Abstract
This study was designed to determine the effects of hormonal manipulation on stress responses in female and male pikeperch. Two-year-old cultured female and male broodstocks with an average weight of 337.4 ± 20.1 (mean ± SE; n = 16) and 318.7 ± 15.1 g (n = 16), respectively, were randomly allocated into four hormonal treatments each containing 4 fish. Two sexual groups of 16 fish for each gender were considered. Sexually mature male and female pikeperch were injected with either physiological saline solution (as control group), common carp pituitary extract (CPE), human chorionic gonadotropin (hCG) and or luteinizing hormone-releasing hormone analog (LHRHa2). The blood samples were taken before hormonal injection and after ovulation and spermiation. Then the plasma levels of stress indices (cortisol, glucose, and lactate) were determined. The results showed that all CPE-, HCG-, and LHRHa2- injected males produced sperm. In females treated with CPE and hCG, three of four ovulated, but none of LHRHa2- and saline-injected fish spawned. Significant changes in cortisol, glucose, and lactate levels were observed among the females injected with different hormones. Plasma cortisol and glucose levels increased significantly in males injected with CPE and females injected with hCG, but no significant change was observed in lactate levels before and after hormonal induction. Comparison of two sexes revealed significant differences in glucose levels for females in some groups before injection, while CPE-injected sexes showed significant changes in cortisol and lactate concentrations. The results indicated that the induction of ovulation or spermiation stimulated stress responses especially in female pikeperch, and therefore, all the procedures should be made to minimize the disturbance during the artificial spawning.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pikeperch, Sander lucioperca, is known as a predatory species in temperate waters of Eurasia and is a valuable species for culture due to its rapid growth, flesh quality, high commercial value and sport fishing. As an innovative potential candidate for intensive aquaculture in Europe, the interest in developing techniques for commercial culture of this species has risen over the last few decades. So that aquaculturists have been attracted to the intensive culture technologies of this valuable species (Kestemont and Melard 2000). For controlled rearing, researchers have been succeeded to establish the protocols for artificial propagation of pikeperch spawners (Zakes and Szczepkowski 2004; Ronyai 2007; Muller-Belecke and Zienert 2008).
Hormonal induction of wild and domesticated fish for final maturation and egg ovulation is a common practice in fish hatcheries (Zohar and Mylonas 2001). The fish can be affected by common husbandry practices such as netting, capturing, crowding, handling, and transporting (Nikoo and Falahatkar 2012). Numerous studies have been demonstrated that such stressors have deleterious effects on the reproductive function and the quality and quantity of gamete and larvae (Pankhurst and Van Der Kraak 1997; Contreras-Sanchez et al. 1998; Haddy and Pankhurst 2000; Schreck et al. 2001; Milla et al. 2009; Pourhosein Sarameh et al. 2012). In addition, it appears that the wild fish is more sensitive to such disturbances than the domesticated fish. Thus, domestication, which is recognized as a very important tool in sustainable aquaculture, may be associated with a reduction in stressful responses and an increase in reproductive effectiveness.
Generally, cortisol concentrations reflect the level of stress in animals, including fish (Wendelaar Bonga 1997; Barton 2002). Although direct and indirect actions of cortisol on hypothalamus–pituitary–gonad (HPG) axis have been evaluated in most species (Leatherland et al. 2010; Schreck 2010; Poursaeid et al. 2012), unfortunately, it is not clear whether the stressor-induced disorders in HPG axis during propagation process impact on the fish ability in responding to exogenous hormonal manipulation.
To improve the artificial propagation of pikeperch and to support wild stocks through restocking, it is crucial to recognize the physiological changes during the final maturation in domesticated fish. Understanding the fish responses to the different stressors during the domestication process may be helpful to improve the management protocols for fish cultivation and eventually for fitness and better growth (Belanger et al. 2001). It is not clear whether different hormonal treatments will alter endocrine responses specially stress indicators. To date, little is known about the factors that affect stress responses in pikeperch and those effects on spawning in cultured fish. Therefore, this study aimed to examine the stress response in cultured female and male pikeperch following different hormonal treatments by measuring plasma cortisol, glucose, and lactate concentrations as the primary and secondary stress indicators in the pre-ovulation and post-spawning.
