Abstract
This study was conducted to determine the effect of oral administration of Zingiber officinale and Aegle marmelos extracts on the growth performance and immunomodulatory activities of the shrimp Penaeus monodon against white spot syndrome virus (WSSV). A methanol extract of the Z. officinale rhizome and A. marmelos leaf was sprayed into a pellet feed at concentrations of 0% (control), 0.05% (treatment 1), and 0.1% (treatment 2), respectively. Shrimps fed the extract (T1) showed substantial improvements in growth performance and feed utilization efficiency when compared to the control group. Extract-fed shrimp also exhibited an increase in immune-related gene expression in comparison to controls. After 4 weeks of feeding, shrimp were injected with the white spot syndrome virus and observed for another 2 weeks to determine mortality. Compared to shrimp fed the control diet, Z. officinale and A. marmelos extract–fed shrimp displayed a decrease in hemolymph clotting time and an increase in immunological parameters such as total hemocyte count, prophenoloxidase activity, and superoxide dismutase activity both before and after viral challenge. Fourteen days after the WSSV challenge, the cumulative mortality of extract-fed shrimp was considerably lower compared to the mortality of the control group which died shortly after the challenge. Therefore, the Z. officinale and A. marmelos extracts could be used as immunostimulants to enhance the growth and resistance of shrimp against the white spot syndrome virus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Shrimps represent the most widely cultivated crustacean species in the world, with the white leg shrimp, Litopenaeus vannamei, and the black tiger shrimp Penaeus monodon dominating >90% of the market (El-Saadony et al. 2022). Penaeus monodon is the most frequently cultivated shrimp species along the coasts of Asia, Australia, and other Pacific island nations, due to its high domestic and global market demand (Rahi et al. 2022; Xie et al. 2022). With the continual expansion of cultural scale, disease outbreaks pose a significant and ubiquitous threat to the viability and advancement of global shrimp farming, mostly as a result of viral infections responsible for an estimated 40% of tropical shrimp loss (Arbon et al. 2022; Prochaska et al. 2022; Zhang et al. 2023). Among shrimp viruses, white spot syndrome virus (WSSV) is an extremely lethal and virulent pathogen with massive mortality of shrimp nearly 100% within a remarkably short time window of 3–10 days. Therefore, the virus was identified as a reportable pathogen by the International Office of Epizootics (OIE) (Peruzza et al. 2020; Patil et al. 2021; Zhang et al. 2023). Taxonomically, WSSV belongs to a new family Nimaviridae containing a single genus Whispovirus, is a big, enclosed, and rod-shaped double-stranded circular coiled DNA virus whose hosts are diverse including shrimp, crab, crayfish, and lobster (Liu et al. 2021; Jian et al. 2021). Because of the rapid transmission of infection among shrimp populations and the diversity of hosts, it appears nearly impossible to control this virus. As such, novel strategies and therapeutic agents to control WSSV are necessary (Balasubramanian et al. 2008a; Zhang et al. 2023). In order to boost shrimp production, antimicrobials and antibiotics are the most popular means of disease prevention. But, antibiotics are ineffective against viruses; thus, the overuse of these antibiotics by inexperienced aquaculture producers actually increases the rate of mortality among sick aquatic animals and worsens water contamination (Watts et al. 2017; Liao et al. 2022).
Since shrimps lack an adaptive immune system, they are dependent on their innate immune system, which does not allow shrimps to provide an efficient defense mechanism against viral diseases (Salehpour et al. 2021; Mengal et al. 2022; Pooljun et al. 2022). Because of their potential benefits (less side-effects, minimal drug residues, and reasonable pricing) in preventing and treating diseases in aquatic animals, medicinal plants have drawn a lot of attention in research (Shan et al. 2021; Liao et al. 2022; Zhang et al. 2023). In recent years, it has been revealed that various plant extracts, including Argemone mexicana (Palanikumar et al. 2018), Agati grandiflora (Bindhu et al. 2014), Cynodon dactylon (Tomazelli Junior et al. 2017; Howlader et al. 2020), several algae such as Gracilaria corticata (Houshmand et al. 2022), Ulva intestinalis (Klongklaew et al. 2021), or compounds such as cuminaldehyde (Zhang et al. 2023) can boost the immune response and decrease mortality in WSSV-infected shrimps. The plant Zingiber officinale Roscoe (Zingiberacae), often called ginger, is commonly used as a spice and folk remedy. The ginger rhizome, which contains gingerol and shogaol, has been the center of interest for decades (Dalsasso et al. 2022; Bitari et al. 2022). Nowadays, this magnificent rhizome is widely valued because of its cholagogue and hepatoprotective characteristics as well as its antibacterial, antifungal, anti-inflammatory, and anti-vomiting effects (Bitari et al. 2022; Mukherje and Karati 2022). In addition to its clinical utility, ginger may also be effective against viruses such human respiratory syncytial virus, hepatitis C virus, and Chikungunya virus (Mao et al. 2019; Kaushik et al. 2020). In aquaculture, it has been shown that Z. officinale extract enhances the growth performance and immune response of common carp (Cyprinus carpio) and shrimp L. vannamei against Photobacterium damselae (Mohammadi et al. 2020; Shahraki et al. 2021).
Another plant Aegle marmelos, commonly called bael belonging to the family of Rutaceae, is an excellent nutritious and medicinal plant in Ayurveda and is extensively distributed throughout the Southeast Asian countries (Chakthong et al. 2012; Wangkahart et al. 2022). It has been found that the plant possesses several therapeutic activities which help to prevent and treat of numerous diseases (Rahman and Parvin 2014; Wangkahart et al. 2022). The seselin compound isolated from A. marmelos showed activity against Bombyx mori nucleopolyhedrovirus (BmNPV) (Somu et al. 2019), and the extract of different parts of A. marmelos was effective against human coxsackieviruses B1–B6 virus (Badam et al. 2002). In aquaculture, it was found that the extract of A. marmelos could improve the growth and immune response of the fish Nile tilapia (Oreochromis niloticus) against Streptococcus agalactiae (Wangkahart et al. 2022), Cyprinus carpio against Aeromonas hydrophila (Pratheepa et al. 2010), and Catla catla against Pseudomonas aeruginosa (Pratheepa et al. 2011). Thus, these plants have been reported to have medicinal efficacy against a wide range of diseases and also to reduce pathogen impacts in aquaculture. However, extensive research on the effects of oral administration of these extracts on the growth and immunological alterations of WSSV-challenged P. monodon is lacking. Therefore, this study was conducted to determine the effect of dietary administration of Z. officinale and A. marmelos extracts on the growth performance and non-specific immune response of shrimp by determining several parameters as well as the resistance capacity of shrimp (P. monodon) against WSSV.
Materials and methods
Preparation of Z. officinale extract using large-scale extraction
A fresh rhizome of Z. officinale and a fresh leaf of A. marmelos were collected from Khulna, Bangladesh, and voucher specimens were deposited in the BNH library. After being washed and chopped up, the rhizome and leaf were dried in an oven set at 40°C and finally ground into a powder using a mechanical grinder. Approximately 150 g of powder was steeped in 1.5 L of 100% methanol (approximately), sonicated four times for 30 min at 6-h intervals, and then filtered with muslin cloth and filter paper (Whatman no. 1). The solvent was evaporated by a rotary vacuum evaporator (Hahnvapor, Hahnshin, Korea). The residues were kept at 4°C until use.
Preparation of test diets
The appropriate amount of dried extract obtained by large-scale extraction of Z. officinale and A. marmelos was diluted in ethanol and sprayed on the pellet feed (protein, crude fat, and moisture were 32, 7, and 12% respectively) and mixed (Balasubramanian et al. 2008b). Two experimental diets were prepared with shrimp feed containing the extract at concentrations of 0.05% and 0.1% respectively, along with the control diet (no extract). After mixing the extract with the feed, the wet pellet was air-dried and then placed in a 40°C oven to complete the drying process. The control diet was prepared by adding the same volume of solvent (ethanol) to the feed that was devoid of extract. The diet was then coated with a binding gel before applying in water to prevent extract loss in water (Balasubramanian et al. 2008a).
