatic than oral gavage and makes it possible to deliver a dose of WERS into the midgut precisely. Therefore, the immunomodulatory effects of WERS associated with each dose on prawn immunity can be accurately compared. For this reason, it is necessary to investigate the immunomodulatory effects of WERS when applied to prawns by RGF.
Materials and methods
Prawn acclimatization
Selected M. rosenbergii (20.0 ± 2.0 g) were purchased from a local commercial shrimp farm (Pingtung, Taiwan). Two weeks before treatment, prawns were maintained at room temperature in a 400 l tank (25 prawns/tank) fitted with a freshwater circulator and fed with commercial feed twice daily, equal to 5% of prawn body weight.
Plant extraction
Selected Radix Scutellariae was purchased from a local market (Pingtung, Taiwan). Following a previous method to prepare WERS (Zhao et al. 2016), 10 g of Radix Scutellariae powder was boiled in 150 ml of double-distilled water at 95 °C for 15 min. Then, the supernatant was obtained by centrifugation at 1000 × g at 4 °C for 10 min before evaporating using a freeze dryer (EYELA FDU-1100). Lyophilized powder of WERS was then stored at 4 °C until used.
Reverse gavage feeding and sample collection
The selected prawns were divided into 4 treatment groups, and each treatment consisted of 3 replications (25 prawns/tank). Before WERS administration, prawns were fasted for 24 h to ensure their intestines were free of feces. Subsequently, a 10 µl mixed solution of PBS and red dye (30: 1 v/v) containing different doses of WERS (0, 1, 5, and 10 µg) was slowly introduced to the anterior midgut via anal route by RGF. The use of red dye to facilitate observation and ensure that the solution sent into the intestine through the anus can reach the anterior midgut.
Based on a previous study (Xie et al. 2017), sampling was carried out 24 h after RGF, consisting of four time intervals (3, 6, 12, and 24 h after RGF). Hemolymph (100 µl) was transferred to a 1.5-ml Eppendorf tube containing 900 μl cooled anticoagulant (0.34 g EDTA, 0.8 g sodium citrate, and 10 μl Tween 80 in 100 ml of distilled H2O, at pH 7.45 with the osmolality adjusted to 490 mOsm kg−1 with NaCl). In addition, digestive organs (hepatopancreas and midgut) were taken out from the prawns’ bodies and stored at − 20 °C in RNAlater™ solution until use.
Measurement of the immune responses
Total hemocyte count (THC) was determined by counting the number of hemocytes in the anticoagulant and hemolymph mixture on the hemocytometer fields under a microscope. Hemocytes found on the fields of the hemocytometer were calculated in three replications.
The respiratory burst (RB) activity was determined using the reduction of nitroblue tetrazolium (NBT) to formazan as a measure of superoxide anion (O2−) production (Song and Hsieh 1994) with some modification. RB activity was expressed as the amount of NBT reduction in 100 µl of hemolymph.
The hydroxamate-based colorimetric method was used to determine the TG activity in M. rosenbergii hemolymph (Gorman and Folk 1980). Initially, 50 µL of hemolymph was incubated with 150 µl of Tris–acetate buffer, 12.5 µl of hydroxylamine hydrochloride 2 M, and 37.5 µl of Z-Glu-Gly at 37 °C for 10 min. Then, 250 µl of coloring agent (contained 15 g trichloroacetic acid and 5 g iron (III) chloride hexahydrate in 100 ml of ddH2O) was added and centrifuged at 10,000 rpm for 5 min. Finally, the supernatant was measured colorimetrically at 525 nm with a microplate reader.
Lysozyme activity was determined by a turbidimetric assay which used a suspension of Micrococcus lysodeikticus as a substrate in a reaction mixture (Shugar 1952). Hemolymph (10 µl) was mixed with 200 µl of M. lysodeikticus in PBS (0.02% (w/v)) at 25 °C. Then, the absorbance at 450 nm was immediately recorded with a microplate reader every minute. Lysozyme from white egg (Amresco, USA) was used as control. Lysozyme activity was quantified in absorbance reduction per minute.
RNA extraction, cDNA synthesis, and qRT-PCR analysis of immune related genes
Total RNA was extracted from the digestive organs (hepatopancreas and midgut) using Trizol® reagent (Ambion®, USA). The RNA samples were suspended in 30 μl DECP-treated water and kept at − 80 °C until use. Total RNA concentration was qualified and quantified using BioSpectrometer ((Eppendorf BioSpectrometer® fitted with Hellma TrayCell Cap, Germany). According to the manufacturer’s instructions, first-strand cDNA was synthesized from 2 µg of total RNA using M-MuLV Reverse Transcriptase (Lucigen®, USA). The cDNA was subsequently stored at − 20 °C.
Quantitative real-time PCR (qRT-PCR) was performed to investigate the expression of immune-related genes in the prawn digestive organs after WERS administration by RGF (Table 1). qRT-PCR was performed using the ABI StepOnePlus™ Real-Time PCR System (Applied Biosystems™, USA). The amplification was performed in a total volume of 10 μl, containing 1 μl of cDNA template, 5 μl of TB Green Premix Ex Taq II (Tli RNaseH Plus) (Takara Bio Inc., Japan), 0.2 μl of each primer, 0.2 μl of ROX reference dye (50x), and 3.4 μl of DEPC treated water. The real-time PCR program was 95 °C for 1 min, followed by 40 cycles of 95 °C for the 15 s and 60 °C for 1 min. Dissociation and melting curves of amplification products were performed at the end of each PCR to confirm that only one PCR product was amplified and detected. The relative expression of related genes were calculated by the 2−ΔΔCT method (Livak and Schmittgen 2001).
