Abstract
This study investigated physiological and behavioral responses of pikeperch broodstock related to sexual interactions under controlled conditions throughout semi-artificial reproduction. Blood samples taken from pond-cultured pikeperch broodstock were analyzed to assess the stress response at different stages of semi-artificial reproduction protocol. Sampling was performed as follows: before hormonal induction (control), 24 h (SP24) and 48 h (SP48) after spawning when males and females were separated, and 24 h (NS24) and 48 h (NS48) after spawning when males and females were kept together. The separation immediately after spawning had a significant effect on physiological state of broodstock. Separation affected indices of erythrocyte count, hematocrit (packed cell volume, PCV), glucose (GLU), and mean corpuscular volume (MCV) in females and levels of lactate and leukocytes in males. Monitoring of pikeperch behavior in groups NS24 and NS48 revealed the strong aggressive paternal behavior of male to females. Attacks mainly targeted the caudal fin and resulted in female mortality in group NS48.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the last few decades, commercial interest in pikeperch culture prioritized research developing reproduction techniques (Blecha et al. 2016; Policar et al. 2016; Samarin et al. 2015). Despite current progress in domestication, wild-caught and pond-cultured pikeperch are still a source of broodstock providing high-quality gametes (Fontaine et al. 2009, 2015; Křišťan et al. 2012, 2018; Ljubobratović et al. 2017; Khendek et al. 2018; Kestemont and Henrotte, 2015). However, the biggest disadvantage of wild and pond-cultured broodstock is their higher post-spawning mortality in comparison to broodstock reared in recirculation aquatic systems (RASs) (Łuczyński et al. 2007; Rónyai 2007; Zakęś and Demska-Zakęś 2009; Zakęś et al. 2013).
Semi-artificial or so-called “nest” spawning is an effective and convenient reproduction method for pond-cultured and wild broodstock. Unlike artificial reproduction (stripping), where post-spawning losses may reach up to 100% in several days after spawning (Gomułka et al. 2007; Łuczyński et al. 2007; Rónyai 2007; Zakęś and Demska-Zakęś 2009). Semi-artificial reproduction in RASs and ponds (Malinovskyi et al. 2018) has significantly less handling requirements, and broodstock survival after recovery treatment can be about 92–100% depending on the sex (Policar et al. 2019).
Stress response is a generically costly process resulting in poor physiological status of broodstock (Schreck et al. 2001; Schreck 2010; Sarameh et al. 2013). Post-spawning losses of pikeperch are mainly caused by exposure to artificial environment and handling procedures resulting in a decrease of immunity level and subsequent outbreak of either protozoan, bacterial, or fungal infections (Gomułka et al. 2007; Muller-Belecke and Zienert 2008; Zakęś and Demska-Zakęś 2009). Antifungal and antibacterial treatments could increase survival (Policar et al. 2019), even though their application aimed to resolve secondary consequences, like diseases, rather than improvement of the poor health status.
Physiological changes and stress responses of pikeperch broodstock on artificial photoperiod, hormonal treatment, and handling were studied during nest or artificial spawning by Sarameh et al. (2012, 2013) and Falahatkar and Poursaeid (2014); however, broodstock physiology and stress response were not documented in relation to broodstock behavioral interaction between males and females. General spawning behavior before and during nest spawning in pikeperch was observed by Drasovean and Blidariu (2013), but neither of the male’s aggression during spawning nor problematic interactions between sexes after spawning were mentioned there.
The aims of this study were (1) to investigate the health and physiological status of pikeperch broodstock during semi-controlled reproduction, (2) to determine the most stressful steps related to broodstock sexual behavior, and finally, (3) to optimize broodstock management in pond-cultured pikeperch during semi-artificial reproduction.
Materials and methods
Fish and experimental conditions
In total, 15 males (total length (TL) = 542 mm ± 37 mm and body weight (BW) = 1487 g ± 434 g) and 15 females (TL = 532 mm ± 40 mm and BW = 1353 g ± 309 g) of matured pikeperch were used in this study. All broodstock fish were captured from the production pond of the fish farm Rybářství Nové Hrady, Ltd., during the autumn harvesting season, before being transferred to an earthen pond at the Faculty of Fisheries and Protection of Waters, University of South Bohemia in Vodňany, Czech Republic, for overwintering. In early April, males and females were randomly selected for the study and stocked into a RAS. Fish were evenly distributed among six tanks with a volume of 350 l—three for males and three for females. After 2 weeks of adaptation, pikeperch broodstock were divided into pairs for sampling throughout reproduction (described in Table 1). Each pair of pikeperch was stocked into separate 350-l tanks connected to a RAS. Throughout the experiment, fish were exposed with artificial luminescent light. The intensity was set on 50–100 lx on water surface with the photoperiod of 13 h light and 11 h dark (13L:11D) in accordance with the latitude of the Czech Republic (at the end of April). Water temperature was constantly set at 15 °C ± 0.3 °C (Blecha et al. 2015), and oxygen saturation was maintained at a level of 107% ± 2.7%. The nests, made from artificial grass, were placed on the bottom of each tank as a spawning substrate (Malinovskyi et al. 2018). For the induction and synchronization of spawning, both sexes were intramuscularly injected with the human chorionic gonadotropin (hCG) (Chorulon; Intervet, Netherlands) at a concentration of 500 IU per kg (Křišťan et al. 2013).