Experimental shrimp collection and maintenance
Healthy, disease-free, and approximately the same-sized experimental shrimp (~6g) were obtained from a commercial shrimp farm in Jhilerdanga, Dumuria, Khulna, and transported to the laboratory under aerated conditions. The shrimp were examined for outward symptoms of WSSV, which was confirmed by PCR. The salinity of rearing water was adjusted to 10 ppt by mixing brine (salinity = 150–200 ppt) purchased from a commercial hatchery with the water. Before filling the experimental tanks (60L) with water, a potable UV device was used to eliminate any microorganisms. In order to maintain the desired water temperature, mini submersible water heaters (RS electrical, 200W) were installed in each tank. Ten juvenile shrimps were placed in each tank and given 10 days to acclimate before the start of the experiment. During the acclimation period, additional shrimp were kept to replace any dead shrimp in experimental tanks. The shrimp were fed at a rate of 5% of body weight twice a day. Before each feeding, waste materials were emptied out using a siphoning process. In order to maintain optimal water quality parameters, 25% of tank water was replaced daily with UV-treated reserved water. In each tank, a single air stone provided constant aeration to maintain the necessary dissolved oxygen (DO) for the entire period. Daily measurements and records of various water quality parameters were made, and the following ranges were maintained; temperature 29–30°C, pH 7.8–8.5, salinity 10–11 ppt, DO >6 mg/L, NH3 <0.1 mg/L (Pholdaeng and Pongsamart 2010).
Preparation of the WSSV inoculum and challenge procedures
The WSSV inoculum was prepared according to Tsai et al. (1999) and Kang et al. (2013). Briefly, 0.5 g of the gill of frozen infected P. monodon (obtained from the Shrimp Research Station, Bagerhat of the Bangladesh Fisheries Research Institute) was crushed and homogenized with 4.5 mL of PBS (phosphate-buffered saline). The material was then centrifuged for 10 min at 400×g and 4°C. The supernatant was collected, filtered through a 0.45-μm pore-size filter, and diluted 10× in order to infect the experimental shrimp at a concentration of 5 μL/g body weight. The injection was administered to the dorso lateral region of the shrimp’s fourth abdominal segment.
Determination of antiviral activity by oral administration of diet
After acclimatization, juvenile shrimp were separated into three experimental groups of ten shrimp in each group, with three replications. In the control group, shrimp were fed the control diet (no extract), in the treatment groups, T1 and T2, shrimp were fed diets containing extracts at concentrations of 0.05% and 0.1%, respectively. Following a 28-day feeding trial, each shrimp cohort was injected with WSSV inoculum and observed for 2 weeks at post-challenge condition. After the challenge, each group continued to receive the same diet as before the challenge until the completion of the experiment.
Data collection and measurement of growth performance
Before the shrimps were challenged with WSSV, growth performance data was collected and several parameters were calculated, such as weight gain (AWG), growth rate (GR), and specific growth rate (SGR). For the purpose of determining feed utilization, parameters such as feed conversion ratio (FCR) and protein efficiency ratio (PER) were utilized. The formulae are as follows (Abdel-Tawwab et al. 2022):
Average weight gain (AWG, g) = Wt − W0
Individual Growth rate (GR, g/day): (Wt − W0)/28
Specific growth rate (SGR; %/day) = 100 [LnWt− LnW0]/28
where Wt designates the final weight (g) and W0 indicates the initial weight (g)
Feed conversion ratio (FCR) = feed intake (g)/weight gain (g)
Protein efficiency ratio (PER) = weight gain (g)/protein intake (g)
Shrimp survival (%) = 100 (final shrimp count/beginning shrimp count)
Hematological analysis and immunological assay
Before and after the challenge, hematological measures such as total hemocyte count (THC), hemolymph clotting time (HCT), and immunological parameters such as prophenoloxidase activity (proPO) and superoxide dismutase activity (SOD) were assessed.
Hemolymph collection and calculation of the total hemocyte count (THC)
Hemolymph (100 μL) was collected from the ventral sinus located at the base of the first abdominal segment of shrimp using a 26-gauge needle of 1-mL syringe with anticoagulant (30 mM trisodium citrate, 10 mM EDTA, 115 mM glucose, 338 mM sodium chloride, pH 7.0) (Le Moullac et al. 1997). Hemolymph was collected and mixed with anticoagulant in a 2:1 ratio (200 μL anticoagulant: 100 μL hemolymph) in a tube to quickly count THC. One drop of Rose Bengal stain solution was added to 20 μL of collected mixed hemolymph to improve the countability of hemocytes by increasing their visibility. To determine the amount of THC, a drop of the combined sample was placed on a hemocytometer (Neubauer improved counting chamber, Precicolor HBG, Germany), covered with glass, and viewed using a microscope (Labomed, USA). THC and dilution correction factor were calculated using the following formulas, where THC was expressed as cells/mL (Japitana et al. 2017).
THC= ((A+B+C+D)/4) × 104 × Dcf
A, B, C, D=block of hemocytometer
Dcf= (volume of anticoagulant + volume of hemolymph)/volume of hemolymph
Determination of hemolymph clotting time (HCT)
The clotting time of hemolymph was determined according to Liu et al. (2019). Shrimp hemolymph was collected in quantities of 100 μL (as described in the “Hemolymph collection and calculation of the total hemocyte count (THC)” section) and placed in a cold Eppendorf tube, from which a 25 μL aliquot was placed into a glass capillary tube that had already been chilled. The tube was positioned vertically so that the hemolymph would flow from the upper end to the lower end due to gravity. When the hemolymph met the lower end of the tube, it was twisted around again, and this process was repeated until the hemolymph coagulated.
Determination of prophenoloxidase (proPO) and superoxide dismutase (SOD) activity
The activity of proPO in hemolymph was measured using a spectrophotometer (C-7200, USA) in accordance with Le Moullac et al. (1997) based on the formation of dopachrome from L-dihydrophenylalanine (L-DOPA). The superoxide dismutase activity of shrimp was measured following the protocol of Creative BioMart, Inc., USA (EC 1.15.1.1), modified from Marklund and Marklund (1974) and Jing and Zhao (1995). The assay is constructed on the basis of the competition between the autoxidation of pyrogallol by O2− and the dismutation of the radical by SOD. One unit of SOD activity is defined as a 50% suppression of the autoxidation of pyrogallol. A TRIS-EDTA buffer solution (solution A) and a 0.2 mM pyrogallol solution (solution B) were produced according to the manufacturer’s instructions (EC 1.15.1.1). The absorbance was measured at 325 nm using a spectrophotometer (Peak instruments, C-7200, USA), and the SOD activity was determined using the formula.
∆A325 blank, autoxidation rate in blank; ∆A325 sample, autoxidation rate in sample; V, volume of sample; D, dilution factor; V1, total volume of the sample; m, weight of the solid sample; R, volume of reaction mixture (4.5 mL).