Statistical analysis
ANOVA (one-way analysis of variance) was applied to evaluate data obtained from the experiments. Multiple comparisons (Duncan test) were conducted to investigate significant differences among treatments using SPSS 17. Results were present as mean ± SD. Differences between different groups at each time point were considered significant at p < 0.05.
Results
Effect of Radix Scutellariae water extract (RSWE) on the immune responses
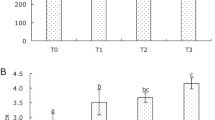
As shown in Fig. 1, after administration with 1 and 5 µg of WERS by RGF, THC levels increased significantly (p < 0.05) compared with the other treatments for 24 h (Fig. 1). Moreover, RSWE administration by RGF was shown to enhance the TG levels of M. rosenbergii, which persist for up to 24 h, and administration of 5 µg RSWE showed the highest TG activity (Fig. 2). Furthermore, RSWE administration by RGF was shown to enhance the RB levels of M. rosenbergii. However, administration of high doses of RSWE (10 µg) by RGF showed excessive RB activity, in which RB activity increased significantly (p < 0.05) 3 h after RGF and persisted for up to 24 h (Fig. 3). In addition, RSWE administration by RGF was shown to enhance the lysozyme levels of M. rosenbergii but administration of high doses of RSWE (5 and 10 µg) by RGF showed an earlier increase in lysozyme activity than low doses of RSWE (Fig. 4).
Effect of Radix Scutellariae water extract (RSWE) on the immune-related genes
Hepatopancreas
The expressions of LGBP in M. rosenbergii HP were decreased post-RGF in all treatments for 24 h, except for an increase of LGBP (2.1-fold) at 12 h post-RGF in T3 (Fig. 5). The downregulation of PE in T2 and T3 was observed as early as 3 h (0.2-fold) post-RGF (Fig. 6). An increase in PE was only shown 12 h post-RGF in T3 (non-significant).
Midgut
The expressions of LGBP in M. rosenbergii MG were downregulated as early as 3 h post-RGF at T2 and T3, while T1 expression was not significantly different to the control group (Fig. 7). The upregulation of LGBP was observed in all treatments at 6 h. Furthermore, an increase of LGBP in MG was also seen at 12 and 24 h post-RGF in T2 and T1 (2.6- and 5.35-fold, respectively). The expression of proPO was upregulated in all treatments for 3 h and only T2 for 12 h (1.77-fold), while at 6 and 24 h showed no significance among treatments (Fig. 8). The upregulation of PE in T1 was observed as early as 3 h (10.64-fold) post-RGF (Fig. 9). An increase in PE also was shown at 6 h post-RGF in T3 (Fig. 9).
Discussion
Most of the immune responses occur in hemolymph, which contains three different types of hemocytes, namely semigranular, granular, and hyaline (Interaminense et al. 2019). Meanwhile, the digestive tract of prawn consists of three main parts, the foregut, midgut, and hindgut (McGaw and Curtis 2013). The midgut is the main absorptive area of the digestive tract, which represents a vital route of pathogen entry (Barreto et al. 2018; Rosa and Barracco 2010) and is closely associated with hemocytes. Another essential organ is the hepatopancreas, the prawn’s primary digestive gland similar to the liver in vertebrates. The hepatopancreas, which occupies a substantial part of the posterior cephalothorax and surrounds the posterior stomach and anterior midgut, is an essential site for the expression of immune-related genes in prawn (Du et al. 2013; Robalino et al. 2007; Zeng et al. 2013).
Effect of Radix Scutellariae water extract (RSWE) on the immune responses
Total hemocyte count (THC)
The circulating hemocytes play primary roles in crustaceans’ immune response to invaded microbial pathogens and external stimulations. Hemocytes conduct various physiological and pathological functions, such as antigen recognition, phagocytosis, encapsulation, nodule formation, and releasing of humoral factors (Chang et al. 2015). However, the number of hemocytes is related to many factors, such as environmental stresses (Perazzolo et al. 2002), infections (Feng et al. 2008), immunostimulant administration (Chen et al. 2014), and endocrine activities (Estrada et al. 2016). In the present study, we evaluated the effects of RSWE administration by RGF on the THC of M. rosenbergii. As shown in Fig. 1, after administration with 1 and 5 µg of RSWE by RGF, THC levels increased significantly compared with the other treatments for 24 h (Fig. 1). The similar results described by Wu et al. (Wu et al. 2015) showed that the water extract of Gynura bicolor could enhance THC of Litopenaeus vannamei at 7th day post-feeding treatment. Noni leaves extract was also shown to increase M. rosenbergii THC at 3rd day post-feeding treatment (Halim et al. 2017). It is supposed that RSWE administration by RGF may trigger immune cell proliferation in prawns, thereby increasing the secretion of cytokines (e.g., astakine), which can increase hemocyte synthesis in hematopoietic tissues.