Materials and methods
Fish and rearing system
Two-year-old cultured females (337.4 ± 20.1 g; 35.8 ± 0.6 cm, n = 16) and males (318.7 ± 15 g; 35 ± 0.7 cm, n = 16) were used in this experiment. These farmed fish were originally hatched from wild broodstocks at the Dr. Yousefpour Fish Hatchery Center in April 2006, and then reared in earthen ponds under natural light regimes and water temperature for 2 years, and fed with bait fish (cyprinids fries and fingerlings). The brood fish were unfed during the experiment.
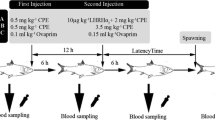
All brood fish were caught from earthen ponds when water temperature reached to 10 °C in March. Sixteen females and sixteen males, which were marked using a uniquely numbered external tag in the first dorsal fin, were allocated in four hormonal treatments including control (0.9 % physiological saline solution; n = 4), common carp pituitary extract (CPE; n = 4), human chorionic gonadotropin (hCG; Corion, LG Life Sciences, India; n = 4), and luteinizing hormone-releasing hormone analog (LHRHa2, Ningbo Sansheng pharmaceutical Co., China; n = 4). These fish were selected for ripeness based on the softness of their abdomen and the macroscopic and microscopic observations of gonadal samples that had been obtained via the genital pore using catheter. Then, the brood fish were separately and randomly distributed into 8 circular concrete tanks (1.95 m diameter, water depth 30 cm, and total volume 0.89 m−3) with a flow rate of 20 ± 0.9 l min−1 and adapted with these conditions for 2 weeks.
Spawning induction
The broodstocks were injected intramuscularly with 1 ml of one of the following agents: CPE, hCG, LHRHa2, and physiological saline solution as control. All females received each hormone in a priming (10 % of total volume) and resolving (90 % of total volume) doses, while the males were stimulated once coincidence with the second injection to females. The amount of this injection was similar to the first injection to females. Females were received 150 IU kg−1 hCG in a priming dose and 500 IU kg−1 in a resolving dose. LHRHa2 was given 3.5 and 10 μg kg−1 in the first and second injection, respectively. Two injections of CPE were conducted at 1.5 and 4.5 mg kg−1 body weight. The whole resolving injections were conducted at a 48-h interval. Hormones were dissolved in physiological saline solution and injected into the dorsal muscle close to the first dorsal fin. For each injection, the broodstocks were anaesthetized with 150 mg l−1 tricaine methanesulfonate (MS-222) after being removed from the tank with a scoop net. Fish were examined by applying gentle abdominal pressure to assess their response to hormonal treatments starting 12 h after the second injection at 2-h intervals for 48 h. Also, the bottom of each tank was controlled for the presence of any released eggs.
Sampling and analysis
The bleeding was synchronously performed on all the females at the first injection and after ovulation (approximately 39 h after receiving the resolving dosage). Blood was also sampled from the rest of females that did not respond to hormonal injections coincidence with the ovulated fish. The blood samples were synchronously taken from all the males at the moment of hormone administration and post-spermiation (about 19-h post-injection). Blood was obtained from the caudal vasculature of anesthetized fish using a 5-ml heparinized syringe equipped with an 18-G needle. Blood samples were transferred into the simple test tubes for extracting plasma. The simple test tubes containing blood were centrifuged for 10 min at 1500×g. Plasma was stored at −20 °C for later determination of cortisol, glucose, and lactate concentrations.
Concentration of cortisol in the plasma samples was determined by radioimmunoassay. The glucose concentration in each plasma sample was measured using a kit, glucose C2-test Wako based on an enzymatic method by glucose oxidase (Wako Pure Chemical Ltd., Osaka, Japan). The linear range of standard glucose was 0.5 to 4 mg ml−1. Plasma lactate was enzymatically determined using Sigma diagnostic kit (St Louis, Mo., USA).
Statistical analyses
The effects of the various hormones on ovulation, spermiation, and plasma metabolites were analyzed by one-way analysis of variance (ANOVA), and the significant difference among the means was evaluated using the Tukey’s post hoc test. Paired samples t test was used to find difference in metabolites between before injection and after ovulation/spermiation. Independent samples t test was considered to find any difference between females and males at each time for each treatment. Statistical analysis was conducted using SPSS (version 13, SPSS Chicago, IL, USA), and differences were considered to be statistically significant at P < 0.05. Data are presented as mean ± standard of error (SE).