Detection of WSSV through PCR
PCR analysis was used to detect the WSSV infection in P. monodon following viral challenge. DNA was extracted from shrimp gills and pleopods using the Monarch® Genomic DNA Purification Kit (Cat no: T3010S, New England Biolabs Inc., USA) according to the manufacturer’s protocol. The purity and concentration of DNA were determined by measuring the A260/280 nm ratio with a Nanodrop (Nabi, Microdigital Co., Ltd, Korea). Each DNA sample was subjected to a first and second round (nested) PCR using the OIE-recommended WSSV primer sets WSSV 146 F1 (ACTACTAACTTCAGCCTATCTAG), WSSV146R1(TAATGCGGGTGTAATGTTCTTACGA),WSSV146F2(GTAACTGCCCCTTCCATCTCCA) and WSSV 146 R2 (TACGGCAGCTGCTGCACCTTGT) (Lo et al. 1996). The reaction mixture contained a total volume of 25 μL comprising HotStarTaq® Master Mix Kit (Cat no: 203443. Qiagen, Germany) that included 0.5 μL of forward and reverse primers, 9.5 μL of DNase/RNase-free water, and 2 μL of DNA template. The Bio-Rad T100 PCR Thermal Cycler was used to complete the PCR, which was set to 94°C for 3 min, 40 cycles of 94°C for 20 s, 62°C for 20 s, and 72°C for 30 s, followed by 72°C for 3 min and held at 4°C. Positive samples yield a 1447bp product in the first round of PCR and a 941bp product in the nested PCR (Bateman 2016). The amplified products were then examined on 2% agarose gels stained with DNA Gel Loading Dye (Thermo Scientific™) and visualized using a UV illuminator (Maestrogen, Model-SML-01, Taiwan).
Analysis of immune genes by qPCR
The expression of immune genes such as lysozyme, prophenoloxidase, and penaeidin was done using qPCR. To do this, total RNA was extracted from shrimp hepatopancreas (Rahi et al. 2022) using Trizol (Cat No: FATRR 001, Favorgen Biotech Corp, Taiwan) according to the manufacturer’s protocol. The A260/280 nm ratio was used to determine the quality and concentration of total RNA using Nanodrop (Nabi, Microdigital Co., Ltd, Korea). The first-strand cDNA was then synthesized using the ProtoScript® II First Strand cDNA Synthesis Kit (Catalog no. E6560S, New England Biolabs Inc., USA) and kept at −80 °C for subsequent real-time RT-qPCR. The full-length sequence with the accession number of GenBank is listed following Deris et al. (2020) and Untergasser et al. (2012). The reaction mixture contained 2× of Luna® Universal qPCR Master Mix (Catalog no. M3003S, New England Biolabs Inc., USA), 0.5 μL forward and reverse primers (Table 1), 6.5 μL of DNase/RNase-free water, and 2 μL of cDNA template in a total volume of 20 μL. qPCR was performed via HYRIS bCUBETM (UK) as follows: 95 °C for 2 min, 40 cycles of 95 °C for 5 s, 53–60 °C for 11 s based on the gene, and 72 °C for 19 s, followed by continual heating from 55 to 95 °C for melting curve analysis. 16S rRNA was utilized as a reference gene (Deris et al. 2020). The relative gene expression was calculated using the formula 2−[ΔCt sample−ΔCt control] (Rahi et al. 2022):
Statistical analysis
The data were presented as mean±SD and analyzed using SPSS (Version 16) and one-way analysis of variance (ANOVA). Tukey’s test for multiple comparisons was used to see whether there were significant differences between experimental groups. Using Student’s t-test, the mean values before and after the challenge were compared. Differences were considered significant at p < 0.05.
Results
Shrimp growth, feed utilization, and survival rate
For each administered plant extract, a significant difference (p < 0.05) in terms of shrimp growth and feed utilization was observed between the treatment and control groups (Table 1). There was no significant difference in the initial weight of shrimp in each group. The weight gain (AWG) and specific growth rate (SGR) of extract-treated shrimp in T1 were significantly higher in each plant (p < 0.05) than those of the control group. No significant difference was found (p > 0.05) between the control group and treatment 2 (T2). Similarly, T1 had the significantly lowest FCR, and the highest PER, while control shrimp had the highest FCR and the lowest PER. Between the treatments of shrimp, no significant difference was observed in terms of FW, FCR, or PER. The survival rate of stocked shrimp was 85 to 90%, which was not significantly different from the control and treatment groups prior to the challenge.
Evaluation of total hemocyte count (THC)
The THC levels of the shrimp in the treatment group differed significantly (p < 0.05) from those of the control group both before and after the WSSV challenge (Fig. 1). The THC of shrimp fed the control diet was significantly lower (p < 0.05) than those fed the diet containing extracts. The THC was highest in T1 (p < 0.05) followed by T2 and control groups both pre- and post-WSSV challenge in each plant extract. The THC dropped in all experimental groups following WSSV exposure, although the difference with the pre-challenge condition was not statistically significant (p > 0.05).
Hemolymph clotting time (HCT)
The dietary extract of Z. officinale and A. marmelos had a significant effect (p < 0.05) on the HCT of P. monodon. The control group displayed the longest clotting time, while the shrimp fed with extract added to their diet had the shortest clotting time before and after the challenge, respectively (Fig. 2). The HCT was significantly higher in post-challenged shrimp compared to pre-challenged shrimp except in T1 of each plant. The clotting time also increased as the concentration of extract increased, but it was low compared to the control group.
Prophenoloxidase activity (proPO)
The ProPO levels differed significantly (p < 0.05) between treated and control shrimp both before and after the WSSV challenge (Fig. 3). The highest concentration of proPO was found in the T1 group of each plant, while the lowest concentration was found in the control group. The proPO values were higher in post-challenged shrimp than in pre-challenge shrimp, but only T1 (p < 0.05) showed a significant difference.
proPO of P. monodon fed with dietary extract–treated and untreated shrimp prior to and following the WSSV challenge. Different superscripts in the same color bar indicate significant differences among the control and treatments of each plant (5% significance level). BC, before challenge; AC, after challenge
Superoxide dismutase (SOD) activity
SOD activity differed significantly (p < 0.05) between treatment and control groups both before and after the WSSV challenge (Fig. 4). The T1 group had the highest level of SOD, while the control group had the lowest. The SOD value increased as the concentration of extract in the diet decreased. When comparing the conditions before and after the challenge, the level of SOD decreased in the control group while it rose in the treatment group, although the difference did not reach statistical significance (p > 0.05).
SOD of P. monodon fed with dietary extracts treated and control shrimp both before and after the challenge with WSSV. Different superscripts in the same color bar indicate significant differences among the control and treatments of each plant (5% significance level). BC, before challenge; AC, after challenge
Relative expression of immune genes
The relative expression of three immune genes (lysozyme, proPO, and penaeidin) was measured before the challenge of shrimp with WSSV, and a significant difference was found between the groups for all genes (Fig. 5). The relative expression of the proPO genes was significantly greater in the T1 group, while the expression of the other two genes was insignificant between treatments. In contrast to the expression of other genes, the proPO gene was more expressed in the treated shrimp.
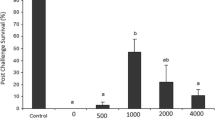
Cumulative mortality of shrimp during the challenge test
After exposure to WSSV, the mortality of shrimp was recorded for up to 2 weeks (Fig. 6). During the whole experiment, no mortality was observed in the unchallenged control group (negative control). There was, however, a significant difference (p < 0.05) in cumulative mortality among the experimental groups of both plants. The cumulative mortality was lowest in treatment 1 (T1), at 20% for both plants treated group, whereas it was 100% in the control group. Shrimp in the control group died within 6 days, exhibiting gross symptoms such as decreased feed consumption and the formation of white spots in the cephalothoracic region. In the control group, the first death occurred 2 days after the challenge, but the treated shrimps’ first deaths happened 5 or 6 days later. The non-challenged and WSSV-challenged shrimp were subjected to PCR analysis to confirm infection. All WSSV-challenged shrimp displayed the typical band at 941 bp, whereas the unchallenged shrimp did not produce a band.