Transglutaminase (TG)
Coagulation is initiated by releasing factors and enzymes from hemocytes, which effectively polymerizes the hemolymph clotting protein (Martin et al. 1991). In crustaceans, clotting is mediated through coagulates presented in the plasma and TG within circulating hemocytes (Liu et al. 2011). TG prevents hemocyte loss during the microbial invasion and inhibits their growth in the hemocoel (Fagutao et al. 2012). Besides, hemolymph clotting is responsible for binding and killing pathogens in horseshoe crabs. This process involves inflammatory responses, wound closure, and healing (Theopold et al. 2004). In the present study, RSWE administration by RGF was shown to enhance the TG levels of M. rosenbergii, which persist for up to 24 h, and administration of 5 µg WERS showed the highest TG activity (Fig. 2). A previous study using Eichhornia crassipes supplemented feed to enhance the immune system of M. rosenbergii also showed a similar result (Chang et al. 2013). Together with an increase in THC, our data support a functional involvement of TG in the acceleration of hemolymph coagulation of M. rosenbergii.
Respiratory burst (RB)
RB is the rapid release of reactive oxygen species (ROS) from phagocytes during phagocytosis. Increased RB activity is related to increased bactericidal activity by phagocytes or hemocytes within the cellular immune system (Song and Hsieh 1994). However, overproduction of ROS may induce oxidative stress that can potentially damage host cells (Pohanka 2013). In this study, RSWE administration by RGF was shown to enhance the RB levels of M. rosenbergii. However, administration of high doses of RSWE (10 µg) by RGF showed excessive RB activity, in which RB activity increased significantly 3 h after RGF and persisted for up to 24 h (Fig. 3). A previous study showed that RB activity of L. vannamei was increased at 24 h post-feeding with P. binghamiae hot water extract at the concentration of 10 µg/g (Chen et al. 2014). Furthermore, crude extract of Cardiospermum halicacubum leaves supplemented with commercial feed enhanced the RB activity of Penaeus monodon from 48 to 120 h after feeding (Rajasekar et al. 2011). These results suggest that the appropriate administration of RSWE by RGF can effectively induce RB activity of hemocytes in prawns.
Lysozyme
Lysozyme is found in hemolymph and most shrimp tissues, but lysozyme production is related to the number of hemocytes in the hemolymph (Burge et al. 2007; Mai and Hu 2009; Qiao et al. 2013). Lysozyme is an antimicrobial agent, and it is proven that the increased activity of lysozyme can protect the host by acting as an essential defense enzyme against infectious diseases (Ragland and Criss 2017; Saurabh and Sahoo 2008). In this study, RSWE administration by RGF was shown to enhance the lysozyme levels of M. rosenbergii. However, administration of high doses of RSWE (5 and 10 µg) by RGF showed an earlier increase in lysozyme activity than low doses of WERS (Fig. 4). An increase in lysozyme activity was also found in P. monodon and L. vannamei, given guava leaf extract and zingerone, respectively (Chang et al. 2012; Yin et al. 2014). In contrast, Radix Scutellariae could not enhance lysozyme activity in tilapia, Oreochromis niloticus (Yin et al. 2006). These results suggest that administering high doses RSWE by RGF can effectively induce RB activity of hemocytes in prawns.
Effect of Radix Scutellariae water extract (RSWE) on the immune related-genes
Lipopolysaccharide- and β-1,3-glucan-binding protein (LGBP).
LGBP was mainly expressed in the hepatopancreas (Pan et al. 2005; Yeh et al. 2009) and is involved in cellular and humoral defenses using PAMP (pathogen-associated molecular patterns) and stimulates a cascade of responses, such as phagocytosis, clotting activation, antibacterial peptides production, and proPO cascades (Iwanaga 1994; Lai et al. 2011; Roux et al. 2002; Yeh et al. 2009). In our study, the significant upregulation of the LGBP gene in hepatopancreas was observed at 12 h after administration of 10 µg RSWE by RGF (Fig. 5). A previous study reported that LGBP expression was increased in the hepatopancreas of L. vannamei at 72 h and 7 days post-feeding treatment of diets containing β-1,3-glucan (Wang et al. 2008). In addition, LGBP expression in the hepatopancreas of M. rosenbergii was upregulated at 12 h after LPS or poly I:C administration (Yeh et al. 2009). Meanwhile, at 6 h after RSWE administration by RGF, the expression of LGBP in the midgut was significantly upregulated, and this trend persisted for prawn groups with 5 and 10 µg RSWE (Fig. 7). The increased expression of LGBP in the midgut is originates from the infiltration of hemocytes (Silveira et al. 2018). Hemocytes perform a migratory behavior to digestive tissues and contribute to gut immunity (Arts 2006; Silveira et al. 2018; Yeh et al. 2009). Based on the statements above, we speculated that the increased LGBP in MG was due to their content of hemocytes. These results suggest that the difference in LGBP expression in the digestive organs was modulated by types of immunostimulants, delivery method, and migratory behavior of hemocytes.
Prophenoloxidase (proPO)
The proPO-activating system is believed to be an essential innate defense in invertebrate immunity (Charoensapsri et al. 2011). Activation of proPO is shown to generate cytotoxic products, melanin production (melanization), and encapsulation of pathogen (Cerenius et al. 2008). The shrimp’s proPO expression was found in the hemolymph, gill, stomach, hepatopancreas, and intestine (Ai et al. 2008; Charoensapsri et al. 2009; Pang et al. 2014). It showed that proPO plays a central role in killing and eliminating invading pathogens (Tassanakajon et al. 2018). Besides, upregulation of proPO expression in diverse tissues was used as a hemocyte marker to determine hemocyte infiltration into various tissues (Soonthornchai et al. 2010).