Results
Three out of four females injected with CPE and hCG ovulated about 39 h after receiving the second injection. None ovulation was observed in fish treated with LHRHa2 and saline solution. All CPE-, hCG- and LHRHa2-injected males produced milt with high motility (67.5–86.7 %) 19-h post-injection. In the control group, only one male produced milt.
There was no significant change in plasma cortisol concentrations among different treatments at the initial time (before injection). Plasma cortisol levels were significantly higher in hCG-injected fish than those in females treated with control and LHRHa2 39 h after the second injection (Fig. 1; P < 0.05). Although cortisol levels elevated in all treatments 39 h after the second injection in comparison with the beginning of experiment, this elevation was significant only in fish injected with hCG (P < 0.001). In males, the plasma cortisol concentrations elevated significantly 19 h after CPE administration relative to before injection (Fig. 1; P < 0.001). Plasma cortisol levels in CPE-treated females were significantly higher than that in CPE-treated males in spawning time (P < 0.05).
Effect of different hormonal treatments on plasma cortisol concentrations in female and male pikeperch. Values that are significantly different in each gender have different lower case letters (P < 0.05). Statistically significant differences from before injection time are marked by asterisks (***P < 0.001). Other upper asterisk shows significant difference between females and males in the same treatment (*P < 0.05). Open bar before injection, filled bar after ovulation
Significant differences were found in glucose concentrations after ovulation among the treatments (Fig. 2; P < 0.05), so that plasma glucose concentrations were significantly higher in females injected CPE and hCG compared to pre-injection time (P < 0.01). The plasma levels of glucose significantly increased in males injected with CPE (P < 0.001) and LHRHa2 (P < 0.05) after spermiation (Fig. 2). There were significant differences between females and males before hormonal induction in LHRHa2 and CPE groups (P < 0.05).
Effect of different hormonal treatments on plasma glucose concentrations in female and male pikeperch. Values that are significantly different in each gender have different lower case letters (P < 0.05). Statistically significant differences from before injection time are marked by asterisks (*P < 0.05; **P < 0.01; ***P < 0.001). Other upper asterisk shows significant difference between females and males in the same treatment (*P < 0.05). Open bar before injection, filled bar after spermiation
Lactate levels were significantly changed 39 h after the second injection among the experimental groups with the highest level of 48.7 ± 4.3 mg dl−1 in females injected LHRHa2 (Fig. 3; P < 0.05), while no significant changes were observed in lactate levels after hormonal induction for males (Fig. 3; P > 0.05). Lactate concentrations in CPE-treated males were higher than that in females injected with this hormone (P < 0.01).
Effect of different hormonal treatments on plasma lactate concentrations in female and male pikeperch. Values that are significantly different in each gender have different lower case letters (P < 0.05). Upper asterisks show significant difference between females and males in the same treatment (**P < 0.01). Open bar before injection, filled bar after spermiation
Discussion
The aim of this study was to examine whether the hormonal treatments can alter physiological stress responses in female and male pikeperch. The results of the present study revealed that the hormonal injections had significant effects on plasma cortisol and glucose concentrations as primary and secondary responses, respectively, especially in females, during the final maturation and spawning.
Typically, classical stress hormones, catecholamines and corticosteroids (mainly cortisol) are involved directly or indirectly in the secondary or tertiary responses for energy mobilization and metabolism, mineral balance and additional physiological functions (Schreck 2010). In the present study, female fish exhibited the higher levels of cortisol in response to spawning induction compared to the males. Contradictory results have been reported regarding the differences between male and female teleost fish in responding to stressors. Binuramesh et al. (2005) showed a less sensitive of female tilapia Oreochromis mossambicus to stressors compared to male. Additional studies on other teleosts have also indicated differences in sensitivity to acute and chronic physical disturbance between male and female, so that male fish have demonstrated higher and quicker stress responsiveness than female (Barcellos et al. 1999; Haddy and Pankhurst 1999; Kubokawa et al. 1999). In contrast, mature male of rainbow trout, Oncorhynchus mykiss, showed a reduced cortisol response to acute and chronic stress, which is relatively linked to elevated plasma androgens, but mature female showed high levels of stress responses when the estrogen levels were high (Pottinger et al. 1995a, b). These findings showed that, unlike male pikeperch, females are sensitive to any handling. Such sensitivity may be caused by changes in physiological condition as a result of having huge volume of mature oocytes in the ovary during ovulation. Females have a large number of mature oocytes in the pre-spawning stage and their body cavity is therefore swollen compare to the male’s body cavity. Consequently, this swelling may cause a high degree of stress response in females, as suggested by Kubokawa et al. (1999). An increase in levels of 11β-hydroxy steroid dehydrogenases (important steroidogenic enzymes for catalyzing the interconversion of active glucocorticoid) and cortisol through ovulation has also been demonstrated by Kusakabe et al. (2003) in rainbow trout. In addition, sex steroid concentrations and also social behavior may be other factors that have caused such differences in cortisol levels between male and female pikeperch. It has been demonstrated when the genders were held separately, all female tilapia had higher cortisol concentrations compare to male (Binuramesh et al. 2005).