Discussion
The White Spot Disease (WSD), which is caused by WSSV and has a high mortality rate as well as a quick transmission speed, is the most dangerous viral disease that is harming shrimp farming (Ruan et al. 2018; Zhang et al. 2023). Because there is now no therapeutic strategy available, it is of the utmost need to identify a therapeutic agent that can be developed and applied to combat against WSSV in shrimp (Zhang et al. 2023). Extracts containing bioactive components can promote growth, defend against viral and bacterial pathogens by boosting natural resistance and facilitate the treatment and prevention of a variety of disorders (Bindhu et al. 2014; Klongklaew et al. 2021). We found that extract-treated shrimp (T1) had considerably greater final weight, weight gain, and specific growth rate (SGR) compared to the control group. The outcome was consistent with some of the findings of prior studies where shrimp growth characteristics were enhanced by feeding them herbal extracts (Klongklaew et al. 2021; Abdel-Tawwab et al. 2022; Abidin et al. 2022). In the current experiment, the diet with the least amount of extracts (T1) led to the most growth. This is similar to what Yin et al. (2014), Abidin et al. (2022), and Yin et al. (2023) found, where low to moderate amounts of extract from Psidium guajava, Moringa oleifera, and Andrographis paniculata led to higher growth compared to high amounts. It has also been reported that ginger extract at comparatively low concentrations can enhance the growth performance and feed consumption efficiency of L. vannamei and Cyprinus carpio (Mohammadi et al. 2020; Shahraki et al. 2021). Moreover, A. marmelos extract-supplemented diet showed the highest growth performance and feed utilization efficiency in Oreochromis niloticus fed with a moderate dose of diet (20 g/kg) compared to the control group (Wangkahart et al. 2022). This implies that a low concentration of these extracts is advantageous for shrimp development and enhanced feed utilization efficiency (low FCR, high PER) as a result of the improved enzymatic function in the shrimp’s stomach, resulting in increased appetite and feed intake, higher feed utilization, more nutrient absorption, and overall better digestion (Platel and Srinivasan 2004; Yin et al. 2023). Due to the presence of toxic or anti-nutritional elements, however, the addition of a large quantity of extracts may inhibit growth compared to a lower amount (Radhakrishnan et al. 2015; Prabu et al. 2018; Kaleo et al. 2019).
Due to the absence of adaptive immune system processes, the defense mechanisms of shrimp are less advanced than those of fish. Consequently, they appear to rely exclusively on innate defense mechanisms (Ghosh et al. 2021; Fajardo et al. 2022). The majority of the cellular immune reaction in shrimp is performed by hemocytes (hyalinocytes, granulocytes, and semi-granulocytes), which are responsible for phagocytosis, hardening of the exoskeleton, coagulation, and elimination of foreign particles (Kumar et al. 2022; Tran et al. 2022). The total number of hemocytes in shrimp confers immunological capacity and can be used as a reflection of the shrimp’s physiological state as a measure of its resistance to pathogens; hence, it is utilized as a measure of shrimp health (Novriadi et al. 2021; Yin et al. 2023). The findings of this study showed that the THC in shrimp treated with extracts was considerably higher than those in control shrimp both before and after being challenged with WSSV. Similar findings have been reported in a number of studies in which THC was elevated in shrimp fed with Cynodon dactylon (Balasubramanian et al. 2008b), Gracilaria corticata (Afsharnasab et al. 2016; Houshmand et al. 2022), or Gynura bicolor (Wu et al. 2015) enriched diets prior to WSSV challenge. The increased production of THC in the treated shrimp likely resulted from the extract’s ability to promote the immune function as well as the multiplication of younger, better functional hemocytes and to induce the apoptosis of older, less useful hemocytes in the shrimp’s hematopoietic tissues (Chen et al. 2014; Salehpour et al. 2021). THC levels, on the other hand, were found to be lower in post-challenge shrimp than in pre-challenge shrimp, and this finding is in line with the findings of earlier studies in which THC levels were shown to be lower after shrimp were exposed to WSSV (Citarasu et al. 2006; Balasubramanian et al. 2008a; Immanuel et al. 2012). Chang et al. (2003) showed that the THC was substantially reduced to 60% after 24 h of WSSV exposure in comparison to pre-infection with P. monodon. The reduction of hemocytes in infected shrimp may have been due to an accumulation of hemocytes at the injection site for wound repair and phagocytosis of foreign particles (Balasubramanian et al. 2008b; Sarathi et al. 2007). The drop in THC could also be due to virus-induced apoptosis or cell lysis triggered by the virus’s budding, as certain viral infections can drive or repress this type of cell “suicide” (Hameed et al. 2006; Balasubramanian et al. 2008a) or as a result of the invasion of WSSV into hematopoietic tissue, which inhibited hematopoiesis (Afsharnasab et al. 2016).
When compared to control shrimps, the HCT in the extract-treated shrimps was considerably lower both before and after being challenged with WSSV. This result is consistent with the findings of Balasubramanian et al. (2008b) and Velmurugan et al. (2015), in which the clotting time was greatly shortened when the shrimp were administered Cynodon dactylon or Enteromorpha flexuosa extract while testing its efficacy against WSSV. Additionally, it has been discovered that clotting time has an inverse association with THC (semi granulocytes) and, hemolymph protein (Jussila et al. 2001). The highest number of hemocytes and, perhaps, the highest concentration of hemolymph protein in the diet with the lowest quantity of extract (T1) resulted in the shortest clotting time (Jussila et al. 2001; Raja Rajeswari et al. 2012). The clotting time was similarly high in the shrimps following the WSSV challenge, which is consistent with findings from Yoganandhan et al. (2003) and Hameed et al. (2006), who found that WSSV-injected shrimps had a longer HCT than shrimps who were not challenged. The WSSV may be causing the inability of the hemolymph to coagulate and the damage to the coagulation mechanism in infecting shrimp and lobsters, as suggested by the current study. Infected shrimp with a high viral load and low hemocyte count in their hemolymph required more time to coagulate than shrimp whose diets included immunostimulants (Citarasu et al. 2006; Balasubramanian et al. 2008a).
Prophenoloxidase (proPO) is present in hemolymph as the inactive pro-enzyme prophenoloxidase, which is considered the most significant enzyme in the crustacean immune system (Iwanaga and Lee 2005; Abidin et al. 2022). The proPO is subsequently activated to become phenoloxidase (PO), which is responsible for several cellular defense mechanisms, including phagocytosis, hemocyte motility, nodule formation, encapsulation, non-self-recognition among others (Amparyup et al. 2013; Viana et al. 2022). Phenoloxidase initiates the oxidation of tyrosine, which results in the creation of poisonous quinone compounds and other chemical intermediates that ultimately lead to melanin which improves hemocytes’ ability to adhere to germs and speeds up the process of eliminating them through nodule formation (Cerenius et al. 2008; Yildirim-Aksoy et al. 2022). In the present study, the proPO activity of extract-treated shrimp was elevated both before and after WSSV exposure compared to the control. Similar to this, proPO activity in shrimps was enhanced when they were fed an enriched diet containing Cynodon dactylon, Argemone mexicana, Gracilaria tenuistipitata, or Gracilaria corticata as opposed to a control diet to prevent WSSV infection (Balasubramanian et al. 2008b; Sirirustananun et al. 2011; Palanikumar et al. 2018; Houshmand et al. 2022). In addition, the extract of Z. officinale and the component zingerone both had a beneficial influence on the PO activity of L. vannamei when it was tested against bacteria (Chang et al. 2012; Shahraki et al. 2021). Therefore, in the present experiment, the extracts of Z. officinale and A. marmelos served as a promoter and helped to boost proPO enzyme secretion. The immunostimulant components in the extract may have interacted with the pattern recognition proteins (PRPs) of the shrimp immune system and triggered the proPO system, resulting in immunostimulation against WSSV (Citarasu et al. 2006; Palanikumar et al. 2018).
