Abstract
Increased oxidative stress (OS) due to in vitro culture conditions can affect the quality of denuded eggs during various assisted reproductive technologies (ARTs). Presence of intact granulosa cells may protect eggs from OS damage under in vitro culture conditions. The present study was aimed to investigate whether encircling granulosa cells could protect against hydrogen peroxide (H2O2)-induced egg apoptosis in ovulated cumulus oocyte complexes (COCs) cultured in vitro. The OS was induced by exposing COCs as well as denuded eggs with various concentrations of H2O2 for 3 h in vitro. The morphological changes, total reactive oxygen species (ROS) as well as catalase expression, Bax/Bcl-2, cytochrome c levels and DNA fragmentation were analysed in COCs as well as denuded eggs. Our results suggest that H2O2 treatment induced morphological apoptotic features in a concentration-dependent manner in denuded eggs cultured in vitro. The 20 µM of H2O2 treatment induced OS by elevating total ROS level, reduced catalase and Bcl-2 expression levels with overexpression of Bax and cytochrome c and induced DNA fragmentation in denuded eggs cultured in vitro. The presence of encircling granulosa cells protected H2O2-induced morphological apoptotic features by preventing the increase of Bax, cytochrome c expression levels and DNA fragmentation in associated egg. However, 20 µM of H2O2 was sufficient to induce peripheral granulosa cell apoptosis in COCs and degeneration in few denuded eggs cultured in vitro. Taken together our data suggest that the presence of encircling granulosa cells could be beneficial to protect ovulated eggs from OS damage under in vitro culture conditions during various ART programs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mammalian ovary generates meiotically competent oocytes required for successful fertilization and early embryonic development [1, 2]. Follicular oocytes are arrested at diplotene stage for long time from birth to puberty. Meiotic competency in these oocytes starts with resumption of meiosis from diplotene arrest and ends with achievement of metaphase-II (M-II) stage. During this period, oocytes are encircled by several layers of granulosa cells that provide nutrients and growth factors required during the achievement of developmental competency inside the follicular microenvironment [2]. These encircling granulosa cells generate several signal molecules, survival factors and a cross talk between encircling granulosa cells and oocytes are essential for survival of both the cell types under in vivo and in vitro culture conditions [3–5].
Generation of reactive oxygen species (ROS) has been reported in cumulus oocyte complexes (COCs) cultured in vitro [4, 5]. The removal of granulosa cells from diplotene-arrested oocytes triggers susceptibility of immature oocytes towards apoptosis in vitro [6, 7]. Our recent studies suggest that granulosa cell apoptosis leads to egg apoptosis in rat [8]. Generation of ROS and/or depletion of antioxidant system could result oxidative stress (OS) and thereby egg apoptosis in rat [9–12]. Based on these studies, we propose that encircling granulosa cells could protect against ROS-induced egg apoptosis cultured in vitro. ROS act as signal molecules in wide variety of cell types including mammalian germ cells [13, 14]. A moderate increase of ROS is beneficial for meiotic cell cycle progression [13–16], while their sustained high levels induce cell cycle arrest and apoptosis [6, 17–19]. In addition, exogenous supplementation of H2O2 in culture medium induces apoptosis in oocytes and zygotes cultured in vitro [6, 20, 21]. Recent studies from our laboratory suggest that high intracellular level of ROS trigger egg apoptosis [22, 23]. Under in vitro culture conditions, several factors can stimulate egg apoptosis. A minor change in culture conditions or culture medium may cause the generation of ROS in cultured cells [4, 24], a possibility exist that the handling of denuded eggs under in vitro culture conditions during various assisted reproductive technologies (ARTs) can generate ROS [25–27], and deteriorate egg quality by inducing apoptosis. Based on these studies, we propose that the generation of ROS particularly hydrogen peroxide (H2O2) could be one of the factors affecting egg quality as well as in vitro fertilization rate. Further, presence of encircling granulosa cells could protect egg from OS-induced apoptosis under in vitro culture conditions. The 20 µM of H2O2 treatment for 3 h in vitro has been reported to induce OS-mediated apoptosis in rat eggs cultured in vitro [6, 9]. Hence, in the present study, we have used this concentration and exposure time to induce OS in COCs as well as denuded eggs under in vitro culture conditions and protective effects of encircling granulosa cells were analysed. The morphological changes, total ROS, catalase, Bax/Bcl-2, cytochrome c levels as well as DNA fragmentation were analysed in COCs as well as denuded eggs cultured for 3 h in vitro.
Materials and methods
Chemicals and reagents
Chemicals used in this study were purchased from Sigma Chemical Co. (St. Louis, MO) unless otherwise stated. Culture medium-199 (HiMedia Laboratories, Mumbai, India) was prepared as per company manual protocol. The culture medium was supplemented with sodium bicarbonate (0.035% w/v) and then pH was adjusted to 7.2 ± 0.1 and osmolarity was found to be 290 ± 5 mOsmol. The culture medium was supplemented with l-glutamine, penicillin and streptomycin (GPS; 1 µl/ml: HiMedia Laboratories, Mumbai, India) antibiotics before use. The final concentrations of H2O2 (0, 2.5, 5, 10 and 20 µM) were prepared by diluting in medium-199. Addition of H2O2 at final concentrations did not alter the osmolarity and pH of culture medium used in the present study.
Experimental animal
The immature female rats (22–24 days old) of Charles Foster strain were subjected to superovulation induction protocol (20 IU pregnant mare’s serum gonadotropin for 48 h followed by 20 IU human chorionic gonadotropin for 14 h, intramuscular injection) to collect COCs and ovulated eggs. This study was approved by Institutional Animal Ethical Committee of the University (vide letter No. F.Sc/IAEC/2014-15/0248 dated 27/08/2014).