All the manipulations with fish during the experiment were done under anesthesia (clove oil, 0.03 ml l−1; according to Křišťan et al. 2014) in accordance with the directive 2010/63/EU on the protection of animals used for scientific purposes. The injection of hormonal agents and samplings of the blood were performed with veterinary guidance in accordance with good veterinary practice. The fish in this experiment have not been used more than once in procedures involving pain, distress, or suffering.
Blood sampling
Three pairs of fish were sampled on different stages of semi-artificial reproduction protocol: before hormonal treatment and 24 h and 48 h after spawning (Table 1). After anesthetizing the fish, blood samples were collected from vena caudalis using a heparinized needle and 1-ml volume syringe. Immediately after collection, the blood was transferred to microtubes rinsed with sodium heparin (Heparin Léčiva inj. sol.; Zentiva, Prague, Czech Republic) 40 IU per ml of blood to prevent coagulation.
The blood samples for biochemical analyses were immediately centrifuged at 1500×g for 10 min in a microcentrifuge (MPW 55; MPW Instruments, Warszawa, Poland) after sampling, and the blood plasma was transferred on ice and stored in − 80 °C before analyses. The blood samples for hematological analyses were processed immediately after sampling. After the sampling procedure, fish were transferred into a RAS with a constant water salinity of 5–10 g l−1 of NaCl for elimination of secondary fungal infection (Policar et al. 2019). Comparison of hematological and biochemical indices was used for evaluation of physiological changes during semi-artificial reproduction with respect to sexual behavior with the aim of optimizing the separation of females after spawning.
Measurement of hematological and biochemical parameters
The hematological profile was evaluated immediately after blood sampling according to Svobodova et al. (2012) and included an erythrocyte (Er) count, hematocrit (packed cell volume, PCV), hemoglobin (Hb), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), and leukocyte (Leuko) count. Cortisol was assayed in the blood plasma by RIA using a commercial antibody kit (cortisol-3-OCMO antiserum; Immunotech, Prague, Czech Republic). The following biochemical parameters in the blood plasma: calcium (Ca2+), glucose (GLU), and lactate (LACT) were assayed by the methods described by Kolářová and Velíšek (2012) using the blood gas analyzer VetTest 8008 (IDEXX Laboratories, Inc., USA).
Monitoring of pikeperch behavior after spawning and evaluation of fin erosion
A waterproof digital camera (LAMAX Action X8 Electra; elem6 s.r.o., Czech Republic) was used for observing pikeperch behavior after nest spawning. Five-minute recordings were randomly collected during the light phase within the following periods: 0–24 h, 24–48 h, and 48–72 h post spawning in groups NS24 and NS48. The total duration of records accounted 135 min for each 0–24-h and 24–48-h period and 120 min for the period 48–72 h after spawning. After recording, completed video files were analyzed to determine specific patterns of sexual behavior after spawning such as the number of male attacks on female per minute.
The caudal fins of both sexes in all groups were photographed and processed throughout the experiment. Evaluation of fin erosion was done by image analysis with QuickPHOTO MICRO 3.0 software. Fin erosion was shown as a percentage of the fragmented and inflamed area of the total caudal fin surface (according to Policar et al. 2016).
After the experiment, all fish were transferred to a RAS with brackish water in a concentration of 5–10 g l−1 for recovery and were fed ad libitum with prey fish (Pseudorasbora parva) according Policar et al. (2019). After 2 weeks of the recovery period, pikeperch broodstock were restocked to the local polyculture pond.
Statistical analysis
Data from 15 pikeperch males and 14 females were analyzed due to the mortality of one female at the end of the experiment. Due to the rareness of matured pikeperch during spawning season, it was possible to get samples of only three individuals for each tested group. Although the test strength of some data points was not high, it was enough to find significant differences among groups in the following parameters.
The normal distribution of data was ensured by the Shapiro–Wilk test. Normal distribution of caudal fin erosion data was achieved by square root transformation. After the parametric assumptions were met, differences in hematological, biochemical, and caudal fin erosion values were estimated using two-way analysis of variance (ANOVA) followed by post hoc Tukey tests to find the differences among tested groups. All analyses were conducted using the statistical package Statistica 13 (StatSoft, Inc.). For all tests, the level of significance was set at p < 0.05. Results were presented as mean ± SD. Statistical differences during reproduction are marked in Tables 2 and 3 according to the numbers of fish groups (described in Table 1).
Results
Hematological profile
Changes of hematological profile in broodstock of each group are summarized in Table 2. Indices of Hb in the blood of females were significantly lower (p < 0.05) in groups NS48 (44.76 g l−1 ± 5.37 g l−1) and SP24 (46.19 g l−1 ± 6.53 g l−1) compared to the control group (58.01 g l−1 ± 4.12 g l−1), while there were no significant differences in males among tested groups. Indices of PCV were significantly lower (p < 0.05) in all tested groups in comparison to control groups in both males and females.
Indices of Er count in blood had significant differences (p < 0.05) only between the males and females from groups NS24 and NS48 and accounted the following: in NS24, 1.86 T l−1 ± 0.18 T l−1 for males and 1.48 T l−1 ± 0.04 T l−1 for females, and in NS48, 1.90 T l−1 ± 0.15 T l−1 for males and 1.43 T l−1 ± 0.16 T l−1 for females.