Superoxide dismutase (SOD) is one of the principal antioxidant enzymes in response to oxidative stress and plays an important defensive role by scavenging superoxide anions and other reactive oxygen species (ROS) produced by hemocytes during phagocytosis. It prevents the buildup of free radicals by accelerating the transformation of superoxide radicals into less dangerous molecules like hydrogen peroxide, which is then converted into water and oxygen by other antioxidant enzymes (Campa-Córdova et al. 2002; Balasubramanian et al. 2008a; Abidin et al. 2022). The present study revealed that dietary supplementation with Z. officinale and A. marmelos extracts increased SOD activity in shrimp, resulting in a robust immunological response against WSSV. In accordance with the findings of other studies, such as Balasubramanian et al. (2008b) and Sirirustananun et al. (2011), the dietary extracts of Cynodon dactylon and Gracilaria tenuistipitata increased the SOD activity of shrimps when challenged with WSSV. Similarly, Gynura bicolor extract and Gracilaria corticata extract could enhance the enzymatic activity of SOD against WSSV when shrimp were fed a diet including the extracts (Wu et al. 2015; Afsharnasab et al. 2016). The ginger extract could also make the shrimp L. vannamei more resistant to Photobacterium damselae by increasing its SOD activity (Shahraki et al. 2021). As ginger includes a variety of phenolic chemicals, such as shogaols, gingerols, volatile oils, flavonoids, and phenolic ketone derivatives, they may reduce lipid peroxidation and increase the antioxidant capacity against free radicals (Kim et al. 2007). Moreover, the extract of A. marmelos also improved the SOD activity of the fish Oreochromis niloticus (Wangkahart et al. 2022) and Catla catla (Pratheepa et al. 2011) when they were challenged with Streptococcus agalactiae and Pseudomonas aeruginosa respectively. Thus, diet-containing extracts were able to increase the enzymatic activity of shrimp relative to control shrimp. Increased antioxidant activity in cells is related to a rapid detoxification response and shows the essential role of SOD in removing excess reactive oxygen species from cells (Moreno et al. 2005; Afsharnasab et al. 2016). In the current study, the higher SOD level in post-challenged shrimp was attributed to the increased expression of enzymatic activity by immunostimulant under stress conditions compared to normal conditions. Increased formation of reactive oxygen species (ROS) occurs after WSSV infection of shrimp, which can lead to oxidative stress and cell damage. The cells of the shrimp may produce more SOD due to this oxidative stress to guard against ROS-caused damage. Therefore, a high level of SOD activity is commonly observed in WSSV-infected post-challenge shrimp, as supported by other researches (Prabu et al. 2018; Sivagnanavelmurugan et al. 2014).
Lysozyme and Penaeidin are different anti-microbial peptides (poly) (AMPs) that degrade the microbial cell wall by hydrolysis and hence play an essential function in the immune system of shrimp (Deris et al. 2020; Destoumieux et al. 2000). In the present study, the immune genes were upregulated in shrimp fed with a dietary Z. officinale and A. marmelos extracts, corroborating the findings of a number of previous studies (Sivagnanavelmurugan et al. 2014; Wu et al. 2015; Sinurat et al. 2016; Liu et al. 2019). The proPO gene was considerably upregulated in shrimp fed Sargassum wightii extract compared to the control group prior to the WSSV challenge (Sivagnanavelmurugan et al. 2014). Several immune-related genes were found to be expressed at higher levels in shrimp fed with dietary extracts of Gracilaria tenuistipitata, Gynura bicolor, and fucodian compounds before and during infection with WSSV (Wu et al. 2015; Sinurat et al. 2016; Liu et al. 2019).
The present investigation demonstrates that the cumulative mortality of extract-treated shrimp was considerably lower than that of control shrimp when challenged with WSSV, which is in line with multiple previous studies. Wu et al. (2015) showed that WSSV-challenged shrimp fed with diets containing Gynura bicolor extract had a reduced mortality rate. Similarly, dietary extracts from the plants Argemone mexicana, Ceriops tagal, and Gracilaria tenuistipitata were able to minimize the mortality of shrimps when they were exposed to WSSV (Sirirustananun et al. 2011; Sudheer et al. 2012; Palanikumar et al. 2018). It has also been observed that the addition of ginger extract and zingerone to the diet of L. vannamei shrimp increased their resistance to Photobacterium damselae and Vibrio alginolyticus (Chang et al. 2012; Shahraki et al. 2021). The extract of A. marmelos also increased the immunity and decreased the cumulative mortality of fish species (Oreochromis niloticus, Cyprinus carpio) infected with bacterial pathogens (Wangkahart et al. 2022; Pratheepa et al. 2010). In general, it has been found that certain plant extracts can boost the resistance capability of shrimps against WSSV, which is the cumulative effect of several immune parameters. The low mortality rate observed in the current study may be attributable to a number of factors, including the extract’s immunostimulant activity, which increased THC, proPO, and antioxidant activity against WSSV infection, reduced WSSV activation due to interaction between extract compounds and viral protein, and reduced WSSV multiplication within host cells (Balasubramanian et al. 2007).
Conclusion
In conclusion, the dietary extract of Z. officinale and A. marmelos stimulated appetite and enzymatic activity, resulting in superior growth performance and feed utilization efficiency. The extracts acted as a potential immunostimulant with the biological activity of increasing total hemocytes (THC), prophenoloxidase activity (proPO), superoxide dismutase activity (SOD), and immune gene expression, resulting in disease resistance capacity of P. monodon against WSSV with decreased mortality. The enrichment of the extracts at a low concentration (0.05%) displayed a significantly better outcome in terms of both growth and immunological parameters. Therefore, these plants have the potential to be employed as an immunostimulant in shrimp against WSSV. Additional research is required for extended growth periods, as well as for the isolation and characterization of the active compounds produced by the plant in response to WSSV.
Data availability
All of the data that were generated or utilized throughout the course of the study are included in the article that was submitted.
References
Abdel-Tawwab M, Selema TAA, Khalil RH, El-Sabbagh N, Eldessouki EA, Fawzy RM, Abd El-Naby AS (2022) The growth performance, antioxidant and immune responses, and disease resistance of Litopenaeus vannamei fed on diets supplemented with Indian ginseng (Withania somnifera). Fish Shellfish Immunol 128:19–27
Abidin Z, Huang HT, Hu YF, Chang JJ, Huang CY, Wu YS, Nan FH (2022) Effect of dietary supplementation with Moringa oleifera leaf extract and Lactobacillus acidophilus on growth performance, intestinal microbiota, immune response, and disease resistance in whiteleg shrimp (Penaeus vannamei). Fish Shellfish Immunol 127:876–890
Afsharnasab M, Kakoolaki S, Mohammadidost M (2016) Immunity enhancement with administration of Gracilaria corticata and Saccharomyces cerevisiae compared to gamma irradiation in expose to WSSV in shrimp, in juvenile Litopenaeus vannamei: a comparative study. Fish Shellfish Immunol 56:21–33
Amparyup P, Charoensapsri W, Tassanakajon A (2013) Prophenoloxidase system and its role in shrimp immune responses against major pathogens. Fish Shellfish Immunol 34(4):990–1001
Arbon PM, Condon K, Martinez MA, Jerry DR (2022) Molecular detection of six viral pathogens from Australian wild sourced giant black tiger shrimp (Penaeus monodon) broodstock. Aquaculture 548:737651
Badam L, Bedekar S, Sonavane KB, Joshi SP (2002) In vitro antiviral activity of bael (Aegle marmelos Corr) upon. J Comm Dis 34(2):88
Balasubramanian G, Sarathi M, Kumar SR, Hameed AS (2007) Screening the antiviral activity of Indian medicinal plants against white spot syndrome virus in shrimp. Aquaculture 263(1-4):15–19
Balasubramanian G, Sarathi M, Venkatesan C, Thomas J, Hameed AS (2008a) Studies on the immunomodulatory effect of extract of Cyanodon dactylon in shrimp, Penaeus monodon, and its efficacy to protect the shrimp from white spot syndrome virus (WSSV). Fish Shellfish Immunol 25(6):820–828
Balasubramanian G, Sarathi M, Venkatesan C, Thomas J, Hameed AS (2008b) Oral administration of antiviral plant extract of Cynodon dactylon on a large scale production against white spot syndrome virus (WSSV) in Penaeus monodon. Aquaculture 279(1-4):2–5
Bateman K. (2016). WSSV Proficiency Test 2016. Detection of white spot syndrome virus (WSSV) in shrimp pleopods. EURL Ring Trial Reference Number: EURL16005.