Collection of COCs and denuded eggs
Ovary along with oviduct was collected from experimental animals subjected to superovulation induction protocol. The ampulla was punctured using 26 gauge needle attached to 1 ml tuberculin syringe and ovulated COCs were isolated in medium-199 under a dissecting microscope (Nikon dissecting microscope, model C-DS; Tokyo, Japan). For collection of eggs, half number of ovulated COCs were picked up using microtubing (inner diameter 2 mm) attached with disposable glass micropipette (inner diameter, 100 µm for denuded eggs; 300 µm for COCs; Clay Adams, NJ) and transferred to culture medium containing 0.01% hyaluronidase at 37 °C and manually pipetted to denude the eggs quickly. The denuded eggs were removed and washed three times with culture medium.
In vitro effects of H2O2 on morphological changes
A group of 12–14 COCs and denuded eggs were transferred separately in a culture medium with various concentrations (0, 2.5, 5, 10 and 20 µM) of H2O2 in CO2 incubator (Galaxy 170R New Brunswick, Eppendorf AG, Hamburg, Germany, UK) for 3 h in vitro. At the end of incubation period, COCs and denuded eggs were removed, washed three times with culture medium and transferred on to a grooved slide with 100 µl of culture medium and then analysed for morphological changes using a phase-contrast microscope (Nikon, Eclipse; E600, Tokyo, Japan) at ×400 magnification. The 20 µM of H2O2 induced apoptosis in majority of eggs cultured in vitro in the present study as well as previous studies [6, 9]. Hence, we selected 20 µM of H2O2 treatment for 3 h to analyse the protective effects of granulosa cells on egg apoptosis in present study.
Analysis of total ROS level in COCs as well as denuded eggs
Total ROS can be measured using 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) dye, which is commonly used as cell permeable fluorescence-based probe that can be detected as green fluorescence in a treated cell. The total ROS level was detected using H2DCFDA following previous published protocol [16] with minor modifications. In brief, control and 20 µM H2O2-treated COCs as well as denuded eggs (12–14) were exposed to H2DCFDA (10 µM) for 15 min at 37 °C in CO2 incubator. At the end of incubation period, COCs and denuded eggs were washed five times with pre-warmed PBS and then DCF fluorescence was measured at 485 nm excitation/520 nm emissions using fluorescence microscope (Model, Ni-U, Nikon Eclipse, Tokyo, Japan).
Detection of catalase, Bax, Bcl-2 and cytochrome c expressions
Immunofluorescence for catalase, Bax, Bcl-2 and cytochrome c expressions were analysed in COCs and denuded eggs using their highly specific antibodies purchased from Santa Cruz Biotechnology, CA, USA as per our published protocol with some modifications [18]. COCs as well as denuded eggs (12–14 in each group) were fixed with 4% buffered formaldehyde for 10 min at room temperature. Slides were washed 3 times with pre-warmed PBS and exposed to triton X-100 (0.01% in PBS) for 10 min at 37 °C for permeabilization. Slides were washed three times with pre-warmed PBS and then treated with sodium citrate solution (0.01 M) at 37 °C for 10 min for better antigen retrieval. Slides were again washed 3 times with pre-warmed PBS and then incubated with blocking buffer (2.5% PBS-BSA solution) at 37 °C for 30 min. Thereafter, slides were exposed to 100 µl of their respective primary antibodies [Catalase (H-9), mouse monoclonal antibody (sc-271803) specific for an epitope mapping between amino acids 471–503 near the C-terminous of catalase of mouse origin; Bax (B-9), mouse monoclonal antibody (sc-7480) raised against amino acids 1–171 of Bax of mouse origin; Bcl-2 (C-9), mouse monoclonal antibody (sc-7382) raised against amino acids 1-205 of Bcl-2 of human origin; Cytochrome c (A-8), mouse monoclonal antibody (sc-13156) raised against amino acids 1–104 of cytochrome c of horse origin; Actin (C-2) mouse monoclonal antibody (sc-8432) specific for an epitope mapping between amino acids 350–375 at the C-terminous of actin of human origin] (1:500 dilutions in blocking buffer) at 37 °C for 1 h. After 4–5 washes with PBS, slides were exposed to 100 µl of fluorescein isothiocyanate (FITC, sc-2010) or tetra methyl rhodamine isothiocyanate (TRITC, sc-3796) labeled secondary antibody (1:1000 dilution in blocking buffer) for 1 h at 37 °C in humidified chamber. After 1 h of incubation, slides were washed five times with pre-warmed PBS, mounted with fluorescence mounting medium and then observed under fluorescence microscope 465 nm (FITC) and 540 (TRITC) at ×400 magnification. FI of β-actin was analyzed in parallel as a control. The experiment was repeated three times to confirm the results.
The corrected total cell fluorescence (CTCF) of egg was carried out by taking the fluorescence intensity (FI) of egg cytoplasm for analysis. All parameters were kept constant for each egg and FI was analyzed using ImageJ software (version 1.44 from National Institute of Health, Bethesda, USA). For this purpose, minimum three different areas of each egg cytoplasm as well as its corresponding background were selected. Total fluorescence per egg was calculated on an Excel sheet by applying the measurements obtained from the analyzed cell using formula i.e. CTCF = Integrated density − (area of selected cell × mean fluorescence of background readings).