In the present study, elevated levels of plasma cortisol in fish injected with CPE were probably due to the existence of adrenocorticotropin hormone in the extract prepared from pituitary gland that can have stimulatory effects on interrenal tissue and increases cortisol secretion into the circulation (Barton 2002). Similarly, the administration of different hormones (e.g., CPE and LHRHa) for spawning induction of sturgeon species has been shown to induce changes in sex steroid levels (Barannikova et al. 1999, 2000a, b; Semenkova et al. 2002; Bayunova et al. 2002) and to increase cortisol level (Bukovskaya et al. 1999; Bayunova et al. 2002; Semenkova et al. 2002). In this study, injection of hCG to brood fish resulted in high secretion of cortisol into the circulation. A significant increase in cortisol levels was also observed in the black bream, Acanthopagrus butcheri, 24 h after hCG injection to induce sexual maturity (Haddy and Pankhurst 2000). The greater sensitivity of brood fish to hCG injections, especially in females, is likely related to the fact that it is a heterologous hormone for fish and can trigger an immune response (Zohar and Mylonas 2001).
No significant changes in cortisol concentration after ovulation in CPE and LHRHa2-injected females, and hCG and LHRHa2-treated males is probably due to interrenal response of fish to experimental conditions. Also, it seems that fish had been exhausted and that was no longer the prospective to secrete extra cortisol and other related metabolites such as lactate.
Glucose levels showed a very typical stress response in brood pikeperch, particularly in females, which is consistent with enhanced cortisol levels through ovulation/spermiation. This result is consistent with earlier study in which the confinement stress in breeding season increased glucose levels in brood sockeye (Kubokawa et al. 1999). During the stressful conditions, catecholamines and corticosteroids act directly on the liver to stimulate glycogenolysis that results in the glucose mobilization (Mommsen et al. 1999). It has been clearly demonstrated that the increase in glucose concentration occurs with an increase in the corticosteroid levels during acute stress (Wendelaar Bonga 1997; Barton 2002; Falahatkar et al. 2012). Since stress is an energy-demanding process, the elevation of plasma glucose in this study may be a useful pathway for coping with increased metabolic demands. Lower cortisol and glucose levels in females injected with LHRHa2 and physiological serum in comparison with CPE- and hCG-treated females may be related to the spawning status. Since no female fish injected with LHRHa2 and physiological serum ovulated; therefore, it confirms this hypothesis that ovulation induced stress responses in female pikeperch.
Lactate is produced during anaerobic energy metabolism to maintain acid–base equilibrium (Barton et al. 1998; Ruane et al. 2002). Surprisingly, the highest lactate concentration was observed in LHRHa2-treated females with the lowest cortisol level. The reason for the large increase of plasma lactate in this treatment compared with other experimental groups is unknown, although it is possible that these females experienced a greater degree of swimming activity. Furthermore, low lactate levels in female treated with CPE may be related to lower storages of glycogen. It is also possible that the glycogen reserved in the body has been used for the glucose synthesis in order to maintain homeostasis. Hormonal treatments on males were not able to produce significant differences in lactate levels after injection. Previous studies have documented rapid increase in lactate levels (30 min to 2 h) after intense exercise and stress (Falahatkar et al. 2012). Therefore, it is possible that lactate levels increased before spermiation and blood sampling and then reached to the basal levels.
Our results reveled that various hormonal treatments caused different responses in inducing ovulation/spermiation, so that CEP and hCG were effective hormones for spawning induction in female pikeperch, while whole hormones applied have high effective in inducing the spermiation. The lack of ovulation in fish treated with LHRHa2 may be related to the kind and dosage of hormone applied in this study.