Values of MCV in females were significantly lower (p < 0.05) in groups SP24 (133.83 fentoliters (fl) ± 13.56 fl) and SP48 (198.00 fl ± 48.16 fl) compared to groups NS24 (222.77 fl ± 9.37 fl) and NS48 (217.48 fl ± 16.69 fl) and the control group (259.91 fl ± 31.04 fl). Indices of MCV in males varied with the same trend and were significantly lower in groups NS24 (187.29 fl ± 19.61 fl), NS48 (181.13 fl ± 24.4 fl), and SP24 (156.99 fl ± 24.82 fl) when compared to the control group.
Indices of Leuko count in the blood of females had no significant differences among tested groups. Values of Leuko count in males were significantly higher in group NS48 (14.33 g l−1 ± 6.25 g l−1) in comparison with groups SP24 (3.67 g l−1 ± 1.25 g l−1) and SP48 (5.50 g l−1 ± 2.45 g l−1). There were no significant differences in indices of MCH and MCHC among tested groups.
Biochemical plasma profile
Changes in indices of biochemical plasma profile of broodstock are summarized in Table 3. Females’ level of cortisol was significantly higher (p < 0.05) in comparison to males in the control group alone (68.34 ng ml−1 ± 17.47 ng ml−1 for males and 228.90 ng ml−1 ± 96.65 ng ml−1 for females). Although the given high variability in cortisol values, there was no significance in tested groups, although the general baseline of cortisol level was higher in females compared to the males.
Indices of Ca2+ in the plasma of females varied without significant differences among tested groups. Values of Ca2+ in the plasma of males from group NS48 (4.18 mmol l−1 ± 2.36 mmol l−1) were significantly higher (p < 0.05) compared to those from groups NS24 (2.32 mmol l−1 ± 0.16 mmol l−1), SP24 (2.25 mmol l−1 ± 0.22 mmol l−1), and SP48 (2.35 mmol l−1 ± 0.15 mmol l−1).
Values of GLU in the plasma of females from group SP24 (1.94 mmol l−1 ± 0.28 mmol l−1) were significantly lower (p < 0.05) compared to group NS48 (8.84 mmol l−1 ± 5.84 mmol l−1) and the control group (7.97 mmol l−1 ± 4.23 mmol l−1). Indices of GLU in the plasma of males were not significantly different among tested groups.
LACT plasma indices of females did not differ significantly among tested groups, while blood LACT indices of males were significantly higher (p < 0.05) in group SP48 (5.80 mmol l−1 ± 2.85 mmol l−1) compared to all tested groups.
Monitoring of pikeperch behavior after spawning and evaluation of fin erosion
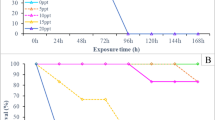
After spawning, all males exhibited strong aggressive paternal behavior to females in groups NS24 and NS48. There was no evidence of female aggressive behavior, and all attacks were initiated by males. The attack frequency accounted 1.68 ± 1.46 attacks per min within 0–24 h after spawning, 2.26 ± 1.14 attacks per min within 24–48 h after spawning, and 2.56 ± 2.36 attacks per min within 48–72 h after spawning without significant differences between groups. The caudal fin and tail of females were the most often attacked, resulting in fin damages (Fig. 1). Significantly higher levels (F(3, 15) = 19.943, p < 0.05) of caudal fin deterioration erosion were observed in females from groups NS48 and NS24 (23.8% ± 2.9% and 15.7% ± 3.5%, respectively). For both males and females in the rest of the groups, this value did not exceed a mean of 3% (Table 4). The experiment was stopped when the first female mortality occurred. The death was caused by the male attacking, and subsequently damaging, body tissue. After the end of the experiment, males and females were separated in different tanks for 2 weeks of the recovery period. No further mortality was observed.
Discussion
This study revealed differences in physiological response between pond-cultured males and females with regard to nest spawning under controlled conditions of the RAS.
The hematological and biochemical profiles are often used for estimation of physiological status and stress response in fish (Kusakabe et al. 2003; Noaksson et al. 2005; Ghosh and Joshi 2008; Westring et al. 2008; Milla et al. 2009; Sarameh et al. 2013; Falahatkar and Poursaeid 2014; Křišťan et al. 2014; Lepič et al. 2014; Svačina et al. 2016). The experimental design elucidated crucial points of pikeperch reproduction for both males and females, considering certain aspects of behavior, i.e., aggressive paternal behavior of males (Lappalainen et al. 2003), when fish were not separated after spawning completion. There is no investigation about this aspect in pikeperch paternal behavior with regard to the controlled condition of reproduction unit. Results from this study could serve for optimization of broodstock management procedures and for decreasing, or even eliminating, issues related to higher mortality of outdoor-reared broodstock and thus minimizing costs needed for production (Zakęś and Demska-Zakęś 2009; Zakęś et al. 2013).