DNA fragmentation analysis
The DNA fragmentation was detected as our previous published protocol [8] using acridine orange/ethidium bromide (AO/EtBr) staining. In brief, COCs as well as denuded eggs (12–14 in each group) were fixed in 4% buffered formaldehyde for 15 min and then air dried. After washing, COCs as well as denuded eggs were labeled using the nucleic acid binding dye mix, 100 µl of 1:1 mixture of AO/EtBr solutions (4 µg/ml) for 1 min. Slides were then washed with PBS and photographs were taken using fluorescence microscope. The viable cells and apoptotic cells were identified depending on their color due to binding of EtBr to the fragmented DNA. Normal cells with intact DNA had green fluorescence of AO. The EtBr binding to fragmented DNA changed the color from green to yellow.
H2O2-induced DNA fragmentation and its protection by encircling granulosa cells were further confirmed using Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay kit purchased from Trevigen Inc. MD. A group of COCs as well denuded eggs (12–14 eggs/group) was transferred and fixed in 4% buffered formaldehyde for 15 min. These slides were subjected to TUNEL assay for DNA fragmentation as per company manual protocol. The experiment was repeated three times to confirm the results.
Statistical analysis
Data are expressed as mean ± standard error of mean (S.E.M) of three independent experiments. All percentage data were subjected to arcsine square-root transformation before statistical analysis. Data were analyzed by One-way analysis of variance (ANOVA) using SPSS software, version 17.0 (SPSS, Inc. Chicago, IL, USA) followed by Bonferroni post hoc analysis. A probability of p < 0.05 was considered to be statistically significant.
Results
Encircling granulosa cells protect against H2O2-induced morphological changes
Figure 1 shows effects of H2O2 on morphological apoptotic features in denuded eggs cultured for 3 h in vitro. Freshly ovulated M-II arrested eggs showed first polar body (PB-I) with normal morphology (A1). Culture of these eggs for 3 h in vitro induced initiation of extrusion of second polar body (PB-II), which is a morphological feature characteristic of spontaneous egg activation (SEA) (data not shown). However, various concentrations of H2O2 inhibited SEA and induced morphological apoptotic features particularly shrinkage and cytoplasmic granulation (A2) followed by degeneration (A3). The H2O2 induced morphological apoptotic features in a concentration-dependent manner (A4: One-way ANOVA followed by Bonferroni post hoc analysis; F = 353.765, p < 0.001). The 20 µM of H2O2 induced morphological apoptotic features in majority of eggs (92.33 ± 1.45%), while few eggs underwent degeneration (7.66 ± 1.45%) after 3 h of in vitro culture. On the other hand, presence of encircling granulosa cells protected against H2O2-induced morphological apoptotic changes in denuded eggs collected from COCs exposed to 20 µM of H2O2 cultured for 3 h in vitro. These denuded eggs (B3) were similar to control denuded eggs (A1) showing normal morphology with PB-I. However, 20 µM of H2O2 altered encircling granulosa cells morphology (B2) as compare to encircling granulosa cells of control COCs (B1). Presence of encircling granulosa cells prevented H2O2-induced morphological apoptotic changes in majority of eggs collected from treated COCs, while granulosa cells could not prevent apoptosis in few eggs (6.67 ± 0.88%) if exposed to higher concentrations of H2O2 for 3 h in vitro (B4: One-way ANOVA followed by Bonferroni post hoc analysis; F = 39.100, p < 0.001). Three independent experiments using 36–42 eggs/COCs were conducted to confirm the observations.
Representative photograph showing the protective effects of encircling granulosa cells on H2O2-induced morphological apoptotic changes in eggs cultured for 3 h in vitro. H2O2-induced morphological apoptotic features (A2) such as shrinkage (red arrow) and cytoplasmic granulation (yellow arrow), followed by degeneration (A3, green arrow) as compare to control egg showing first polar body with normal morphology (A1, blue arrow). H2O2-induced morphological apoptotic features in a concentration-dependent manner (A4). Presence of encircling granulosa cells protected against H2O2-induced morphological apoptotic changes in denuded egg collected from treated COCs (B3) but the encircling granulosa cells showed deterioration in their morphology (B2, blue arrow) as compare to encircling granulosa cells of control COCs that showed normal morphology (B1, green arrow). Encircling granulosa cells prevented H2O2-induced morphological apoptotic features in treated eggs at lower concentrations, while higher concentrations induced apoptosis in few eggs cultured in vitro (B4). Data are mean ± SEM of three independent experiments using 36–42 eggs/COCs and analyzed by One-way ANOVA followed by Bonferroni post hoc analysis (*p < 0.05). Denuded egg, Bar 20 µm; COCs, Bar 60 µm. (Color figure online)
Granulosa cells protect against H2O2-mediated increase of total ROS and decrease of catalase expression in treated eggs
As shown in Fig. 2, H2O2 (20 µM) significantly increased total ROS level in encircling granulosa cells of treated COCs and denuded egg as evidenced by increased DCF FI (Fig. 2, A2) as compare to control COCs (Fig. 2, A1) and denuded egg (C, in box). Presence of encircling granulosa cells protected H2O2-mediated increase of total ROS level in denuded egg (H2O2 + GC, in box) of treated COCs (A2) and DCF FI was similar to denuded egg (C + GC, in box) collected from control COCs (A1). The total ROS level was comparatively high in denuded eggs (C and H2O2, in box) cultured in vitro as compare to denuded eggs (C + H2O2 and H2O2 + GC) collected from COCs of treated as well as control groups. On the other hand, catalase level was significantly decreased as evidenced by reduced catalase FI in denuded egg (H2O2, in box) as well as COCs cultured in vitro (B2) as compare to their respective denuded egg (C, in box) and COCs (B1) control. The catalase level was comparatively high in denuded eggs (C + GC and H2O2 + GC, in box) collected from COCs as compare to denuded eggs cultured in vitro (C and H2O2, in box).The CTCF analysis of 36–42 eggs/COCs from three independent experiments using One-way ANOVA followed by Bonferroni post hoc analysis further confirms the protective effects of granulosa cells on H2O2-mediated increase of total ROS level (A3) and decrease of catalase level (B3).
