Abstract
The present study was aimed to find out whether increased level of reactive oxygen species (ROS) particularity hydrogen peroxide (H2O2) could persuade postovulatory aging-mediated abortive spontaneous egg activation (SEA) in rat eggs cultured in vitro. For this purpose, ROS and H2O2 levels, mitochondria distribution and its membrane potential, p286-CaMK-II, Emi2, Thr-161 phophorylated cyclin-dependent protein kinase1 (Cdk1) as well as cyclin B1 levels, in vitro effects of 3-tert-butyl-4 hydroxy anisole (BHA), pentoxifylline and dibutyryl-adenosine 3′,5′-cyclic monophosphate (db-cAMP) were analyzed during postovulatory aging-induced abortive SEA in vitro. Data of the present study suggest that postovulatory aging increased H2O2 levels, disturbed mitochondrial distribution pattern and mitochondrial membrane potential (MMP) in eggs. There was an significant increase of p286-CaMK-II level, while Emi2 level reduced significantly during egg aging in vitro. The reduced Emi2 level was associated with decreased Thr-161 phosphorylated cyclin-dependent kinase-1 (Cdk1) as well as cyclin B1 level in aged eggs that underwent abortive SEA. Further, supplementation of pentoxifylline, db-cAMP, and BHA protected postovulatory aging-mediated abortive SEA in concentration-dependent manner. These data suggest that postovulatory aging increased H2O2 levels, reduced MMP, and increased p286-CaMK-II. The increased p286-CaMK-II was associated with reduced Emi2 level and maturation-promoting factor levels during postovulatory aging-mediated abortive SEA. Drugs that elevate cAMP directly or indirectly and BHA protected postovulatory aging-mediated abortive SEA possibly by reducing ROS level in rat eggs cultured in vitro.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Freshly ovulated eggs are arrested at metaphase-II (M-II) stage with first polar body (PB1) in several mammalian species. These eggs remain in the ampula of fallopian tube for a short window period and wait for fertilization (Yanagimachi 1988). If the fertilization does not occur within the window period, postovulatory aging causes spontaneous exit from M-II arrest (Premkumar and Chaube 2013). In rat, more than 80% of ovulated eggs undergo spontaneous exit from M-II arrest and initiate the extrusion of a second polar body, so-called abortive spontaneous egg activation (SEA) (Keefer and Schuetz 1982; Zernika-Goetz 1991; Ross et al. 2006; Premkumar and Chaube 2013). The postovulatory aging directly affects egg quality (Miao et al. 2009) and developmental potentials of ovulated eggs (Tarin et al. 2000). Hence, protection of postovulatory aging-mediated abortive SEA under in vitro culture conditions is very important to achieve the desired assisted reproduction technology(s) (ART) outcome. The postovulatory aging-mediated abortive SEA has been reported in several mammalian species including rat (Zernika-Goetz 1991), mouse (Kubiak 1989), bovine (Sergeev and Norman 2003), porcine (Ito and Shimada 2003), and human (Lu et al. 2006) but the possible players and pathways that drives abortive SEA remains poorly understood.
A growing body of evidence suggests that postovulatory aging reduces the fitness of an egg both in vivo (Tarin et al. 2000) as well as under in vitro culture conditions (Keefer and Schuetz 1982; Zernika-Goetz 1991; Chaube et al. 2007; Chebotareva et al. 2011; Premkumar and Chaube 2015). Recent studies from our laboratory suggest that Ca2+ (Premkumar and Chaube 2013) and nitric oxide (NO) are possible players during postovulatory aging-induced abortive SEA in rat (Premkumar and Chaube 2015). An insufficient increase of cytosolic free Ca2+ level triggers abortive SEA as well as M-III like arrest, which is characterized by scattered chromosome in egg cytoplasm (Keefer and Schuetz 1982; Zernika-Goetz 1991; Ross et al. 2006; Premkumar and Chaube 2013), while high sustained level of cytosolic free Ca2+ is required for complete egg activation (Ozil 1998).
Increased cytosolic free calcium Ca2+ level during postovulatory aging may be associated with the generation of ROS (Premkumar and Chaube 2014) as well as activation of CaM-dependent kinase-II (CaMK-II) (Ito et al. 2006; Yoo and Smith 2007). The CaMK-II activates anaphase promoting complex/cyclosome (APC/C) by releasing endogenous meiotic inhibitor 2 (Emi2; a cytostatic factor, CSF) and Wee 1, a tyrosine kinase (Kubiak et al. 2008; Oh et al. 2011). The active Wee 1 destabilizes maturation-promoting factor (MPF) by inducing Thr-161 phosphorylation of Cdk1 and degradation of cyclin B1 (Verlhac et al. 2002; Kubiak et al. 2008; Oh et al. 2011). The destabilized MPF finally triggers abortive SEA during postovulatory egg aging (Madgwick and Jones 2007; Premkumar and Chaube 2014). Based on these studies, we propose that an increased level of cytosolic free Ca2+ level could be associated with the increase of H2O2 level. The increased level of ROS could modulate MMP and thereby postovulatory aging-mediated abortive SEA possibly through CaMK-II pathway. In addition, drugs that can elevate cAMP directly or indirectly and antioxidants may scavenge ROS and could protect against postovulatory aging-mediated abortive SEA in rat eggs cultured in vitro. Therefore, the present study was designed to investigate whether generation of ROS can modulate the mitochondrial membrane potential (MMP) and reduces MPF through the activation of CaMK-II pathway during postovulatory-mediated abortive SEA. If yes, whether phosphodiesterase inhibitors, dibutyryl-adenosine 3′,5′-cyclic monophosphate (db-cAMP) and 3-tert-butyl-4 hydroxy anisole (BHA) could protect against postovulatory aging-mediated abortive SEA in rat eggs cultured in vitro.