Regards the fact that in which life span and with what intensity a stress is held and for how long a stressor lasts, those factors may affect reproduction in different ways (Schreck 2010). Also, nutritional factors (Zohar et al. 1995; Pereira et al. 1998; Siddiqui et al. 1998); age, spawning status and sexual maturity (Kjorsvik 1994; Navas et al. 1995; Brooks et al. 1997); the intensity of stress (Campbell et al. 1992; Contreras-Sanchez et al. 1998; Schreck et al. 2001); and strain (Bromage et al. 1990) could directly affect the reaction and gamete quantity and quality.
Conclusion
The results of the present study revealed that there were different responses between male and female in response to hormonal inductions, which may be due to sex-specific reactions, different gonadal structure and volume, sex steroid concentrations and also social environment. Regarding the changes of plasma cortisol and glucose levels in pikeperch, the results have provided useful information regarding to stressful effects of hormonal induction on endocrine functions in brooders. Our results have clearly demonstrated that cultured pikeperch, especially females, are extremely sensitive to handling stress and thus must be maintained with minimal disturbances particularly during the artificial spawning.
References
Barannikova IA, Boev AA, Bayunova LV, Dyubin VP, Saenko II (1999) Sex steroid levels in blood serum in Stellate sturgeon (Acipenser stellatus) at beginning of anadromous migration and during final maturation after hormonal treatment. Vopr Ichtiol 39:111–116 (in Russian)
Barannikova IA, Artyukhin E N, Bayunova LV, Dyubin VP, Semenkova TB (2000a) Steroid profiles in giant sturgeon females [Huso huso (L.)] at the beginning of anadromous migration and at induced ovulation after reservation. In: Norberg B, Kjesbi OS, Tarranger GL, Andersson E, Steffanson SO (eds) Proceedings of the 6th international symposium on the reproductive physiology of fish. 4–9 July 1999, Bergen, p 420
Barannikova IA, Dyubin VP, Bayunova LV, Semenkova TB (2000b) Steroids in reproductive function control in fish. Russian J Physiol 86:968–978 (In Russian, with English abstract)
Barcellos LJ, Nicolaiewsky S, Souza SM, Lulhier F (1999) The effects of stocking density and social interaction on acute stress response in Nile tilapia Oreochromis niloticus (L.) fingerlings. Aquac Res 30:887–892. doi:10.1046/j.1365-2109.1999.00419.x
Barton BA (2002) Stress in fishes: a diversity of response with particular reference to changes in circulating corticosteroids. Integr Comp Biol 42:517–525. doi:10.1093/icb/42.3.517
Barton BA, Rahn AB, Feist G, Bollig H, Schreck CB (1998) Physiological stress responses of the freshwater chondrostean paddlefish (Polydon spathula) to acute physical disturbances. Comp Biochem Physiol 120A:355–363. doi:10.1016/S1095-6433(98)10036-3
Bayunova L, Barannikova I, Semenkova T (2002) Sturgeon stress reactions in aquaculture. J Appl Ichthyol 18:397–404. doi:10.1046/j.1439-0426.2002.00410.x
Belanger JM, Son JH, Laugero KD, Moberg GP, Doroshov SI, Lankford SE, Cech JJ Jr (2001) Effects of short-term management stress and ACTH injections on plasma cortisol levels in cultured white sturgeon, Acipensor transmontanus. Aquaculture 203:165–176. doi:10.1016/S0044-8486(01)00620-2
Binuramesh C, Prabakaran M, Steinhagen D, Dinakaran Michael R (2005) Effect of chronic confinement stress on the immune responses in different sex ration groups of Oreochromis mossambicus (Peters). Aquaculture 250:47–59. doi:org/10.1016/j.aquaculture.2005.03.043
Bromage N, Hardiman P, Jones J, Springate J, Bye V (1990) Fecundity, egg size and total egg volume differences in 12 stocks of rainbow trout, Oncorhynchus mykiss Richardson. Aquac Fish Manage 21:269–284. doi:10.1111/j.1365-2109.1990.tb00465.x