In this study, strong and aggressive behavior of males drastically affected the physiological status of the females. Our results are in line with other authors reporting higher sensitivity of female pikeperch during reproduction (Rónyai 2007; Falahatkar and Poursaeid 2014; Falahatkar et al. 2014; Sarameh et al. 2013; Ljubobratović et al. 2017). Falahatkar and Poursaeid (2014) observed higher levels of cortisol in pikeperch females from control groups as well as in response to the hormonal induction compared to the males. The highest values were found in females treated with hCG. Similar pattern was found in our study, where both of the sexes were treated with hCG and females exhibited higher cortisol levels in blood compared to the males. However, due to the high variability, those differences were significant only in a control group. The baseline of cortisol concentrations was in range as described by Sarameh et al. (2013), although they were lower than those in juveniles reported by Falahatkar et al. (2012), which could be explained by the age of pikeperch used for the investigation.
Changes in blood plasma cortisol levels with regard to maturation are common in fish (Milla et al. 2009). Physiological changes occurring during the spawning period induced cortisol level fluctuation. Furthermore, the literature validates the essential role of corticosteroids for regulation of reproductive mechanisms (Cook et al. 1980; Pickering and Christie 1981; Bry 1985; Kime and Dolben 1985; Andersen 2002; Milla et al. 2009). In this study, higher cortisol level in the blood of females may not necessarily relate to stress alone but may stem from physiological changes in the final stages of maturation (Andersen 2002). Current fish literature reports a broad increase in blood cortisol levels during late maturation and spawning (Kusakabe et al. 2003; Noaksson et al. 2005; Westring et al. 2008; Milla et al. 2009; Faught and Vijayan 2018).
In response to physiological stress, cortisol released in fish body induces both glycogenesis and gluconeogenesis resulting in increased levels of GLU in the blood plasma which is directly related to the energy metabolism (Ghosh and Joshi 2008; Milla et al. 2009). This study observed consistently high levels of GLU in both males and females throughout the semi-controlled reproduction procedure. The only significant decrease of GLU was found in females from group SP24 that could indicate the positive effect of separation after spawning. Despite the absence of significance, there was an overall tendency of higher levels of GLU in groups NS24 and NS48 for both males and females. This could confirm that higher stress level was a consequence of keeping male and female together and concomitant male aggressive behavior (Lappalainen et al. 2003).
There is a lack of information regarding Ca2+ metabolism in fish; however, an increase in Ca2+ ion concentration is rather related to acute respiratory acidosis (Ghosh and Joshi 2008). This combined with lower numbers of erythrocytes could lead to insufficient nutrient transport and low tissue oxygenation with subsequent anemia (Nakayasu et al. 2002; Witeska 2015). In this study, levels of Ca2+ were significantly higher in males that were not separated from the females (NS48) after spawning and indicated higher movement activity in comparison to separated fish.
Physiological stress is often combined with increased movement activity of the fish (Thomas et al. 1999; Sarameh et al. 2013). Subsequent diminished oxygen availability leads to an increase of LACT in the blood plasma (Omlin and Weber 2010; Svačina et al. 2016). In this study, the higher LACT levels were found in males from group SP48, which is contradictory to our observations of intensive movement of the males due to aggressive behavior. The higher LACT level in separated males could be explained not only with increased movement activity but also with the stimulation of anaerobic glycolysis or the reduction of the lactate utilization rate (Omlin and Weber 2010). Apparently, concentration measurements of LACT in this study are not enough to fully reveal the changes in fish body while direct measurements of glycolytic flux would help to interpret results.
Significant differences were recorded in hematological parameters between fish that were separated after spawning and those kept together. In this study, significant changes of MCV were observed in both males and females which were not separated from each other after spawning. This difference might indicate an osmoregulatory failure of blood cells, leading to their deformation. Subsequently, insufficient function of these cells may lead to destruction of erythrocytes and concomitant anemia (Nakayasu et al. 2002; Gomułka et al. 2015; Witeska 2015).
During the experiment, males from groups NS24 and NS48 exhibited a significantly higher count of Leuko compare to groups SP24 and SP48. Our results are in line with Sarameh et al. (2013) who observed a high count of Leuko in pikeperch broodstock exposed to handling and different photoperiods. Observed changes of this parameter are more likely to be an outcome of the stress that fish experienced during reproduction. From the other hand, consistently high count of Leuko in female’s plasma could be a result of a general fragility of their immune system during reproduction (Espelid et al. 1996; Bowden 2008).
Although there was no significant differences in Er count in females of any experimental group, the separation had a positive effect on this parameter, and 48 h after spawning, the numbers of Er count increased back to their at the beginning of the experiment (Table 2). This normalization period may represent approximate time required for broodstock to restore their physiological state.
Separation of females during semi-artificial reproduction under RAS conditions is a necessary step with regard to fish welfare practices. During the semi-artificial reproduction protocol, when limited space is available, females are often subjected to attack (Zakęś and Demska-Zakęś 2009). When both sexes are kept together, they are likely to exhibit skin damages caused by attacking, increasing vulnerability to infection and disease (Gomułka et al. 2007; Łuczyński et al. 2007; Rónyai 2007).