Representative photograph showing total ROS and catalase expression levels in COCs as well as denuded egg cultured in vitro. H2O2 significantly increased total ROS level as evidenced by increased DCF FI in granulosa cells of treated COCs (A2, white arrow) as well as denuded egg (H2O2, in box) as compare to their respective control COCs (A1) and denuded egg (C, in box). Presence of encircling granulosa cells protected H2O2-mediated increase of total ROS level in denuded egg (H2O2 + GC, in box) of treated COCs and DCF FI was similar to denuded egg collected from control COCs (C + GC, in box). On the other hand, catalase level was significantly decreased as evidenced by reduced catalase FI in encircling granulosa cells of treated COCs (B2, white arrow) as well as denuded egg (H2O2, in box) as compare to their respective control COCs (B1) and denuded egg (C, in box). Presence of encircling granulosa cells protected ROS-mediated decrease of catalase level in denuded egg (H2O2 + GC, in box) of treated COCs. One-way ANOVA followed by Bonferroni post hoc analysis of CTCF data further confirms our results (A3, B3). Data are mean ± SEM of three independent experiments using 36–42 eggs/COCs. *p < 0.05 (C vs. C + GC), #p < 0.05 (C + GC vs. H2O2), Ϯp < 0.05 (H2O2 vs. H2O2 + GC). Bar 70 µm
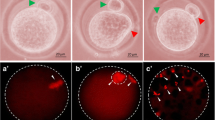
Granulosa cells protect against H2O2-mediated increase of Bax and cytochrome c level and decrease of Bcl-2 in treated eggs
As shown in Fig. 3, H2O2 (20 µM) increased FI of Bax (A2) as compare to control eggs (A1). Encircling granulosa cells protected H2O2-mediated increase of Bax expression in denuded eggs (A3) collected from treated COCs and FI was similar to control eggs (A1). On the other hand, H2O2 (20 µM) significantly reduced FI of Bcl-2 in treated eggs (B2) as compare to control eggs (B1). However, granulosa cells prevented the H2O2-mediated decrease of Bcl-2 expression in denuded eggs collected from treated COCs (B3). Further, cytochrome c expression was significantly increased in H2O2-treated eggs (C2) as compare to control eggs (C1) as evidenced by increased FI. Encircling granulosa cells prevented H2O2-mediated increase of cytochrome c expression in denuded eggs (C3) collected from treated COCs and FI was similar to control eggs (C1). However, the control protein β-actin expression level remains unchanged (D1–D3) suggesting that all parameters were kept constant during immunofluorescence studies. The CTCF analysis using 36–42 eggs/COCs from three independent experiments using One-way ANOVA followed by Bonferroni post hoc analysis further confirms the protective effects of granulosa cells on H2O2-mediated increase of Bax (A4), decrease of Bcl-2 (B4) and increase of cytochrome c levels (C4), while β-actin expression level did not change (D4).
Representative photograph showing the protective effects of encircling granulosa cells on Bax, Bcl-2 and cytochrome c expression levels in eggs cultured in vitro. H2O2 significantly increased FI of Bax in treated eggs (A2) as compare to control eggs (A1). Encircling granulosa cells protected H2O2-mediated increase of Bax expression in denuded eggs (A3) collected from treated COCs and FI was similar to control eggs (A1). On the other hand, H2O2 treatment significantly reduced FI of Bcl-2 in treated eggs (B2) as compare to control eggs (B1). However, granulosa cells prevented the decrease of Bcl-2 expression level in denuded eggs (B3) collected from treated COCs. The cytochrome c expression level was significantly increased in H2O2-treated eggs (C2) as compare to control eggs (C1). Encircling granulosa cells prevented H2O2-mediated increase of cytochrome c expression in denuded eggs (C3) collected from treated COCs and FI was similar to control eggs (C1). The lower panel shows β-actin FI as a control for upper panel photographs (D1–3). The CTCF analysis of FI using 36–42 eggs/COCs from three independent experiments further confirm above observations (A4, B4, C4, D4). Data are mean ± SEM of three independent experiments and subjected to One-way ANOVA followed by Bonferroni post hoc analysis. *p < 0.001 (C vs. H2O2), #p < 0.001 (H2O2 vs. H2O2 + GC). Bar 80 µm
Encircling granulosa cells protect against H2O2-induced DNA fragmentation in eggs
As shown in Fig. 4, H2O2 treatment induced DNA fragmentation as evidenced by yellow/orange color of EtBr (A2) and TUNEL fluor green fluorescence (B2) in treated eggs as compare to their respective controls (A1 and B1). On the other hand, encircling granulosa cells protected H2O2-mediated DNA fragmentation in eggs of treated COCs as evidenced by background green fluorescence of AO (A4) and TUNEL fluor negative staining (B4) in the cytoplasm of treated egg that showed FI similar to their respective controls (A3 and B3). Although encircling granulosa cells protected H2O2-induced DNA fragmentation in eggs but peripheral granulosa cells themselves underwent apoptosis as evidenced by yellowish orange color of EtBr that indicates apoptosis (A4) and TUNEL fluor positive green staining (B4). Three independent experiments using 36–42 eggs/COCs were conducted to confirm the observations.