In the present study, proPO was expressed in midgut M. rosenbergii. However, in the midgut, the expression of proPO showed a rapid on–off response after stimulation with RSWE by RGF (Fig. 8). This result is consistent with previous studies on Morinda citrifolia (Halim et al. 2017), Musa acuminate (Rattanavichai and Cheng 2015), and Solanum nigrum (Harikrishnan et al. 2011). In contrast, another study showed that the regulatory mechanism for proPO gene expression after stimulation with pUC57-CpG is a long-lasting response (Yi et al. 2014). Besides, the present study considered the upregulation of proPO in the midgut caused by infiltrating hemocytes in the midgut because proPO is synthesized in granular hemocytes and converted into an active PO form by a serine proteinase (Okumura 2007).
Peroxinectin (PE)
Peroxinectin is a cell adhesion protein essential to invertebrates for many physiological processes, including immune responses (Johansson 1999). In addition, active PE can bind to integrin receptors on the cell surface to encourage the degranulation process in a positive reaction loop and play a multifunctional agent during pathogen invasion (Sricharoen et al. 2005). In the present study, the expression of PE in the hepatopancreas of M. rosenbergii was significantly upregulated after administration of RSWE, particularly when stimulated with 1 and 10 µg RSWE at 3 and 12 h after RGF (Fig. 6). Meanwhile, the expression of PE in the midgut has a similar pattern to the expression of proPO in the midgut (Figs. 8 and 9). These circumstances are understandable since PE is a protein associated with the proPO system, and its cell adhesion activity is triggered when the proPO system is activated (Sritunyalucksana et al. 2001).
Overall, there was no correlation between the administered doses of RSWE by RGF with the immune responses and the expression of immune-related genes on M. rosenbergii. Moreover, there is no single dose of RSWE that can simultaneously boost all immune responses in circulating hemocytes and the expression of immune-related genes in the digestive organs of M. rosenbergii. However, stimulation with 5 μg RSWE by RGF consistently increased immune responses and immune-related genes in M. rosenbergii. In contrast, administration of 10 μg RSWE may inhibit some immune responses and immune-related genes in M. rosenbergii.
Conclusion
The results demonstrated that administration of RSWE by RGF improved immune responses (THC, TG, RB, and lysozyme activity) and induced the expression of immune-related genes (LGBP, proPO, and PE) in the hepatopancreas and midgut. The RGF method can be used to investigate the immunomodulatory effect of RSWE on shrimp, and the immune response associated with each dose can be compared precisely.
Data availability
All the required data are available in the manuscript.
Code availability
Not applicable.
References
Ai H-S, Huang Y-C, Li S-D, Weng S-P, Yu X-Q, He J-G (2008) Characterization of a prophenoloxidase from hemocytes of the shrimp Litopenaeus vannamei that is down-regulated by white spot syndrome virus. Fish Shellfish Immunol 25(1–2):28–39
Aranguren F, Lightner D (2009) Reverse gavage: a new standardized technique for infecting shrimp suitable for challenge tests [Abstracts]. Aquaculture America 2009, Seattle, Washington
Arts JA (2006) Immune defence white spot syndrome virus infected shrimp, Penaeus monodon
Assefa A, Abunna F (2018) Maintenance of fish health in aquaculture: review of epidemiological approaches for prevention and control of infectious disease of fish. Vet Med Int 2018:1–10
Barreto C, Coelho JdR, Yuan J, Xiang J, Perazzolo LM, Rosa RD (2018) Specific molecular signatures for type ii crustins in penaeid shrimp uncovered by the identification of crustin-like antimicrobial peptides in Litopenaeus vannamei. Mar Drugs 16(1):31. https://doi.org/10.3390/md16010031
Burge EJ, Madigan DJ, Burnett LE, Burnett KG (2007) Lysozyme gene expression by hemocytes of Pacific white shrimp, Litopenaeus vannamei, after injection with Vibrio. Fish Shellfish Immunol 22(4):327–339
Cerenius L, Lee BL, Soderhall K (2008) The proPO-system: pros and cons for its role in invertebrate immunity. Trends Immunol 29(6):263–271. https://doi.org/10.1016/j.it.2008.02.009
Chang Y-P, Liu C-H, Wu C-C, Chiang C-M, Lian J-L, Hsieh S-L (2012) Dietary administration of zingerone to enhance growth, non-specific immune response, and resistance to Vibrio alginolyticus in Pacific white shrimp (Litopenaeus vannamei) juveniles. Fish Shellfish Immunol 32(2):284–290. https://doi.org/10.1016/j.fsi.2011.11.017