Despite the growing interest in pikeperch as a commercial fish species, there are only a few studies concerning behavioral monitoring in the context of artificial and semi-artificial reproduction (Drasovean and Blidariu 2013; Grozea et al. 2016; Baekelandt et al. 2019). Over the past decade, technology of digital recording has improved, significantly allowing behavioral monitoring of aquatic organism at lower cost (Huse and Skiftesvik 1990; Lucas and Baras 2000; Papadakis et al. 2012; Steen and Ski 2014). Although behavioral monitoring of fish is widely used in aquaculture, these studies are still rare for pikeperch (Drasovean and Blidariu 2013; Grozea et al. 2016). Hence, this study was focused on paternal behavior of pikeperch males and provided useful data necessary to understand the physiological changes occurring in the bodies of pikeperch broodstock. Photo analyses indicated much higher fin damage levels in females that were kept together with males after spawning compared to fish from other experimental groups (Fig. 1). The effect of male aggressive behavior was reflected in some of the hematological and biochemical parameters, while video recordings and photo analyses made clear a necessity of separating females after spawning in limited area.
Developing techniques for behavioral monitoring will help to improve semi-artificial reproduction protocol for pikeperch in terms of welfare. Remote monitoring systems are currently used for aquaculture (Steen and Ski 2014; Kuklina et al. 2018) and are useful in conditions when long-term observations are necessary or when human presence disturbs the animals. Pikeperch spawning is accompanied with intensive movement of the fish around the nest (Drasovean and Blidariu 2013). Using this system to determine intensive movements of fish in the tank could serve as an alarm indicating necessary human involvement, which, in case of this study, means separation of females immediately after spawning. Evaluation of fin erosion level was previously used in the assessment of rearing condition for pikeperch juveniles (Policar et al. 2016) and Eurasian perch (Perca fluviatilis; Stejskal et al. 2011) during intensive culture. Adámek et al. (2007) used the same principle of digital photo analysis to evaluate body injuries of fish that had escaped cormorant (Phalacrocorax carbo sinensis) attacks. The approach in this study permitted significant improvements of broodstock health status during reproduction and minimized, or even totally prevented, mortality after spawning.
Post-spawning losses of pikeperch broodstock is a common consequence of high sensitivity to manipulation and stress during reproduction (Gomułka et al. 2007; Łuczyński et al. 2007; Rónyai 2007; Zakęś and Demska-Zakęś 2009). Cortisol released in to the bloodstream demonstrates immunosuppressive effect resulting in low effectivity of the immune system (Witeska 2015). Without application of special treatments, subsequent mortality of broodstock could reach 100% in several days after spawning (Policar et al. 2019). In this study, experimental trials were stopped after occurrence of first mortality. A female died after being kept with male for 48 h in one tank. Male’s protective behavior to the nest is well known, and in limited space, females are often targeted which leads to various injures and a decrease in health status (Zakęś and Demska-Zakęś 2009; Policar et al. 2019). In this study, relatively small-volume tanks were used for the reproduction (350 l). It may be important to understand how the volume of water and subsequent available space can affect aggressive behavior in male pikeperch for a managerial point of view. Further research is needed to investigate the size of the territory guarded by male pikeperch to enhance our understanding of reproduction techniques and thereby improve broodstock management practices during reproduction in controlled conditions.
Hematological and biochemical alterations are useful indicators for evaluating fish physiological status; however, among pikeperch, these indices vary depending on age, maturation, and general health status (Falahatkar et al. 2012; Křišťan et al. 2012; Sarameh et al. 2013; Falahatkar and Poursaeid 2014). This study contributed to our understanding of semi-artificial reproduction of pikeperch broodstock caught from natural environment, when fish are continually exposed to multiple stressors. Broodstock behavior monitoring allowed us to minimize mortality after spawning by separating females, thereby significantly improving their physiological status during reproduction. Semi-artificial reproduction is an effective and widely used protocol in pikeperch aquaculture (Blecha et al. 2016; Malinovskyi et al. 2018). This method of reproduction results in high spawning success of broodstock and improved egg incubation, thereby improving the hatching rate by about 72% (Blecha et al. 2016). Further investigations focusing on physiological changes will improve our understanding of the stress response and risks associated with reproduction. Investigation of ecological and behavioral features of pikeperch species could be used to minimize negative consequences of reproduction in captivity, including reducing broodstock mortality, and may dramatically improve the effectiveness of broodstock management procedures.
Change history
25 June 2019
The original article unfortunately contains interchanged first and family name of the authors.
25 June 2019
The original article unfortunately contains interchanged first and family name of the authors.