Representative photograph showing H2O2-induced DNA fragmentation in eggs and its prevention by encircling granulosa cells cultured in vitro. H2O2 induced apoptosis as evidenced by yellow fluorescence of EtBr (A2, white arrow) and TUNEL fluor green fluorescence (B2, white arrow) in treated eggs as compare to their respective controls (A1, B1; white arrow). Presence of encircling granulosa cells protected H2O2-mediated DNA fragmentation in eggs of treated COCs as evidenced by green fluorescence of AO (A4, white arrow) and TUNEL fluor negative staining (B4, white arrow) that show FI similar to their respective controls (A3, B3; white arrow). However, peripheral encircling granulosa cells show DNA fragmentation as evidenced by yellowish orange color of EtBr (A4, blue arrows) as well as TUNEL fluor positive staining (B4, yellow arrows). Three independent experiments using 36–42 eggs/COCs were conducted to confirm the results. Denuded egg, Bar 30 µm; COCs, Bar 60 µm. (Color figure online)
Discussion
Mammalian ovary is a dynamic organ that generates excess amount of ROS during final stages of folliculogenesis [13–17]. However, ovary has its own antioxidant system through which it maintains the redox status by scavenging free radicals generated during final stages of follicular development [28]. If the antioxidant system of the ovary fails to do so or increased level of ROS in the follicular fluid or under in vitro culture conditions may deteriorate egg quality by inducing apoptosis. This notion is supported by our previous study that H2O2 induces cell cycle arrest and apoptosis in rat immature oocytes cultured in vitro [6]. However, it remains unclear whether the increased level of ROS under in vitro culture conditions could affect egg quality by inducing apoptosis. If yes, whether encircling granulosa cells could protect H2O2-induced egg apoptosis? Data of the present study suggest that the majority of control eggs (55%) were arrested at M-II stage possessing PB-I, while remaining underwent spontaneous egg activation (45%) by extruding PB-II. The SEA in the present study could be due to generation of ROS under in vitro culture conditions [29]. Physical factors such as temperature, oxygen level and other in vitro culture conditions may also induce SEA [30, 31]. The removal of cumulus cells at the time of denudation (shear force) and handling of eggs under in vitro culture conditions may accelerate SEA in ovulated eggs [32–34].
Generation of ROS beyond physiological level under in vitro culture conditions or exogenous supplementation of H2O2 may cause OS, which could induce cell cycle arrest and apoptosis. This possibility was further strengthened by observations that exogenous H2O2 inhibited SEA and induce morphological apoptotic features in a concentration-dependent manner in denuded eggs, while few eggs underwent degeneration if exposed to 20 µM H2O2 for 3 h cultured in vitro. Presence of encircling granulosa cells protected H2O2-induced morphological apoptotic features in eggs of treated COCs. Only few eggs and encircling granulosa cells underwent apoptosis at higher concentrations of H2O2 (10 and 20 µM). These data suggest the protective effects of encircling granulosa cells on ROS-mediated apoptosis under in vitro culture conditions. Handling of cumulus enclosed oocytes instead of denuded oocytes could protect oocytes from OS damage due to in vitro culture conditions. Cumulus cells closely interact and provide support to the maturing oocyte, shares the oocyte’s microenvironment and minimize the damage by ROS [35]. Cumulus cells are able to produce antioxidants that protect oocyte from OS damage [36]. These observations corroborates previous findings that high level of H2O2 induces morphological apoptotic features in rat eggs cultured in vitro [9, 10, 18, 22] and presence of encircling granulosa cells prevent egg from ROS-mediated OS damage in mouse [37–40], rat [8, 41, 42], bovine [43, 44], porcine [4] and human [45] oocytes cultured in vitro.
The increased level of ROS causes OS damage hence, ovary protects oocytes from OS damage by several antioxidant enzymes [46, 47]. Encircling granulosa cells inside the follicular microenvironment protect oocytes from OS damage by activating its own enzymatic antioxidant system [4, 8, 24]. The total ROS can be measured using H2DCFDA dye, which can be used as cell permeable fluorescence-based probe for ROS. H2DCFDA is first deacetylated by endogenous esterases to dichlorofluorescein (DCFH), which can further react with several ROS to form the fluorophore DCF which can be measured fluorimetrically [48]. Studies suggest that there is a limit for the cumulus cells to protect against OS damage [49]. Hence, in the present study, we used 20 μM of H2O2 and protective effects of encircling granulosa cells were analysed under in vitro culture conditions. The DCF intensity in the present study suggest that 20 µM H2O2 increased total ROS level in the granulosa cells of treated COCs and denuded eggs cultured in vitro. However, total ROS level was significantly low in denuded eggs collected from control as well as treated COCs as compare to denuded eggs collected from both the groups after 3 h of in vitro culture. These data together with previous studies suggest that in vitro culture conditions generate ROS [24] and encircling granulosa cells prevent increase of ROS in associated egg [35, 36].
Catalase is one of the major enzymatic antioxidants that decompose H2O2 into non toxic molecular oxygen and water [50]. We propose that catalase could be one of the enzymes present in granulosa cells to scavenge H2O2 generated under in vitro culture conditions during various ART programs. Hence in the present study, we analysed catalase expression level in COCs as well as denuded eggs cultured in vitro. Data of the present study suggest that exogenous 20 µM H2O2 reduced catalase expression in encircling granulosa cells of COCs and denuded eggs cultured in vitro. A significant increase of catalase expression level in denuded eggs collected from treated COCs as compare to treated denuded eggs suggest the role of granulosa cell catalase in H2O2 scavenging process under in vitro culture conditions. Similarly, the protective effects of catalase from OS damage have been reported in maturing gilts and mouse oocytes cultured in vitro [8, 50]. On the other hand, the increased production of ROS has been associated with decreased viability of encircling cumulus cells and antioxidants level [40, 51].