Bindhu F, Velmurugan S, Donio MBS, Michaelbabu M, Citarasu T (2014) Influence of Agathi grandiflora active principles inhibit viral multiplication and stimulate immune system in Indian white shrimp Fenneropenaeus indicus against white spot syndrome virus infection. Fish Shellfish Immunol 41(2):482–492
Bitari A, Oualdi I, Touzani R, Elachouri M, and Legssyer A. (2022). Zingiber officinale Roscoe: a comprehensive review of clinical properties. Materials Today: Proceedings.
Campa-Córdova AI, Hernández-Saavedra NY, Ascencio F (2002) Superoxide dismutase as modulator of immune function in American white shrimp (Litopenaeus vannamei). Comp Biochem Physiol Part C: Toxicol Pharmacol 133(4):557–565
Cerenius L, Lee BL, Söderhäll K (2008) The proPO-system: pros and cons for its role in invertebrate immunity. Trends in Immunol 29(6):263–271
Chakthong S, Weaaryee P, Puangphet P, Mahabusarakam W, Plodpai P, Voravuthikunchai SP, Kanjana-Opas A (2012) Alkaloid and coumarins from the green fruits of Aegle marmelos. Phytochemistry 75:108–113
Chang YP, Liu CH, Wu CC, Chiang CM, Lian JL, Hsieh SL (2012) Dietary administration of zingerone to enhance growth, non-specific immune response, and resistance to Vibrio alginolyticus in Pacific white shrimp (Litopenaeus vannamei) juveniles. Fish Shellfish Immunol 32(2):284–290
Chen YY, Chen JC, Lin YC, Putra DF, Kitikiew S, Li CC et al (2014) Shrimp that have received carrageenan via immersion and diet exhibit immunocompetence in phagocytosis despite a post-plateau in immune parameters. Fish Shellfish Immunol 36(2):352–366
Citarasu T, Sivaram V, Immanuel G, Rout N, Murugan V (2006) Influence of selected Indian immunostimulant herbs against white spot syndrome virus (WSSV) infection in black tiger shrimp, Penaeus monodon with reference to haematological, biochemical and immunological changes. Fish Shellfish Immunol 21(4):372–384
Dalsasso RR, Valencia GA, Monteiro AR (2022) Impact of drying and extractions processes on the recovery of gingerols and shogaols, the main bioactive compounds of ginger. Food Res Int:111043
Deris ZM, Iehata S, Ikhwanuddin M, Sahimi MBMK, Do TD, Sorgeloos P et al (2020) Immune and bacterial toxin genes expression in different giant tiger prawn, Penaeus monodon post-larvae stages following AHPND-causing strain of Vibrio parahaemolyticus challenge. Aquacult Rep 16:100248
Destoumieux D, Muñoz M, Cosseau C, Rodriguez J, Bulet P, Comps M, Bachère E (2000) Penaeidins, antimicrobial peptides with chitin-binding activity, are produced and stored in shrimp granulocytes and released after microbial challenge. J Cell Sci 113(3):461–469
El-Saadony MT, Swelum AA, Ghanima MMA, Shukry M, Omar AA, Taha AE, ... and Abd El-Hack ME, (2022). Shrimp production, the most important diseases that threaten it, and the role of probiotics in confronting these diseases: a review. Res Vet Sci.
Fajardo C, Martinez-Rodriguez G, Costas B, Mancera JM, Fernandez-Boo S, Rodulfo H, De Donato M (2022) Shrimp immune response: a transcriptomic perspective. Rev Aquacult 14(3):1136–1149
Ghosh AK, Panda SK, Luyten W (2021) Anti-vibrio and immune-enhancing activity of medicinal plants in shrimp: a comprehensive review. Fish Shellfish Immunol 117:192–210
Hameed AS, Sarathi M, Sudhakaran R, Balasubramanian G, Musthaq SS (2006) Quantitative assessment of apoptotic hemocytes in white spot syndrome virus (WSSV)-infected penaeid shrimp, Penaeus monodon and Penaeus indicus, by flow cytometric analysis. Aquaculture 256(1-4):111–120
Houshmand H, Ahangarzadeh M, Seyedmortezaei SR, Mohammadidust M, Kakoolaki S, Mohseninejad L (2022) Dietary supplementation of Gracilaria corticata extracts improved immunity of white leg shrimp exposed to white spot virus. Utiliz Cultivat Aqua 11(2):101–117
Howlader P, Ghosh AK, Islam SS, Bir J, Banu GR (2020) Antiviral activity of Cynodon dactylon on white spot syndrome virus (WSSV)-infected shrimp: an attempt to mitigate risk in shrimp farming. Aquacult Int 28(4):1725–1738
Immanuel G, Sivagnanavelmurugan M, Marudhupandi T, Radhakrishnan S, Palavesam A (2012) The effect of fucoidan from brown seaweed Sargassum wightii on WSSV resistance and immune activity in shrimp Penaeus monodon (Fab). Fish Shellfish Immunol 32(4):551–564
Iwanaga S, Lee BL (2005) Recent advances in the innate immunity of invertebrate animals. BMB Rep 38(2):128–150
Japitana F, Haro S, Iwag L, Pilota C, Rapiz A (2017) Shrimp hemolymph extraction. Undergraduate Prerequisite. University of the Philippines Visayas
Jian JT, Liu LK, Liu HP (2021) Autophagy and white spot syndrome virus infection in crustaceans. Fish Shellfish Immunol Rep 100047
Jing TY, Zhao XY (1995) The improved pyrogallol method by using terminating agent for superoxide dismutase measurement. Progr Biochem Biophys 22(1):84–86
Jussila J, McBride S, Jago J, Evans LH (2001) Hemolymph clotting time as an indicator of stress in western rock lobster (Panulirus cygnus George). Aquaculture 199(1-2):185–193
Kaleo IV, Gao Q, Liu B, Sun C, Zhou Q, Zhang H et al (2019) Effects of Moringa oleifera leaf extract on growth performance, physiological and immune response, and related immune gene expression of Macrobrachium rosenbergii with Vibrio anguillarum and ammonia stress. Fish Shellfish Immunol 89:603–613
Kang ST, Wang HC, Yang YT, Kou GH, Lo CF (2013) The DNA virus white spot syndrome virus uses an internal ribosome entry site for translation of the highly expressed nonstructural protein ICP35. J Virol 87(24):13263–13278
Kaushik S, Jangra G, Kundu V, Yadav JP, Kaushik S (2020) Anti-viral activity of Zingiber officinale (Ginger) ingredients against the Chikungunya virus. Virus Dis 31(3):270–276
Kim JK, Kim Y, Na KM, Surh YJ, Kim TY (2007) [6]-Gingerol prevents UVB-induced ROS production and COX-2 expression in vitro and in vivo. Free Rad Res 41(5):603–614
Klongklaew N, Praiboon J, Tamtin M, Srisapoome P (2021) Chemical composition of a hot water crude extract (HWCE) from Ulva intestinalis and its potential effects on growth performance, immune responses, and resistance to white spot syndrome virus and yellowhead virus in Pacific white shrimp (Litopenaeus vannamei). Fish Shellfish Immunol 112:8–22
Kumar S, Verma AK, Singh SP, Awasthi A (2022) Immunostimulants for shrimp aquaculture: paving pathway towards shrimp sustainability. Environ Sci Pollut Res:1–19
Le Moullac GILLES, Le Groumellec MARC, Ansquer D, Froissard S, Levy P (1997) Haematological and phenoloxidase activity changes in the shrimp Penaeus stylirostrisin relation with the moult cycle: protection against vibriosis. Fish Shellfish Immunol 7(4):227–234
Liao W, Huang L, Han S, Hu D, Xu Y, Liu M et al (2022) Review of medicinal plants and active pharmaceutical ingredients against aquatic pathogenic viruses. Viruses 14(6):1281
Liu PC, Lin PW, Huang CL, Hsu CH, Chen JC (2019) Long-term administration of diets containing Gracilaria tenuistipitata extract induce the expression of immune-related genes and increase the immune response and resistance against Vibrio harveyi in white shrimp Litopenaeus vannamei. Gene Rep 15:100378
Liu LK, Liu MJ, Li DL, Liu HP (2021) Recent insights into anti-WSSV immunity in crayfish. Dev Comp Immunol 116:103947
Lo CF, Leu JH, Ho CH, Chen CH, Peng SE, Chen YT et al (1996) Detection of baculovirus associated with white spot syndrome (WSBV) in penaeid shrimps using polymerase chain reaction. Dis Aquat Org 25(1-2):133–141
Mao QQ, Xu XY, Cao SY, Gan RY, Corke H, Beta T, Li HB (2019) Bioactive compounds and bioactivities of ginger (Zingiber officinale Roscoe). Foods 8(6):185
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47(3):469–474
Mengal, K., Kor, G., Kozák, P., and Niksirat, H. (2022). Effects of environmental factors on the cellular and molecular parameters of the immune system in decapods. Comparative Biochemistry and Physiology Part A: Molecular and Integrative Physiology, 111332.