References
Adámek Z, Kortan J, Flajšhans M (2007) Computer-assisted image analysis in the evaluation of fish wounding by cormorant [Phalacrocorax carbo sinensis (L.)]. Aquacult Int 15:211–216. https://doi.org/10.1007/s10499-007-9087-0
Andersen CY (2002) Possible new mechanism of cortisol action in female reproductive organs: physiological implications of the free hormone hypothesis. J Endocrinol 173:211–217. https://doi.org/10.1677/joe.0.1730211
Baekelandt S, Mandiki SNM, Schmitz M, Kestemont P (2019) Influence of the light spectrum on the daily rhythms of stress and humoral innate immune markers in pikeperch Sander lucioperca. Aquaculture 499:358–363. https://doi.org/10.1016/j.aquaculture.2018.09.046
Blecha M, Kristan J, Samarin AM, Rodina M, Policar T (2015) Quality and quantity of pikeperch (Sander lucioperca) spermatozoa after varying cold water treatments. J Appl Ichthyol 31 (Suppl. 2:75–78. https://doi.org/10.1111/jai.12853
Blecha M, Samarin AM, Kristan J et al (2016) Benefits of hormone treatment of both sexes in semi-artificial reproduction of pikeperch (Sander lucioperca L.). Czech J Anim Sci 61:203–208. https://doi.org/10.17221/60/2015-CJAS
Bowden TJ (2008) Modulation of the immune system of fish by their environment. Fish Shellfish Immunol 25:373–383
Bry C (1985) Plasma cortisol levels of female rainbow trout (Salmo gairdneri) at the end of the reproductive cycle: relationship with oocyte stages. Gen Comp Endocrinol 57:47–52. https://doi.org/10.1016/0016-6480(85)90199-6
Cook AF, Stacey NE, Peter RE (1980) Periovulatory changes in serum cortisol levels in the goldfish, Carassius auratus. Gen Comp Endocrinol 40:507–510. https://doi.org/10.1016/0016-6480(80)90015-5
Drasovean AG, Blidariu FC (2013) The naturally conducted reproductive behavior of Sander lucioperca L. Bull UASVM Anim Sci Biotechnol 70(2):255–262
Espelid S, Lokken GB, Steiro K, Bogwald J (1996) Effects of cortisol and stress on the immune system in Atlantic salmon (Salmo salar L). Fish Shellfish Immunol 6:95–110
Falahatkar B, Poursaeid S (2014) Effects of hormonal manipulation on stress responses in male and female broodstocks of pikeperch Sander lucioperca. Aquac Int 22:235–244. https://doi.org/10.1007/s10499-013-9678-x
Falahatkar B, Akhavan S, Efatpanah I, Meknatkhah B (2012) Primary and secondary responses of juveniles of a teleostean, pikeperch Sander lucioperca, and a chondrostean, Persian sturgeon Acipenser persicus, to handling during transport. N Am J Aquac 74:241–250. https://doi.org/10.1080/15222055.2012.675988
Falahatkar B, Eslamloo K, Yokoyama S (2014) Suppression of stress responses in Siberian sturgeon, Acipenser baeri, juveniles by the dietary administration of bovine lactoferrin. J World Aquacult Soc 45:699–708. https://doi.org/10.1111/jwas.12153
Faught E, Vijayan MM (2018) Maternal stress and fish reproduction: the role of cortisol revisited. Fish Fish 19(6):1016–1030. https://doi.org/10.1111/faf.12309
Fontaine P, Legendre M, Vandeputte M, Fostier A (2009) Domestication of new species and sustainable development in fish culture. Cah Agric 18:119–124. https://doi.org/10.1684/agr.2009.0293
Fontaine P, Wang N, Hermelink B (2015) Chapter 3: Broodstock management and control of the reproduction cycle. In: Kestemont P, Dabrowski K, Summerfelt RC (eds) Biology and culture of percid fishes—principles and practices. Springer, Dordrecht, pp 103–122. https://doi.org/10.1007/978-94-017-7227-3_3
Ghosh AK, Joshi SR (2008) Disorders of calcium, phosphorus and magnesium metabolism. J Assoc Physicians India 56:613–621
Gomułka P, Kucharczyk D, Szczerbowski A et al (2007) Chapter 9: Artificial pikeperch propagation—veterinary purposes. In: Kucharczyk D, Kestemont P, Mamcarz A (eds) Artificial reproduction of pikeperch. Mercurius Olsztyn, Poland, pp 67–74
Gomułka P, Dagowski J, Wlasow T et al (2015) Haematological and biochemical blood profile in Russian sturgeon following propofol and eugenol anesthesia. Turk J Fish Aquat Sci 15:13–17. https://doi.org/10.4194/1303-2712-v15_1_02
Grozea A, Drasovean A, Lalescu D et al (2016) The pike perch (Sander lucioperca) background color first choice in the recirculating aquaculture systems. Turk J Fish Aquat Sci 16:891–897. https://doi.org/10.4194/1303-2712-v16_4_16
Huse I, Skiftesvik AB (1990) A pc-aided video based system for behavior observation of fish larvae and small aquatic invertebrates. Aquac Eng 9:131–142. https://doi.org/10.1016/0144-8609(90)90016-S
Kestemont P, Henrotte E (2015) Chapter 20: Nutritional requirements and feeding of broodstock and early life stages of Eurasian perch and pikeperch. In: Kestemont P, Dabrowski K, Summerfelt RC (eds) Biology and culture of percid fishes—principles and practices. Springer, New York, pp 539–564. https://doi.org/10.1007/978-94-017-7227-3_3
Khendek A, Chakraborty A, Roche J, Ledoré Y, Personne A, Policar T, Żarski D, Mandiki R, Kestemont P, Milla S, Fontaine P (2018) Rearing conditions and life history influence the progress of gametogenesis and reproduction performances in pikeperch males and females. Animal 12:2335–2346. https://doi.org/10.1017/s1751731118000010