The increased levels of ROS can modulate mitochondrial membrane potential and alter Bax/Bcl-2 ratio in the plasma membrane of treated eggs [6]. This notion is strengthened by our data that H2O2 treatment modulated Bax/Bcl-2 ratio by reducing expression of anti-apoptotic protein such as Bcl-2 and inducing the expression of proapoptotic protein such as Bax in treated eggs. However, presence of encircling granulosa cells prevented the decrease of Bcl-2 and increase of Bax protein expression in the present study suggesting that granulosa cells prevent ROS-mediated changes in Bax/Bcl-2 ratio, which is required for the initiation of apoptosis. The role of granulosa cells in the prevention of ROS-Bax/Bcl-2-mediated apoptosis has been reported in rat COCs cultured in vitro [9]. Change in Bax/Bcl-2 ratio within a cell disrupts mitochondria membrane potential and induces cytochrome c release, which initiate apoptosis in wide variety of cells [2, 11, 12, 52, 53]. Our data suggest that H2O2 treatment increased cytochrome c level in treated eggs. However, the presence of granulosa cells prevented increase of cytochrome c level in eggs collected from treated COCs suggesting the role of encircling granulosa cells in the prevention of ROS-induced Bax/Bcl-2-mediated apoptotic pathway in eggs cultured in vitro. Similarly, ROS-mediated increase of cytochrome c level has been reported to induce oocyte/egg apoptosis in rat [2, 5, 10]. In the present study, presence of encircling granulosa cells prevented H2O2-induced overexpression of Bax, underexpression of Bcl-2 and increased cytochrome c levels in eggs collected from treated COCs as compare to their respective controls. However, Bax and cytochrome c expression levels were still high and Bcl-2 expression level was still low as compare to their respective controls. A little high but insignificant increase of Bax and cytochrome c levels and decrease of Bcl-2 level suggest that the encircling granulosa cells can protect egg from OS damage upto certain limit [49]. Our data suggest that encircling granulosa cells are unable to protect egg if COCs are exposed to ≥20 μM H2O2 for 3 h under in vitro culture conditions.
The increased cytochrome c level in egg can induce apoptosis by activating upstream and downstream caspases [54]. Although we did not analyse caspases activity in the present study, our previous reports indicate that H2O2 supplementation induces caspase-9 as well as caspase-3 in rat oocytes cultured in vitro [6, 9, 18, 19]. The increased caspases activities may cleave key structural and regulatory proteins that result in DNA fragmentation in 180–200 base-pair, a hallmark feature of apoptosis [6, 8, 9]. Our results suggest that H2O2 induced DNA fragmentation as evidenced by AO/EtBr as well as TUNEL fluor positive staining in treated denuded eggs cultured in vitro. On the other hand, presence of encircling granulosa cells protected egg apoptosis as evidenced by green background of AO and negative TUNEL fluor staining. However, 20 µM H2O2 was sufficient to induce peripheral granulosa cell apoptosis as evidenced by yellow/orange color of EtBr and TUNEL fluor positive staining in COCs cultured in vitro. These data support our observations that the encircling granulosa cells prevented OS-mediated egg apoptosis in vitro.
Conclusions
Our results suggest that the exogenous H2O2 induced ROS and thereby OS under in vitro culture conditions. The OS induced apoptosis through the activation of Bax/Bcl-2-cytochrome c-mediated apoptotic pathway in denuded eggs. However, presence of encircling granulosa cells prevented increase of ROS, Bax/Bcl-2 ratio, cytochrome c level and DNA fragmentation in eggs isolated from COCs cultured in vitro. Hence, we propose that presence of encircling granulosa cells could be beneficial to prevent ROS-mediated apoptosis and thereby deterioration of egg quality under in vitro culture conditions during various ART programs.