Chang C-C, Tan H-C, Cheng W (2013) Effects of dietary administration of water hyacinth (Eichhornia crassipes) extracts on the immune responses and disease resistance of giant freshwater prawn, Macrobrachium Rosenbergii. Fish Shellfish Immunol 35(1):92–100. https://doi.org/10.1016/j.fsi.2013.04.008
Chang C-C, Tsai W-L, Jiang J-R, Cheng W (2015) The acute modulation of norepinephrine on immune responses and genes expressions via adrenergic receptors in the giant freshwater prawn, Macrobrachium Rosenbergii. Fish Shellfish Immunol 46(2):459–467. https://doi.org/10.1016/j.fsi.2015.07.015
Charoensapsri W, Amparyup P, Hirono I, Aoki T, Tassanakajon A (2009) Gene silencing of a prophenoloxidase activating enzyme in the shrimp, Penaeus monodon, increases susceptibility to Vibrio harveyi infection. Dev Comp Immunol 33(7):811–820
Charoensapsri W, Amparyup P, Hirono I, Aoki T, Tassanakajon A (2011) PmPPAE2, a new class of crustacean prophenoloxidase (proPO)-activating enzyme and its role in PO activation. Dev Comp Immunol 35(1):115–124. https://doi.org/10.1016/j.dci.2010.09.002
Chen W, Wang CH (2001) The susceptibility of the giant freshwater prawn Macrobrachium rosenbergii to Lactococcus garvieae and its resistance under copper sulfate stress. Dis Aquat Organ 47(2):137–144. https://doi.org/10.3354/dao047137
Chen SC, Lin YD, Liaw LL, Wang PC (2001b) Lactococcus garvieae infection in the giant freshwater prawn Macrobranchium rosenbergii confirmed by polymerase chain reaction and 16S rDNA sequencing. Dis Aquat Organ 45(1):45–52. https://doi.org/10.3354/dao045045
Chen Y, Chen J, Lin Y, Yeh S, Chao K, Lee C (2014) White shrimp Litopenaeus vannamei that have received Petalonia binghamiae extract activate immunity, increase immune response and resistance against Vibrio alginolyticus. J Aquac Res Dev 5(6):1
Chen S-J, Lin YD, Liaw LL, Wang PC (2001a) Lactococcus garvieae infection in the giant freshwater prawn Macrobranchium rosenbergii confirmed by polymerase chain reaction and 16S rDNA sequencing. 45. https://doi.org/10.3354/dao045045
Cho SH, Jeon GH, Kim HS, Kim DS, Kim C (2013) Effects of Dietary Scutellaria baicalensis Extract on Growth, Feed Utilization and Challenge Test of Olive Flounder (Paralichthys olivaceus). Asian Australas J Anim Sci 26(1):90–96. https://doi.org/10.5713/ajas.2012.12147
Direkbusarakom S, Ezura Y, Yoshimizu M, Herunsalee A (1998) Efficacy of Thai traditional herb extracts against fish and shrimp pathogenic bacteria. Fish Pathol 33(4):437–441
Disch L, Drewe J, Fricker G (2017) Dissolution testing of herbal medicines: challenges and regulatory standards in Europe, the United States, Canada, and Asia. Dissolut Technol 24(2):6–12. https://doi.org/10.14227/DT240217P6
Du J, Zhu H, Liu P, Chen J, Xiu Y, Yao W, … Wang W (2013) Immune responses and gene expression in hepatopancreas from Macrobrachium rosenbergii challenged by a novel pathogen spiroplasma MR-1008. Fish Shellfish Immunol 34(1):315–323. https://doi.org/10.1016/j.fsi.2012.11.009
Estrada N, Velázquez E, Rodríguez-Jaramillo C, Ascencio F (2016) Carbohydrate Moieties and cytoenzymatic characterization of hemocytes in whiteleg shrimp Litopenaeus vannamei. Int J Cell Biol 2016:9032181. https://doi.org/10.1155/2016/9032181
Fagutao FF, Maningas MBB, Kondo H, Aoki T, Hirono I (2012) Transglutaminase regulates immune-related genes in shrimp. Fish Shellfish Immunol 32(5):711–715
Feng S, Zhan W, Xing J, Li J, Yang K, Wang J (2008) Hematological changes in white spot syndrome virus-infected shrimp, Fenneropenaeus chinensis (Osbeck). J Ocean Univ China 7(3):287–293
Galindo-Villegas J, Hosokawa H (2004) Immunostimulants: towards temporary prevention of diseases in marine fish. Advances en Nutricion. Acuicola VII Memorias del VII Simposium Internationale de Nutricion Acuícola, 16–19
Gorman JJ, Folk JE (1980) Transglutaminase amine substrates for photochemical labeling and cleavable cross-linking of proteins. J Biol Chem 255(3):1175–1180
Guo JJ, Her BY, Chou RL, Chen TI (2011) Screening of Modern Herbal Medicines in White Shrimp (Litopenaeus vannamei) against Vibrio harveyi Infection (Vol. 63)
Halim AM, Lee P-P, Chang Z-W, Chang C-C (2017) The hot-water extract of leaves of noni, Morinda citrifolia, promotes the immunocompetence of giant freshwater prawn, Macrobrachium rosenbergii. Fish Shellfish Immunol 64:457–468
Harikrishnan R, Balasundaram C, Jawahar S, Heo M-S (2011) Solanum nigrum enhancement of the immune response and disease resistance of tiger shrimp, Penaeus Monodon against Vibrio Harveyi. Aquaculture 318(1–2):67–73