Mohammadi G, Rashidian G, Hoseinifar SH, Naserabad SS, Van Doan H (2020) Ginger (Zingiber officinale) extract affects growth performance, body composition, haematology, serum and mucosal immune parameters in common carp (Cyprinus carpio). Fish Shellfish Immunol 99:267–273
Moreno I, Pichardo S, Jos A, Gómez-Amores L, Mate A, Vazquez CM, Camean AM (2005) Antioxidant enzyme activity and lipid peroxidation in liver and kidney of rats exposed to microcystin-LR administered intraperitoneally. Toxicon 45(4):395–402
Mukherje S, Karati D (2022) A mechanistic view on phytochemistry, pharmacognostic properties, and pharmacological activities of phytocompounds present in zingiber officinale: a comprehensive review. Pharmacol Res –Mod Chinese Med:100173
Novriadi R, Fadhilah R, Wahyudi AE, Trullas C (2021) Effects of hydrolysable tannins on the growth performance, total haemocyte counts and lysozyme activity of pacific white leg shrimp Litopenaeus vannamei. Aquacult Rep 21:100796
Palanikumar P, Benitta DJD, Lelin C, Thirumalaikumar E, Michaelbabu M, Citarasu T (2018) Effect of Argemone mexicana active principles on inhibiting viral multiplication and stimulating immune system in Pacific white leg shrimp Litopenaeus vannamei against white spot syndrome virus. Fish Shellfish Immunol 75:243–252
Patil PK, Geetha R, Ravisankar T, Avunje S, Solanki HG, Abraham TJ et al (2021) Economic loss due to diseases in Indian shrimp farming with special reference to Enterocytozoon hepatopenaei (EHP) and white spot syndrome virus (WSSV). Aquaculture 533:736231
Peruzza L, Thamizhvanan S, Vimal S, Kumar KV, Shekhar MS, Smith VJ et al (2020) A comparative synthesis of transcriptomic analyses reveals major differences between WSSV-susceptible Litopenaeus vannamei and WSSV-refractory Macrobrachium rosenbergii. Dev Comp Immunol 104:103564
Pholdaeng K, Pongsamart S (2010) Studies on the immunomodulatory effect of polysaccharide gel extracted from Durio zibethinus in Penaeus monodon shrimp against Vibrio harveyi and WSSV. Fish Shellfish Immunol 28(4):555–561
Platel K, Srinivasan K (2004) Digestive stimulant action of spices: a myth or reality? Ind J Med Res 119(5):167
Pooljun C, Jariyapong P, Wongtawan T, Hirono I, Wuthisuthimethavee S (2022) Effect of feeding different types of β-glucans derived from two marine diatoms (Chaetoceros muelleri and Thalassiosira weissflogii) on growth performance and immunity of banana shrimp (Penaeus merguiensis). Fish Shellfish Immunol 130:512–519
Prabu DL, Chandrasekar S, Ambashankar K, Dayal JS, Ebeneezar S, Ramachandran K et al (2018) Effect of dietary Syzygium cumini leaf powder on growth and non-specific immunity of Litopenaeus vannamei (Boone 1931) and defense against virulent strain of Vibrio parahaemolyticus. Aquaculture 489:9–20
Pratheepa V, Ramesh S, Sukumaran N (2010) Immunomodulatory effect of Aegle marmelos leaf extract on freshwater fish Cyprinus carpio infected by bacterial pathogen Aeromonas hydrophila. Pharmaceut Biol 48(11):1224–1239
Pratheepa V, Madasamy D, Sukumaran N (2011) Immunomodulatory activity of Aegle marmelos in freshwater fish (Catla catla) by non-specific protection. Pharmaceut Biol 49(1):73–77
Prochaska J, Poompuang S, Koonawootrittriron S, Sukhavachana S, Na-Nakorn U (2022) Evaluation of a commercial SPF Litopenaeus vannamei shrimp breeding program: Resistance to infectious myonecrosis virus (IMNV), Taura syndrome virus (TSV), and white spot syndrome virus (WSSV) from laboratory challenges. Aquaculture 554:738145
Radhakrishnan S, Saravana Bhavan P, Seenivasan C, Muralisankar T, Shanthi R (2015) Effects of native medicinal herbs (Alternanthera sessilis, Eclipta alba and Cissus quadrangularis) on growth performance, digestive enzymes and biochemical constituents of the monsoon river prawn Macrobrachium malcolmsonii. Aquacult Nutr 21(4):496–506
Rahi ML, Sabbir W, Salin KR, Aziz D, Hurwood DA (2022) Physiological, biochemical and genetic responses of black tiger shrimp (Penaeus monodon) to differential exposure to white spot syndrome virus and Vibrio parahaemolyticus. Aquaculture 546:737337
Rahman S, Parvin R (2014) Therapeutic potential of Aegle marmelos (L.)-an overview. Asian Pac J Tropic Dis 4(1):71–77
Raja Rajeswari P, Velmurugan S, Michael Babu M, Albin Dhas S, Kesavan K, Citarasu T (2012) A study on the influence of selected Indian herbal active principles on enhancing the immune system in Fenneropenaeus indicus against Vibrio harveyi infection. Aquacult Int 20(5):1009–1020
Ruan L, Liu H, Shi H (2018) Characterization and function of GSK3β from Litopenaeus vannamei in WSSV infection. Fish Shellfish Immunol 82:220–228
Salehpour R, Biuki NA, Mohammadi M, Dashtiannasab A, Ebrahimnejad P (2021) The dietary effect of fucoidan extracted from brown seaweed, Cystoseira trinodis (C. Agardh) on growth and disease resistance to WSSV in shrimp Litopenaeus vannamei. Fish Shellfish Immunol 119:84–95
Sarathi M, Ahmed VI, Venkatesan C, Balasubramanian G, Prabavathy J, Hameed AS (2007) Comparative study on immune response of Fenneropenaeus indicus to Vibrio alginolyticus and white spot syndrome virus. Aquaculture 271(1-4):8–20
Shahraki N, Imanpour MR, Akbary P, Safari R, Jafari V (2021) Dietary administration of aqueous Zingiber officinale extract on growth performance, antioxidant activity and resistance of shrimp Litopenaeus vannamei against Photobacterium damselae. Iran J Fisheries Sci 20(1):32–44
Shan LP, Zhou Y, Yan MC, Liu L, Chen J, Chen JP (2021) A novel antiviral coumarin derivative as a potential agent against WSSV infection in shrimp seedling culture. Virus Res 297:198387
Sinurat E, Saepudin E, Peranginangin R, Hudiyono S (2016) Immunostimulatory activity of brown seaweed-derived fucoidans at different molecular weights and purity levels towards white spot syndrome virus (WSSV) in shrimp Litopenaeus vannamei. J Appl Pharmaceut Sci 6(10):82–91
Sirirustananun N, Chen JC, Lin YC, Yeh ST, Liou CH, Chen LL et al (2011) Dietary administration of a Gracilaria tenuistipitata extract enhances the immune response and resistance against Vibrio alginolyticus and white spot syndrome virus in the white shrimp Litopenaeus vannamei. Fish Shellfish Immunol 31(6):848–855