References
Tripathi A, Premkumar KV, Chaube SK (2010) Meiotic cell cycle arrest in mammalian oocytes. J Cell Physiol 223:592–600
Tiwari M, Prasad S, Tripathi A et al (2015) Apoptosis in mammalian oocytes: a review. Apoptosis 20:1019–1025
Albertini DF (2011) A cell for every season: the ovarian granulosa cell. J Assist Reprod Genet 28:877–878
Tatemoto H, Sakurai N, Muto N (2000) Protection of porcine oocytes against apoptotic cell death caused by oxidative stress during in vitro maturation: role of cumulus cells. Biol Reprod 63:805–810
Takahashi T, Igarashi H, Kawagoe J, Amita M, Hara S, Kurachi H (2009) Poor embryo development in mouse oocytes aged in vitro is associated with impaired calcium homeostasis. Biol Reprod 80:493–502
Chaube SK, Prasad PV, Thakur SC, Shrivastav TG (2005) Hydrogen peroxide modulates meiotic cell cycle and induces morphological features characteristic of apoptosis in rat oocytes cultured in vitro. Apoptosis 10:863–874
Chaube SK, Prasad PV, Thakur SC, Shrivastav TG (2005) Estradiol protects clomiphene citrate-induced apoptosis in ovarian follicular cells and ovulated cumulus-oocyte complexes. Fertil Steril 84:1163–1172
Tripathi A, Shrivastav TG, Chaube SK (2013) An increase of granulosa cell apoptosis mediates aqueous neem (Azadirachta indica) leaf extract-induced oocyte apoptosis in rat. Int J Appl Basic Med Res 3:27–36
Tripathi A, Chaube SK (2012) High cytosolic free calcium level signals apoptosis through mitochondria-caspase mediated pathway in rat eggs cultured in vitro. Apoptosis 17:439–448
Tripathi A, Shrivastav TG, Chaube SK (2012) Aqueous extract of Azadirachta indica (neem) leaf induces generation of reactive oxygen species and mitochondria-mediated apoptosis in rat oocytes. J Assist Reprod Genet 29:15–23
Chaube SK, Shrivastav TG, Prasad S et al (2014) Clomiphene citrate induces ROS-mediated apoptosis in mammalian oocytes. Open J Apop 3:52–58
Chaube SK, Shrivastav TG, Tiwari M, Prasad S, Tripathi A, Pandey AK (2014) Neem (Azadirachta indica L.) leaf extract deteriorates oocyte quality by inducing ROS-mediated apoptosis in mammals. SpringerPlus 3:464 (1–4)
Pandey AN, Tripathi A, Premkumar KV, Shrivastav TG, Chaube SK (2010) Reactive oxygen and nitrogen species during meiotic resumption from diplotene arrest in mammalian oocytes. J Cell Biochem 111:521–528
Pandey AN, Chaube SK (2014) A moderate increase of hydrogen peroxide level is beneficial for spontaneous resumption of meiosis from diplotene arrest in rat oocytes cultured in vitro. Bio Res Open Access 3:183–191
Prasad S, Tiwari M, Tripathi A, Pandey AN, Chaube SK (2015) Changes in signal molecules and maturation promoting factor levels associate with spontaneous resumption of meiosis in rat oocytes. Cell Biol Int 39:759–769
Tiwari M, Chaube SK (2016) Moderate increase of reactive oxygen species triggers meiotic resumption in rat follicular oocytes. J Obstet Gynaecol Res 42:536–546
Tiwari M, Prasad S, Tripathi A et al (2016) Involvement of reactive oxygen species in meiotic cell cycle regulation and apoptosis in mammalian oocytes. React Oxyg Species 1:110–116
Tripathi A, Chaube SK (2015) Roscovitine inhibits extrusion of second polar body and induces apoptosis in rat eggs cultured in vitro. Pharmacol Rep 67:866–874
Tripathi A, Chaube SK (2015) Reduction of phosphorylated Thr-161 Cdk1 level participates in roscovitine-induced Fas ligand-mediated apoptosis pathway in rat eggs cultured in vitro. In Vitro Cell Dev Biol Anim 51:174–182
Liu L, Keefe DL (2000) Cytoplasm mediated both developmental and oxidation-induced apoptotic cell death in mouse zygotes. Biol Reprod 62:1828–1834
Chan PJ, Calinisan JH, Corselli JU, Patton WC, King A (2001) Updating quality control assays in the assisted reproductive technologies laboratory with a cryopreserved hamster oocyte DNA cytogenotoxic assay. J Assist Reprod Genet 18:129–134
Chaube SK, Tripathi A, Khatun S, Mishra SK, Prasad PV, Shrivastav TG (2009) Extracellular calcium protects against verapamil-induced metaphase-II arrest and initiation of apoptosis in aged rat eggs. Cell Biol Int 33:337–343
Tripathi A, Khatun S, Pandey AN et al (2009) Intracellular levels of hydrogen peroxide and nitric oxide in oocytes at various stages of meiotic cell cycle and apoptosis. Free Radic Res 43:287–294
Uy B, McGlashan SR, Shaikh SB (2011) Measurement of reactive oxygen species in the culture media using Acridan Lumigen PS-3 assay. J Biomol Tech 22:95–107
Parazzini F, Cipriani S, Bulfoni G et al (2015) The risk of birth defects after assisted reproduction. J Assist Reprod Genet 32:379–385
Mendoza R, Perez S, de Los Santos MJ et al (2015) Congenital malformations, chromosomal abnormalities and perinatal results in IVF/ICSI newborns resulting from very poor quality embryos: a case-control study. Gynecol Obstet Invest 79:83–89
Opuwari CS, Henkel RR (2016) An update on oxidative damage to spermatozoa and oocytes. Biomed Res Int. doi:10.1155/2016/9540142
Oyawoye O, Abdel Gadir A, Garner A, Constantinovici N, Perrett C, Hardiman P (2003) Antioxidants and reactive oxygen species in follicular fluid of women undergoing IVF: relationship to outcome. Hum Reprod 18:2270–2274
Premkumar KV, Chaube SK (2016) Increased level of reactive oxygen species persuades postovulatory aging mediated spontaneous egg activation in rat eggs cultured in vitro. In Vitro Cell Dev Biol Anim 52:576–588
Chebotareva T, Taylor J, Mullins JJ, Wilmut I (2011) Rat eggs cannot wait: spontaneous exit from meiotic metaphase-II arrest. Mol Reprod Dev 78:795–807