Brooks S, Tyler CR, Sumpter JP (1997) Eggs quality in fish: what makes a good egg? Rev Fish Biol Fish 7:387–416. doi:10.1023/A:1018400130692
Bukovskaya OS, Bayunova LV, Blokhin SV, Boev AA (1999) The effect of acute stress on hormonal serum levels in Russian and stellate sturgeons during induced maturation. J Appl Ichthyol 15:308–309. doi:10.1111/j.1439-0426.1999.tb00308.x
Campbell PM, Pottinger TG, Sumpter JP (1992) Stress reduces the quality of gametes produced by rainbow trout. Biol Reprod 47:1140–1150. doi:10.1095/biolreprod47.6.1140
Contreras-Sanchez WM, Schreck CB, Fitzpatrick MS, Pereira CB (1998) Effects of stress on the reproductive performance of rainbow trout Oncorhynchus mykiss. Biol Reprod 58:439–447. doi:10.1095/biolreprod58.2.439
Falahatkar B, Akhavan SR, Efatpanah I, Meknatkhah B (2012) Primary and secondary responses of a teleostean, pikeperch Sander lucioperca, and a chondrostean, Persian sturgeon Acipenser persicus juveniles, to handling during. N Am J Aquac 74:241–250. doi:10.1080/15222055.2012.675988
Haddy JA, Pankhurst NW (1999) Stress-induced changes in concentrations of plasma sex steroids in black bream. J Fish Biol 55:1304–1316. doi:10.1111/j.1095-8649.1999.tb02077.x
Haddy JA, Pankhurst NW (2000) The efficacy of exogenous hormones in stimulating changes in plasma steroids and ovulation in wild black bream Acanthopagrus butcheri is improved by treatment at capture. Aquaculture 191:351–366. doi:10.1016/S0044-8486(00)00445-2
Kestemont P, Melard C (2000) Aquaculture. In: Craig JF (ed) Percid fishes systematic, ecology and exploitation. Blackwell Science, Oxford, pp 191–224
Kjorsvik E (1994) Egg quality in wild and broodstock cod Gadus morhua L. J World Aquac Soc 25:22–29. doi:10.1111/j.1749-7345.1994.tb00800.x
Kubokawa K, Watanabe T, Yoshioka M, Iwata M (1999) Effects of acute stress on plasma cortisol, sex steroid hormone and glucose levels in male and female sockeye salmon during the breeding season. Aquaculture 172:335–349. doi:10.1016/S0044-8486(98)00504-3
Kusakabe M, Nakamura I, Young G (2003) 11β-Hydroxysteroid dehydrogenase complementary deoxyribonucleic acid in rainbow trout: cloning, sites of expression, and seasonal changes in gonads. Endocrinology 144:2534–2545. doi:10.1210/en.2002-220446
Leatherland JF, Li M, Barkataki S (2010) Stressors, glucocorticoids and ovarian function in teleosts. J Fish Biol 76:86–111. doi:10.1111/j.1095-8649.2009.02514.x
Milla S, Wang N, Mandiki SNM, Kestemont P (2009) Corticosteroids: friends or foes of teleost fish reproduction? Comp Biochem Physiol 153A:242–251. doi:org/10.1016/j
Mommsen TP, Vijayan MM, Moon TW (1999) Cortisol in teleosts: dynamics, mechanisms of action, and metabolic regulation. Rev Fish Biol Fish 9:211–268. doi:10.1023/A:1008924418720
Muller-Belecke A, Zienert S (2008) Out-of-season spawning of pikeperch Sander lucioperca (L.) without the need for hormonal treatments. Aquac Res 39:1279–1285. doi:10.1111/j.1365-2109.2008.01991.x
Navas J, Thrush M, Ramos J, Bruce M, Carillo M, Zanuy S, Bromage N (1995) The effect of seasonal alteration in the lipid composition of broodstock diets on egg quality in the European sea bass, Dicentrarchus labrax. In: Goetz FW, Thomas P (eds) Proceeding of the Fifth Proc. International Symposium on the Reproductive Physiology of Fish. Austin, Texas: Fish Symposium ‘95, pp. 108–110
Nikoo M, Falahatkar B (2012) Physiological responses in wild broodstocks of the Caspian Kutum Rutilus frisii kutum subjected to transportation stress. J Appl Anim Wel Sic 15:372–382. doi:10.1080/10888705.2012.709156
Pankhurst NW, Van Der Kraak G (1997) Effects of stress on reproduction and growth of fish. In: Iwama GK, Pickering AD, Sumpter JP, Schreck CB (eds) Fish stress and health in aquaculture, society for experimental biology seminar series 62. Cambridge University Press, Cambridge, pp. 73–93