Kime DE, Dolben IP (1985) Hormonal changes during induces ovulation of the carp Cyprinus carpio. Gen Comp Endocrinol 58:137–149. https://doi.org/10.1016/0016-6480(85)90147-9
Kolářová J, Velíšek J (2012) Determination and assessment of biochemical profile of fish blood. FFPW Vodnany, edition of practical handbooks, USB FFPW 135, 58 pp
Křišťan J, Stejskal V, Policar T (2012) Comparison of reproduction characteristics and broodstock mortality in farmed and wild Eurasian perch (Perca fluviatilis L.) females during reproduction under controlled conditions. Turk J Fish Aquat Sci 12:191–197. https://doi.org/10.4194/1303-2712-v12_2_01
Křišťan J, Alavi SMH, Stejskal V et al (2013) Hormonal induction of ovulation in pikeperch (Sander lucioperca L.) using human chorionic gonadotropin (hCG) and mammalian GnRH without dopamine inhibitor. Aquac Int 21:811–818. https://doi.org/10.1007/s10499-012-9572-y
Křišťan J, Stara A, Polgesek M et al (2014) Efficiency of different anesthetics for pikeperch (Sander lucioperca L.) in relation to water temperature. Neuroendocrinol. Lett. 35 (Suppl. 2:81–85
Křišťan J, Zarski D, Blecha M et al (2018) Fertilizing ability of gametes at different post-activation times and the sperm-oocyte-ratio in the artificial reproduction of pikeperch Sander lucioperca. Aquac Res 49:1383–1388. https://doi.org/10.1111/are.13570
Kuklina I, Ložek F, Císař P, Kouba A, Kozák P (2018) Crayfish can distinguish between natural and chemical stimuli as assessed by cardiac and locomotor reactions. Environ Sci Pollut Res 25(9):8396–8403. https://doi.org/10.1007/s11356-017-1183-8
Kusakabe M, Nakamura I, Young G (2003) 11beta-hydroxysteroid dehydrogenase complementary deoxyribonucleic acid in rainbow trout: cloning, sites of expression, and seasonal changes in gonads. Endocrinology 144:2534–2545. https://doi.org/10.1210/en.2002-220446
Lappalainen J, Dörner H, Wysujack K (2003) Reproductive biology of pikeperch (Sander lucioperca L.)—a review. Ecol Freshw Fish 12:95–106. https://doi.org/10.1034/j.1600-0633.2003.00005.x
Lepič P, Stara A, Turek J et al (2014) The effects of four anaesthetics on haematological and blood biochemical profiles in vimba bream, Vimba vimba. Vet Med 59:81–87. https://doi.org/10.17221/7317-VETMED
Ljubobratović U, Péter G, Horváth Z, Żarski D, Ristović T, Percze V, Sándor Z, Lengyel S, Rónyai A (2017) Reproductive performance of indoor-reared pikeperch (Sander lucioperca) females after wintering in outdoor earthen ponds. Aquac Res 48:4851–4863. https://doi.org/10.1111/are.13305
Lucas MC, Baras E (2000) Methods for studying spatial behavior of freshwater fishes in the natural environment. Fish Fish 4:283–316. https://doi.org/10.1046/j.1467-2979.2000.00028.x
Łuczyński J, Szkudlarek M, Szczerbowski A et al (2007) Chapter 3: Spawners and handling. In: Kucharczyk D, Kestemont P, Mamcarz A (eds) Artificial reproduction of pikeperch. Mercurius Olsztyn, Poland, pp 17–22
Malinovskyi O, Veselý L, Blecha M, Křišťan J, Policar T (2018) The substrate selection and spawning behavior of pikeperch Sander lucioperca L. broodstock under pond conditions. Aquac Res 49:3541–3547. https://doi.org/10.1111/are.13819
Milla S, Wang N, Mandiki SNM, Kestemont P (2009) Corticosteroids: friends or foes of teleost fish reproduction? Comp Biochem Physiol A 153:242–251. https://doi.org/10.1016/j.cbpa.2009.02.027
Muller-Belecke A, Zienert S (2008) Out-of-season spawning of pikeperch (Sander lucioperca L.) without the need of hormonal treatments. Aquacult Res 39:1279–1285. https://doi.org/10.1111/j.1365-2109.2008.01991.x
Nakayasu C, Youshinaga T, Kumagai A (2002) Hematology of anemia experimentally induced by repeated bleedings in Japanese flounder with comments on the cause of flounder anemia recently prevailing in Japan. Fish Pathol 37(3):125–130. https://doi.org/10.3147/jsfp.37.125
Noaksson E, Linderoth M, Gustavsson B et al (2005) Reproductive status in female perch (Perca fluviatilis) outside a sewage treatment plant processing leachate from a refuse dump. Sci Total Environ 340:97–112. https://doi.org/10.1016/j.scitotenv.2004.08.010
Omlin T, Weber JM (2010) Hypoxia stimulates lactate disposal in rainbow trout. J Exp Biol 213:3802–3809. https://doi.org/10.1242/jeb.048512
Papadakis VM, Papadakis IE, Lamprianidou F, Glaropoulos A, Kentouri M (2012) A computer-vision system and methodology for the analysis of fish behavior. Aquacult Eng 46:53–59. https://doi.org/10.1016/j.aquaeng.2011.11.002
Pickering AD, Christie P (1981) Changes in the concentrations of plasma cortisol and thyroxine during sexual maturation of the hatchery-reared brown trout, Salmo trutta. Gen Comp Endocrinol 44:488–496. https://doi.org/10.1016/0016-6480(81)90337-3