Cui W, Zhang J, Lian HY et al (2012) Roles of MAPK and spindle assembly checkpoint in spontaneous activation and MIII arrest of rat oocytes. PLoS One 7:e32044
Keefer CL, Schuetz AW (1982) Spontaneous activation of ovulated rat oocytes during in vitro culture. J Exp Zool 224:371–377
Cui W, Zhang J, Zhang CX et al (2013) Control of spontaneous activation of rat oocytes by regulating plasma membrane Na+/Ca2+ exchanger activities. Biol Reprod 88:1–9
Prasad S, Tiwari M, Koch B, Chaube SK (2015) Morphological, cellular and molecular changes during postovulatory aging in mammals. J Biomed Sci 22:36
Agarwal A, Durairajanayagam D, du Plessis SS (2014) Utility of antioxidants during assisted reproductive techniques: an evidence based review. Reprod Biol Endocrinol 12:112
Huang Z, Wells D (2010) The human oocyte and cumulus cells relationship: new insights from the cumulus cell transcriptome. Mol Hum Reprod 16:715–725
Li Q, Miao DQ, Zhou P et al (2011) Glucose metabolism in mouse cumulus cells prevents oocyte aging by maintaining both energy supply and the intracellular redox potential. Biol Reprod 84:1111–1118
Li Q, Wang G, Zhang J et al (2012) Combined inhibitory effects of pyruvate and low temperature on postovulatory aging of mouse oocytes. Biol Reprod 87:105
Zhou P, Lian HY, Cui W et al (2012) Maternal-restraint stress increases oocyte aneuploidy by impairing metaphase I spindle assembly and reducing spindle assembly checkpoint proteins in mice. Biol Reprod 86:83
Jiao GZ, Cao XY, Cui W et al (2013) Developmental potential of prepubertal mouse oocytes is compromised due mainly to their impaired synthesis of glutathione. PLoS One 8:e58018
Zhang C-X, Cui W, Zhang M et al (2014) Role of Na+/Ca2+ Exchanger (NCX) in modulating postovulatory aging of mouse and rat oocytes. PLoS ONE 9:e93446
Jiao GZ, Cui W, Yang R et al (2016) Optimized protocols for in vitro maturation of rat oocytes dramatically improve their developmental competence to a level similar to that of ovulated oocytes. Cell Reprogram 18:17–29
Cetica PD, Pintos LN, Dalvit GC, Beconi MT (2001) Antioxidant enzyme activity and oxidative stress in bovine oocyte in vitro maturation. IUBMB Life 51:57–64
Morado SA, Cetica PD, Beconi MT, Dalvit GC (2009) Reactive oxygen species in bovine oocyte maturation in vitro. Reprod Fertil Dev 21(4):608–614
Goud PT, Goud AP, Qian C et al (1998) In-vitro maturation of human germinal vesicle stage oocytes: role of cumulus cells and epidermal growth factor in the culture medium. Hum Reprod 13:1638–1644
Agarwal A, Gupta S, Sharma R (2005) Oxidative stress and its implications in female infertility-a clinician’s perspective. Reprod Biomed Online 11:641–650
Prasad S, Tiwari M, Tripathi A, Pandey AN, Shrivastav TG, Chaube SK (2016) Impact of stress on oocyte quality and reproductive outcome. J Biomed Sci 23:36
Myhre O, Andersen JM, Aarnes H, Fonnum F (2003) Evaluation of the probes 2′,7′ dichlorofluorescin diacetate, luminol, and lucigenin as indicators of reactive species formation. Biochem Pharmacol 65:1575–1582
Shaeib F, Banerjee J, Maitra D, Diamond MP, Abu-Soud HM (2013) Impact of hydrogen peroxide-driven Fenton reaction on mouse oocyte quality. Free Radic Biol Med 58:154–159
Goud AP, Goud PT, Diamond MP, Gonik B, Abu-Soud HM (2008) Reactive oxygen species and oocyte aging: role of superoxide, hydrogen peroxide, and hypochlorous acid. Free Radic Biol Med 44:1295–1304
De Matos DG, Furnus CC, Moses DF (1997) Glutathione synthesis during in vitro maturation of bovine oocytes: role of cumulus cells. Biol Reprod 57:1420–1425
Eskes R, Antonsson B, Osen-Sand A et al (1998) Bax-induced cytochrome C release from mitochondria is independent of the permeability transition pore but highly dependent on Mg2+ ions. J Cell Biol 143:217–224
Gómez-Crisóstomo NP, López-Marure R, Zapata E, Zazueta C, Martínez-Abundis E (2013) Bax induces cytochrome c release by multiple mechanisms in mitochondria from MCF7 cells. J Bioenerg Biomembr 45:441–448
Braun T, Dar S, Vorobiov D, Lindenboim L, Dascal N, Stein R (2003) Expression of Bcl-x(S) in Xenopus oocytes induces BH3-dependent and caspase-dependent cytochrome c release and apoptosis. Mol Cancer Res 1:186–194
Acknowledgements
This study was financially supported by Department of Science and Technology, Ministry of Science and Technology, Government of India (Grant No. EMR/2014/000702).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no conflict of interest exist.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10495_2016_1324_MOESM1_ESM.tif
Fig. 1 Representative photograph showing protective effects of encircling granulosa cells on H2O2-induced changes in Bax, Bcl-2 and cytochrome c expression levels in COCs cultured for 3 h in vitro. Encircling granulosa cells prevented H2O2-mediated increased expression of Bax (A2, white arrow), reduced expression of Bcl-2 (B2, white arrow) and increased expression of cytochrome c (C2, white arrow) as compare to their respective controls (A1, B1 and C1). Lower panel shows β-actin expression level as a control for upper panel photographs (D1 and D2). Three independent experiments using 36-42 COCs were conducted to confirm the observations. Bar 80 µm (TIF 2628 KB)
Rights and permissions
About this article
Cite this article
Tiwari, M., Tripathi, A. & Chaube, S.K. Presence of encircling granulosa cells protects against oxidative stress-induced apoptosis in rat eggs cultured in vitro. Apoptosis 22, 98–107 (2017). https://doi.org/10.1007/s10495-016-1324-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-016-1324-4