Sivagnanavelmurugan M, Thaddaeus BJ, Palavesam A, Immanuel G (2014) Dietary effect of Sargassum wightii fucoidan to enhance growth, prophenoloxidase gene expression of Penaeus monodon and immune resistance to Vibrio parahaemolyticus. Fish Shellfish Immunol 39(2):439–449
Somu C, Karuppiah H, Sundaram J (2019) Antiviral activity of seselin from Aegle marmelos against nuclear polyhedrosis virus infection in the larvae of silkworm, Bombyx mori. J Ethnopharmacol 245:112155
Sudheer NS, Philip R, Bright Singh IS (2012) Anti–white spot syndrome virus activity of Ceriops tagal aqueous extract in giant tiger shrimp Penaeus monodon. Arch Virol 157(9):1665–1675
Tomazelli Junior O, Kuhn F, Mendonça Padilha PJ, Mota Vicente LR, Winckler da Costa S, Corrêa da Silva B et al (2017) Effect of Cynodon dactylon extract on white spot virus-infected Litopenaeus vannamei. Aquacult Int 25(3):1107–1122
Tran NT, Liang H, Zhang M, Bakky MAH, Zhang Y, Li S (2022) Role of cellular receptors in the innate immune system of crustaceans in response to white spot syndrome virus. Viruses 14(4):743
Tsai MF, Kou GH, Liu HC, Liu KF, Chang CF, Peng SE et al (1999) Long-term presence of white spot syndrome virus (WSSV) in a cultivated shrimp population without disease outbreaks. Dis Aquat Org 38(2):107–114
Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, Rozen SG (2012) Primer3—new capabilities and interfaces. Nucl Acids Res 40(15):e115–e115
Velmurugan S, Jerin N, Michael Babu M, Bindhu F, Albin Dhas S, Citarasu T (2015) Screening and characterization of antiviral compounds from Enteromorpha flexuosa against white spot syndrome virus (WSSV) and its in vivo influence on Indian white shrimp Fenneropenaeus indicus. Aquacult Int 23(1):65–80
Viana JT, dos Santos Rocha R, Maggioni R (2022) Structural and functional diversity of lectins associated with immunity in the marine shrimp Litopenaeus vannamei. Fish Shellfish Immunol
Wangkahart E, Wachiraamonloed S, Lee PT, Subramani PA, Qi Z, Wang B (2022) Impacts of Aegle marmelos fruit extract as a medicinal herb on growth performance, antioxidant and immune responses, digestive enzymes, and disease resistance against Streptococcus agalactiae in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol 120:402–410
Watts JE, Schreier HJ, Lanska L, Hale MS (2017) The rising tide of antimicrobial resistance in aquaculture: sources, sinks and solutions. Mar Drugs 15(6):158
Wu CC, Chang YP, Wang JJ, Liu CH, Wong SL, Jiang CM, Hsieh SL (2015) Dietary administration of Gynura bicolor (Roxb. Willd.) DC water extract enhances immune response and survival rate against Vibrio alginolyticus and white spot syndrome virus in white shrimp Litopeneaus vannamei. Fish Shellfish Immunol 42(1):25–33
Xie S, Wei D, Liu Y, Tian L, Niu J (2022) Dietary fish oil levels modulated lipid metabolism, immune response, intestinal health and salinity stress resistance of juvenile Penaeus monodon fed a low fish-meal diet. Animal Feed Sci Technol 289:115321
Yildirim-Aksoy M, Eljack R, Peatman E, Beck BH (2022) Immunological and biochemical changes in Pacific white shrimp, Litopenaeus vannamei, challenged with Vibrio parahaemolyticus. Micro Pathogen 172:105787
Yin XL, Li ZJ, Yang K, Lin HZ, Guo ZX (2014) Effect of guava leaves on growth and the non-specific immune response of Penaeus monodon. Fish Shellfish Immunol 40(1):190–196
Yin X, Zhuang X, Liao M, Cui Q, Yan C, Huang J et al (2023) Andrographis paniculata improves growth and non-specific immunity of shrimp Litopenaeus vannamei, and protects it from Vibrio alginolyticus by reducing oxidative stress and apoptosis. Dev Comp Immunol 139:104542
Yoganandhan K, Thirupathi S, Hameed AS (2003) Biochemical, physiological and hematological changes in white spot syndrome virus-infected shrimp. Penaeus indicus. Aquacult 221(1-4):1–11
Zhang X, Hu LH, Song DW, Hu Y, Chen J (2023) Evaluation on prevention and treatment of cuminaldehyde in culture of shrimp against white spot syndrome virus. Aquaculture 562:738760
Acknowledgements
The authors are grateful to the Head of the Fisheries and Marine Resource Technology Discipline at Khulna University, Bangladesh, for facilitating the use of the laboratory facilities. The authors would like to thank the Director General and Station Chief of the Shrimp research station at the Bangladesh Fisheries Research Institute in Bagerhat for their logistical support. We appreciate Md. Sohel Rana and Md. Omar Ali’s technical assistance.
Funding
A. K. Ghosh was funded by the Prime Minister Fellowship from the Government of the People’s Republic of Bangladesh. W. Luyten largely supported himself.
Author information
Authors and Affiliations
Contributions
Alokesh Kumar Ghosh: conceptualization, methodology, software, data curation, writing—original draft preparation. Shaikh Shaon Ahmmed: methodology, data curation. H. M. Rakibul Islam: methodology, data curation. Md. Abir Hasan: methodology, data curation. Ghausiatur Reza Banu: supervision, writing—review & editing. Sujogya Kumar Panda: supervision, writing—review & editing. Liliane Schoofs: supervision, writing—review & editing. Walter Luyten: supervision, funding acquisition, writing—review & editing.
Corresponding author
Ethics declarations
Ethical approval
The authors affirm that all experiments were conducted in accordance with all applicable rules and guidelines.
Competing interests
The authors declare no competing interests.
Additional information
Handling Editor: Brian Austin
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ghosh, A.K., Ahmmed, S.S., Islam, H.M.R. et al. Oral administration of Zingiber officinale and Aegle marmelos extracts enhances growth and immune functions of the shrimp Penaeus monodon against the white spot syndrome virus (WSSV). Aquacult Int 32, 613–632 (2024). https://doi.org/10.1007/s10499-023-01177-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-023-01177-6