Hsu JP, Huang C, Liao CM, Hsuan SL, Hung HH, Chien MS (2005) Engulfed pathogen-induced apoptosis in haemocytes of giant freshwater prawn, Macrobrachium rosenbergii. J Fish Dis 28(12):729–735
Interaminense JA, Vogeley JL, Gouveia CK, Portela RS, Oliveira JP, Silva SMBC, … Bezerra RS (2019) Effects of dietary Bacillus subtilis and Shewanella algae in expression profile of immune-related genes from hemolymph of Litopenaeus vannamei challenged with Vibrio parahaemolyticus. Fish Shellfish Immunol 86:253–259. https://doi.org/10.1016/j.fsi.2018.11.051
Iwanaga S (1994) Clotting cascade in the immune response of horseshoe crab. Phylogenetic perspectives in immunity: the insect host defense, 79–96
Jian J, Wu Z (2004) Influences of traditional Chinese medicine on non-specific immunity of Jian carp (Cyprinus carpio var. Jian). Fish Shellfish Immunol 16(2):185–191
Johansson MW (1999) Cell adhesion molecules in invertebrate immunity. Dev Comp Immunol 23(4–5):303–315. https://doi.org/10.1016/s0145-305x(99)00013-0
Lai X, Kong J, Wang Q, Wang W, Meng X (2011) Cloning and characterization of a β-1, 3-glucan-binding protein from shrimp Fenneropenaeus chinensis. Mol Biol Rep 38(7):4527–4535
Lakshmi B, Viswanath B, Sai Gopal DVR (2013) Probiotics as antiviral agents in shrimp aquaculture. J Pathog 2013:13, Article 424123. https://doi.org/10.1155/2013/424123
Lalrinsanga P, Pillai BR, Patra G, Mohanty S, Naik NK, Das RR, … Nelliyoura R (2014) Yield characteristics and morphometric relationships of giant freshwater prawn, Macrobrachium rosenbergii (de Man, 1879). Aquacult Int 22(3):1053–1066
Li C, Lin G, Zuo Z (2011) Pharmacological effects and pharmacokinetics properties of Radix Scutellariae and its bioactive flavones. Biopharm Drug Dispos 32(8):427–445. https://doi.org/10.1002/bdd.771
Liu C-H, Chang C-C, Chiu Y-C, Cheng W, Yeh M-S (2011) Identification and cloning of a transglutaminase from giant freshwater prawn, Macrobrachium rosenbergii, and its transcription during pathogen infection and moulting. Fish Shellfish Immunol 31(6):871–880
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25(4):402–408
Mai W, Hu C (2009) cDNA cloning, expression and antibacterial activity of lysozyme C in the blue shrimp (Litopenaeus stylirostris). Prog Nat Sci 19(7):837–844
Martin GG, Omori JEHS, Chong C, Hoodbhoy T, McKrell N (1991) Localization and roles of coagulogen and transglutaminase in hemolymph coagulation in decapod crustaceans. Comp Biochem Physiol B: Comp Biochem 100(3):517–522. https://doi.org/10.1016/0305-0491(91)90213-W
McGaw IJ, Curtis DL (2013) A review of gastric processing in decapod crustaceans [journal article]. J Comp Physiol B 183(4):443–465. https://doi.org/10.1007/s00360-012-0730-3
Nguyen TMT, Chen T-Y, Shiau C-Y, Cheng Y-T, Chang Y-W (2019) Study on biochemical divergences of the meat and egg of freshwater prawns (Macrobrachium rosenbergii). Food Sci Nutr 7(6):2017–2023. https://doi.org/10.1002/fsn3.1031
Okumura T (2007) Effects of lipopolysaccharide on gene expression of antimicrobial peptides (penaeidins and crustin), serine proteinase and prophenoloxidase in haemocytes of the Pacific white shrimp. Litopenaeus Vannamei Fish & Shellfish Immunology 22(1):68–76. https://doi.org/10.1016/j.fsi.2006.03.013
Pan D, He N, Yang Z, Liu H, Xu X (2005) Differential gene expression profile in hepatopancreas of WSSV-resistant shrimp (Penaeus japonicus) by suppression subtractive hybridization. Dev Comp Immunol 29(2):103–112
Pang Z, Kim SK, Yu J, Jang IK (2014) Distinct regulation patterns of the two prophenoloxidase activating enzymes corresponding to bacteria challenge and their compensatory over expression feature in white shrimp (Litopenaeus vannamei). Fish Shellfish Immunol 39(2):158–167. https://doi.org/10.1016/j.fsi.2014.04.026
Perazzolo L, Gargioni R, Ogliari P, Barracco M (2002) Evaluation of some hemato-immunological parameters in shrimp Farfantepenaeus paulensis submitted to environmental and physiological stress. 214. https://doi.org/10.1016/S0044-8486(02)00137-0
Pohanka M (2013) Role of oxidative stress in infectious diseases. A review. Folia Microbiol (praha) 58(6):503–513. https://doi.org/10.1007/s12223-013-0239-5
Qiao G, Kim S-K, Cho Y-R, Kim S, Jang I-K (2013) Expression of c-type lysozyme from the fleshy shrimp Fenneropenaeus chinensis is upregulated following Vibrio anguillarum and lipopolysaccharide injection. Fisheries and Aquatic Sciences 16(4):267–272
Ragland SA, Criss AK (2017) From bacterial killing to immune modulation: recent insights into the functions of lysozyme. PLoS Pathog 13(9):e1006512