Policar T, Blecha M, Křišťan J, Mráz J, Velíšek J, Stará A, Stejskal V, Malinovskyi O, Svačina P, Samarin AM (2016) Comparison of production efficiency and quality of differently cultured pikeperch (Sander lucioperca L.) juveniles as a valuable product for ongrowing culture. Aquac Int 24:1607–1626. https://doi.org/10.1007/s10499-016-0050-9
Policar T, Malinovskyi O, Křišťan J, Stejskal V, Samarin AM (2019) Post-spawning bath treatments to reduce morbidity and mortality of pond-cultured pikeperch (Sander lucioperca L.) broodstock. Aquac Int
Rónyai A (2007) Induced out-of-season and seasonal tank spawning and stripping of pike perch (Sander lucioperca L.). Aquac Res 38:1144–1151. https://doi.org/10.1111/j.1365-2109.2007.01778.x
Samarin AM, Blecha M, Bytyutskyy D, Policar T (2015) Post-ovulatory oocyte ageing in pikeperch (Sander lucioperca L.) and its effect on egg viability rates and the occurrence of larval malformations and ploidy anomalies. Turk J Fish Aquat Sci 15:435–441. https://doi.org/10.4194/1303-2712-v15_2_29
Sarameh S, Falahatkar B, Takami GA, Efatpanah I (2012) Effects of different photoperiods and handling stress on spawning and reproductive performance of pikeperch Sander lucioperca. Anim Reprod Sci 132:213–222. https://doi.org/10.1016/j.anireprosci.2012.05.011
Sarameh SP, Falahatkar B, Takami GA et al (2013) Physiological changes in male and female pikeperch Sander lucioperca (Linnaeus, 1758) subjected to different photoperiods and handling stress during the reproductive season. Fish Physiol Biochem 39:1253–1266. https://doi.org/10.1007/s10695-013-9780-z
Schreck CB (2010) Stress and fish reproduction: the roles of allostasis and hormesis. Gen Comp Endocrinol 165:549–556. https://doi.org/10.1016/j.ygcen.2009.07.004
Schreck CB, Contreras-Sanchez W, Fitzpatrick MS (2001) Effects of stress on fish reproduction, gamete quality, and progeny. Aquaculture 197:3–24. https://doi.org/10.1016/S0044-8486(01)00580-4
Steen R, Ski S (2014) Video-surveillance system for remote long-term in situ observations: recording diel cavity use and behavior of wild European lobsters (Homarus gammarus). Mar Freshw Res 65(12):1094–1101. https://doi.org/10.1071/MF13139
Stejskal V, Policar T, Křišťan J, Kouřil J, Hamáčková J (2011) Fin condition in intensively cultured Eurasian perch (Perca fluviatilis L.). Folia Zool 60(2):122–128
Svačina P, Priborsky J, Blecha M et al (2016) Haematological and biochemical response of burbot (Lota lota L.) exposed to four different anesthetics. Czech J Anim Sci 61(9):414–420. https://doi.org/10.17221/14/2016-CJAS
Svobodova Z, Pravda D, Modra H (2012) Methods of haematological examination of fish. Edition of practical handbooks, USB FFPW 122, 38 s
Thomas PM, Pankhurst NW, Bremner HA (1999) The effect of stress and exercise on post-mortem biochemistry of Atlantic salmon and rainbow trout. J Fish Biol 54:1177–1196. https://doi.org/10.1111/j.1095-8649.1999.tb02047.x
Westring CG, Ando H, Kitahashi T, Bhandari RK, Ueda H, Urano A, Dores RM, Sher AA, Danielson PB (2008) Seasonal changes in CRF-I and urotensin I transcript levels in masu salmon: correlation with cortisol secretion during spawning. Gen Comp Endocrinol 155:126–140. https://doi.org/10.1016/j.ygcen.2007.03.013
Witeska M (2015) Anemia in teleost fishes. Bull Eur Assoc Fish Pathol 35(4)
Zakęś Z, Demska-Zakęś K (2009) Controlled reproduction of pikeperch Sander lucioperca (L.): a review. Arch Pol Fish 17:153–170. https://doi.org/10.2478/v10086-009-0014-z
Zakęś Z, Szczepkowski M, Partyka K, Wunderlich K (2013) Effect of gonadotropin hormonal stimulation on out-of-season propagation success of different year classes of indoor-reared pikeperch (Sander lucioperca (L.)). Aquac Int 21:801–810. https://doi.org/10.1007/s10499-012-9562
Funding
The study was financially supported by the Ministry of Education, Youth and Sports of the Czech Republic (projects CENAKVA [No. CZ.1.05/2.1.00/01.0024] and Biodiversity [No. CZ.02.1.01/0.0/0.0/16_025/0007370]) and by the Ministry of Agriculture of the Czech Republic (project NAZV QK1710310).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethic statement
The study performed and the experimental broodstock handled were in accordance with national and international guidelines for the protection of animal welfare (EU-harmonized Animal Welfare Act of the Czech Republic). The experimental unit is licensed (No. 2293/2015-MZE-17214 and No. 55187/2016-MZE-17214 within the project NAZV QK1710310) according to the Czech National Directive (the Law against Animal Cruelty, No. 246/1992).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised due to interchanged first and family name of the authors.
Rights and permissions
About this article
Cite this article
Malinovskyi, O., Kolářová, J., Blecha, M. et al. Behavior and physiological status of pond-cultured pikeperch (Sander lucioperca) broodstock effected by sexual interactions throughout semi-artificial reproduction. Aquacult Int 27, 1093–1107 (2019). https://doi.org/10.1007/s10499-019-00401-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-019-00401-6