Pereira JOB, Reis-Henriques MA, Sanchez JL, Costa JM (1998) Effect of protein source on the reproductive performance of female rainbow trout, Oncorhynchus mykiss (Walbaum). Aquac Res 29:751–760. doi:10.1046/j.1365-2109.1998.29100751.x
Pottinger TG, Balm PHM, Pickering AD (1995a) Sexual maturity modifies the responsiveness of the pituitary-interrenal axis to stress in male rainbow trout. Gen Comp Endocrinol 98:311–320. doi:1006/gcen.1995.1073
Pottinger TG, Carrick TR, Hughes SE, Balm PHM (1995b) Testosterone, 11-ketotestosterone, and estradiol-17β modify baseline and stress-induced interrenal and corticotropic activity in trout. Gen Comp Endocrinol 104:284–295. doi:10.1006/gcen.1996.0173
Pourhosein Sarameh S, Falahatkar B, Azari Takami G, Efatpanah I (2012) Effects of different photoperiods and handling stress on spawning and reproductive performance of pikeperch Sander lucioperca. Anim Rep Sci 132:213–222. doi:10.1016/j.anireprosci.2012.05.011
Poursaeid S, Falahatkar B, Mojazi Amiri B, Van Der Kraak G (2012) Effects of long-term cortisol treatments on gonadal development, sex steroids levels and ovarian cortisol content in cultured great sturgeon Huso huso. Comp Biochem Physiol 163:111–119. doi:org/10.1016/j.cbpa.2012.05.202
Ronyai A (2007) Induced out-off-season and seasonal tank spawning and stripping of pike perch (Sander lucioperca L.). Aquac Res 38:1144–1151. doi:10.1111/j.1365-2109.2007.01778.x
Ruane NM, Carballo EC, Komen J (2002) Increased stocking density influences the acute physiological stress response of common carp Cyprinus carpio. Aquac Res 33:777–784. doi:10.1046/j.1365-2109.2002.00717.x
Schreck CB (2010) Stress and fish reproduction: the role of allostatsis and hormesis. Gen Comp Endocrinol 165:549–556. doi:10.1016/j.ygcen.2009.07.004
Schreck CB, Contreras-Sanchez W, Fitzpatrick MS (2001) Effects of stress on fish reproduction, gamete quality, and progeny. Aquaculture 197:3–24. doi:10.1016/S0044-8486(01)00580-4
Semenkova TB, Barannikova IA, Kime DE, McAllister BG, Bayunova LV, Dybin VP, Kolmakov NN (2002) Sex steroids profiles in female and male stellate sturgeon during final maturation induced by hormonal treatment. J Appl Ichthyol 18:375–382. doi:10.1046/j.1439-0426.2002.00368.x
Siddiqui AQ, Al-Hafedh YS, Ali SA (1998) Effect of dietary protein level on the reproductive performance of Nile tilapia, Oreochromis niloticus (L). Aquac Res 29:349–358. doi:10.1046/j.1365-2109.1998.00206.x
Wendelaar Bonga SE (1997) The stress response in fish. Physiol Rev 11:591–625
Zakes Z, Szczepkowski M (2004) Induction of out-of-season spawning of pikeperch, Sander lucioperca (L.). Aquac Int 12:11–18. doi:10.1023/B:AQUI.0000017183.40691.7d
Zohar Y, Mylonas CC (2001) Endocrine manipulations of spawning in cultured fish: from hormones to genes. Aquaculture 197:99–136. doi:10.1016/S0044-8486(01)00584-1
Zohar Y, Harel M, Hassin S, Tandler A (1995) Gilt-head sea bream, Sparus aurata. In: Bromage NR, Roberts RJ (eds) Broodstock management and egg and larval quality. Blackwell Science, pp 94–117
Acknowledgments
This study was supported by Iranian Fisheries Organization (SHILAT), and we would like to thank specially I. Efatpanah, B. Meknatkhah, S.R. Akhavan, and Z. Arzboo for providing fish, facilities, and other helps during the spawning season.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Falahatkar, B., Poursaeid, S. Effects of hormonal manipulation on stress responses in male and female broodstocks of pikeperch Sander lucioperca . Aquacult Int 22, 235–244 (2014). https://doi.org/10.1007/s10499-013-9678-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-013-9678-x