Rajasekar T, Usharani J, Sakthivel M, Deivasigamani B (2011) Immunostimulatory effects of Cardiospermum halicacubum against Vibrio parahaemolyticus on tiger shrimp Penaeus monodon. J Chem Pharm Res 3(5):501–513
Rattanavichai W, Cheng W (2015) Dietary supplement of banana (Musa acuminata) peels hot-water extract to enhance the growth, anti-hypothermal stress, immunity and disease resistance of the giant freshwater prawn, Macrobrachium Rosenbergii. Fish Shellfish Immunol 43(2):415–426. https://doi.org/10.1016/j.fsi.2015.01.011
Reverter M, Bontemps N, Lecchini D, Banaigs B, Sasal P (2014) Use of plant extracts in fish aquaculture as an alternative to chemotherapy: current status and future perspectives. Aquaculture 433:50–61
Robalino J, Almeida JS, McKillen D, Colglazier J, Trent HF, Chen YA, … Gross PS (2007) Insights into the immune transcriptome of the shrimp Litopenaeus vannamei: tissue-specific expression profiles and transcriptomic responses to immune challenge. 29(1):44–56. https://doi.org/10.1152/physiolgenomics.00165.2006
Rosa RD, Barracco MA (2010) Antimicrobial peptides in crustaceans. Invertebr Surviv J 7(2):262–284
Roux MM, Pain A, Klimpel KR, Dhar AK (2002) The lipopolysaccharide and β-1, 3-glucan binding protein gene is upregulated in white spot virus-infected shrimp (Penaeus stylirostris). J Virol 76(14):7140–7149
Saurabh S, Sahoo P (2008) Lysozyme: an important defence molecule of fish innate immune system. Aquac Res 39(3):223–239
Serrano PH (2005) Responsible use of antibiotics in aquaculture. FAO
Shugar D (1952) The measurement of lysozyme activity and the ultra-violet inactivation of lysozyme. Biochim Biophys Acta 8(C):302–309
Silveira AS, Matos GM, Falchetti M, Ribeiro FS, Bressan A, Bachère E, … Rosa RD (2018) An immune-related gene expression atlas of the shrimp digestive system in response to two major pathogens brings insights into the involvement of hemocytes in gut immunity. Dev Comp Immunol 79:44–50
Sivaram V, Babu M, Immanuel G, Murugadass S, Citarasu T, Marian MP (2004) Growth and immune response of juvenile greasy groupers (Epinephelus tauvina) fed with herbal antibacterial active principle supplemented diets against Vibrio harveyi infections. Aquaculture 237(1–4):9–20
Song Y-L, Hsieh Y-T (1994) Immunostimulation of tiger shrimp (Penaeus monodon) hemocytes for generation of microbicidal substances: analysis of reactive oxygen species. Dev Comp Immunol 18(3):201–209. https://doi.org/10.1016/0145-305X(94)90012-4
Soonthornchai W, Rungrassamee W, Karoonuthaisiri N, Jarayabhand P, Klinbunga S, Söderhäll K, Jiravanichpaisal P (2010) Expression of immune-related genes in the digestive organ of shrimp, Penaeus monodon, after an oral infection by Vibrio harveyi. Dev Comp Immunol 34(1):19–28. https://doi.org/10.1016/j.dci.2009.07.007
Sørum M, Johnsen PJ, Aasnes B, Rosvoll T, Kruse H, Sundsfjord A, Simonsen GS (2006) Prevalence, persistence, and molecular characterization of glycopeptide-resistant enterococci in Norwegian poultry and poultry farmers 3 to 8 years after the ban on avoparcin. Appl Environ Microbiol 72(1):516–521. https://doi.org/10.1128/AEM.72.1.516-521.2006
Sricharoen S, Kim JJ, Tunkijjanukij S, Soderhall I (2005) Exocytosis and proteomic analysis of the vesicle content of granular hemocytes from a crayfish. Dev Comp Immunol 29(12):1017–1031. https://doi.org/10.1016/j.dci.2005.03.010
Sritunyalucksana K, Wongsuebsantati K, Johansson MW, Söderhäll K (2001) Peroxinectin, a cell adhesive protein associated with the proPO system from the black tiger shrimp, Penaeus Monodon. Dev Comp Immunol 25(5):353–363. https://doi.org/10.1016/S0145-305X(01)00009-X
Suanyuk N, Dangwetngam M (2014) Identification and pathology of Lactococcus garvieae isolated from cultured and wild giant freshwater prawns (Macrobrachium rosenbergii de Man) in Thailand. Thai J Vet Med 44(3):325
Tassanakajon A, Rimphanitchayakit V, Visetnan S, Amparyup P, Somboonwiwat K, Charoensapsri W, Tang S (2018) Shrimp humoral responses against pathogens: antimicrobial peptides and melanization. Dev Comp Immunol 80:81–93. https://doi.org/10.1016/j.dci.2017.05.009
Theopold U, Schmidt O, Söderhäll K, Dushay MS (2004) Coagulation in arthropods: defence, wound closure and healing. Trends Immunol 25(6):289–294
Tran L, Nunan L, Redman RM, Mohney LL, Pantoja CR, Fitzsimmons K, Lightner DV (2013) Determination of the infectious nature of the agent of acute hepatopancreatic necrosis syndrome affecting penaeid shrimp. Dis Aquat Org 105(1):45–55 (https://www.int-res.com/abstracts/dao/v105/n1/p45-55/)
Valladão G, Gallani S, Pilarski F (2015) Phytotherapy as an alternative for treating fish disease. J Vet Pharmacol Ther 38(5):417–428
Wang Y-C, Chang P-S, Chen H-Y (2008) Differential time-series expression of immune-related genes of Pacific white shrimp Litopenaeus vannamei in response to dietary inclusion of β-1, 3-glucan. Fish Shellfish Immunol 24(1):113–121
Wu C-C, Chang Y-P, Wang J-J, Liu C-H, Wong S-L, Jiang C-M, Hsieh S-L (2015) Dietary administration of Gynura bicolor (Roxb. Willd.) DC water extract enhances immune response and survival rate against Vibrio alginolyticus and white spot syndrome virus in white shrimp Litopeneaus vannamei. Fish Shellfish Immunol 42(1):25–33. https://doi.org/10.1016/j.fsi.2014.10.016
Xie S, Li F, Zhang X, Zhang J, Xiang J (2017) Peritrophin-like protein from Litopenaeus vannamei (LvPT) involved in white spot syndrome virus (WSSV) infection in digestive tract challenged with reverse gavage [journal article]. Chin J Ocean Limnol 35(6):1524–1530. https://doi.org/10.1007/s00343-017-6109-2
Yahaya R, Dash GK, Abdullah MS, Mathews A (2015) Clinacanthus nutans (burm. F.) Lindau: an useful medicinal plant of south-east Asia. Int J Pharmacogn Phytochem Res 7(6):1244–1250
Yan X, Zhang Y, Peng Y, Li X (2022) The water extract of Radix scutellariae, its total flavonoids and baicalin inhibited CYP7A1 expression, improved bile acid, and glycolipid metabolism in T2DM mice. J Ethnopharmacol 293:115238. https://doi.org/10.1016/j.jep.2022.115238
Yeh M-S, Chang C-C, Cheng W (2009) Molecular cloning and characterization of lipopolysaccharide- and beta-1,3-glucan-binding protein from the giant freshwater prawn Macrobrachium rosenbergii and its transcription in relation to foreign material injection and the molt stage. Fish Shellfish Immunol 27(6):701–706. https://doi.org/10.1016/j.fsi.2009.08.014
Yi Q, Liu R, Sun R, Wang L, Zhou Z, Wang M, Liu Y, Sun J, Madzak C, Song L (2014) The protection of CpG ODNs and Yarrowia lipolytica harboring VP28 for shrimp Litopenaeus vannamei against White spot syndrome virus infection. Isj-Invertebr Surviv J 11:119–131
Yin G, Jeney G, Racz T, Xu P, Jun X, Jeney Z (2006) Effect of two Chinese herbs (Astragalus radix and Scutellaria radix) on non-specific immune response of tilapia, Oreochromis niloticus. Aquaculture 253(1–4):39–47. https://doi.org/10.1016/j.aquaculture.2005.06.038
Yin XL, Li ZJ, Yang K, Lin HZ, Guo ZX (2014) Effect of guava leaves on growth and the non-specific immune response of Penaeus monodon. Fish Shellfish Immunol 40(1):190–196. https://doi.org/10.1016/j.fsi.2014.07.001
Zeng D, Chen X, Xie D, Zhao Y, Yang C, Li Y, … Chen X (2013) Transcriptome analysis of Pacific white shrimp (Litopenaeus vannamei) hepatopancreas in response to Taura syndrome virus (TSV) experimental infection. PLoS One 8(2):e57515. https://doi.org/10.1371/journal.pone.0057515
Zhao Q, Chen X-Y, Martin C (2016) Scutellaria baicalensis, the golden herb from the garden of Chinese medicinal plants. Sci Bull 61(18):1391–1398. https://doi.org/10.1007/s11434-016-1136-5
Acknowledgements
The authors are grateful to the Double Degree program, Faculty of Fisheries and Marine Sciences, Brawijaya University, Indonesia, and the Laboratory of Molecular Fish Immunology and Genetics, Department of Tropical Agriculture and International Cooperation, NPUST, Taiwan.
Funding
The Research Center for Animal Biologics supported this research under project number 107–3017-F-020–001.
Author information
Authors and Affiliations
Contributions
Soni Andriawan: concept, methods, critical evaluation, initial draft writing, and writing—review and editing. Hung Tran Bao: methodology, writing—review and editing. Ating Yuniarti: writing—review. Wahyu Purbiantoro: writing—review and editing. Hso Chi Chaung: writing—review. Tsair-Bor Yen: writing—review. Ta-Chih Cheng: concept, methods, critical evaluation, initial draft writing, and writing—review and editing.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Human and animal ethics
Not applicable.
Consent to participate
Not applicable.
Consent for publication
According to the publication.
Competing interests
The authors declare no competing interests.
Additional information
Handling Editor: Gavin Burnell
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Andriawan, S., Bao, H.T., Purbiantoro, W. et al. Reverse-gavage feeding as a novel administration to investigate the immunomodulatory effects of Radix Scutellaria water extract on Macrobrachium rosenbergii immunity. Aquacult Int 31, 1805–1820 (2023). https://doi.org/10.1007/s10499-023-01058-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-023-01058-y