Materials and Methods
Chemicals and preparation of working solutions
All reagents used in this study were from Sigma Chemical Co. (St. Louis, MO), unless otherwise stated. The BHA was initially dissolved in 100 μl of 95% ethanol to get stock solution of 1 mg/ml. The stock solution was further diluted in Ca2+-free medium (Medium-199, AL043A, HiMedia Laboratories, Mumbai, India) to get working concentrations of BHA (0, 12.5, 25, 50, and 100 μM). The freshly prepared working concentrations were kept at 37°C for 5 min before use. Addition of BHA did not alter the osmolarity (290 ± 5 m Osmol) and pH (7.2 ± 0.2) of culture medium. Initially, Fluo-3 AM was dissolved in 100 μl of DMSO and then in culture medium to obtain 50 μM of working solution. The stock solutions (1 μg/ml) of dihydro-dichlorofluorescein-diacetate (hereafter, H2DCF) and Rhodamine-123 (hereafter, Rho-123) were further diluted in Ca2+-free medium to get final concentrations of 10 μM for in vitro studies. Since ethanol and DMSO were used as solvents, respective control groups received equivalent dilution of highest concentration (<0.05% of ethanol and <0.1% DMSO) of these solvents.
Experimental animals and collection of eggs
Immature (22–24 days old) female rats of Rattus norvegicus (Charles-Foster strain) were separated out from existing colony and kept in a light-controlled room with food and water ad libitum. Maximum numbers of eggs were collected if non-cyclic immature female rats were subjected to superovulation induction protocol published earlier (Premkumar and Chaube 2013). The project has been approved by Institutional Animal Ethical Committee, Faculty of Science (letter no. F.Sc./IAEC/2013-14/0341/2199. Dated: 23 Sept 2013) of the University. Ovulated eggs were collected from oviduct by puncturing with the help of a 26-gauge needle and transferred to Ca2+-free medium (Medium-199, Cat. no. AL043A, HiMedia Laboratories, Mumbai, India). All ovulated eggs were picked up using micropipette (Clay Adams; B&D and Co., New Jersey) and transferred to fresh culture medium containing 0.01% hyaluronidase at 37°C for 3 min and denuded by repeated manual pipetting. Denuded eggs were washed three times with culture medium and then used for in vitro studies.
Analysis of postovulatory aging-mediated morphological changes
To find out in vitro effects of postovulatory egg aging on morphological and meiotic stage changes, freshly ovulated M-II arrested eggs (12–15 eggs) collected after 14 h post-hCG surge were cultured in plain medium for various times (0, 1, and 3 h) in humidified incubator with 5% CO2 (Galaxy 170R, Eppendorf, New Brunswick, Germany). At the end of the incubation period, eggs were examined for morphological changes using phase-contrast microscope at ×400 magnification. The meiotic status of eggs were examined by treating eggs with propidium iodide (10 μg/ml) for 10 min at 37°C and examined under epifluorescence microscope (Nikon, Ni-U, Japan) using ex530 nm and em620 nm at ×400 magnification.
Quantitative analysis of H2O2 concentration and total ROS level
The H2O2 concentration was analyzed in control (M-II) and eggs that underwent abortive SEA using H2O2 assay kit (purchased from BioVision, CA) as per company manual protocol. In brief, control and aged eggs were transferred to a microcentrifuge tube containing 100 μl of lysis buffer (5 Mm Tris, 20 mM EDTA, 0.5% Triton X-100, pH 8) for 1 h on ice for lysis. Lysates were centrifuged at 10,000×g at 4°C for 15 min and supernatant was diluted five-fold with sample diluent and immediately processed for the quantitative estimation of H2O2. The optical density (OD) was determined using a microplate reader (Micro Scan MS5608A, ECIL, Hyderabad; India) set at 560 nm. All samples were run in one assay to avoid inter-assay variation and intra-assay variation was found to be 1.9%.
H2DCF was used to analyze ROS level in aged eggs cultured in vitro. The intracellular oxidation of H2DCF to dihydro-dichloro-fluorescein (hereafter, DCF) is documented over time. H2DCF is a stable non-polar, non-fluorescence dye that readily diffuses into cells and is converted to DCF upon hydrolyzing by intracellular esterase (Wang and Joseph 1999). The oxidation of this molecule to the DCF results in green fluorescence. The fluorescence intensity reflects the total ROS level in eggs. The ROS level was measured following published protocol (Takahashi et al. 2003). In brief, 12–15 eggs from each group were incubated for 30 min at 37°C in culture medium supplemented with 10 μM H2DCF. After washing three times with culture medium, eggs were mounted onto a slide and observed under epifluorescence microscope using excitation at 480 nm, emission at 520 nm (Nikon Ni-U, Tokyo, Japan). A total of 36–45 eggs from three independent experiments were used in each group and 5–8 eggs from each experiment were used for the corrected total cell fluorescence (CTCF) analysis following the method published earlier using Image J Software (version 1.44 from National Institute of Health, MD, USA) (Premkumar and Chaube 2013). All parameters were kept constant, and for each egg the whole area was selected. Fluorescence intensity was analyzed using ImageJ software (National Institutes of Health, Bethesda, MD).
Effects of BHA on postovulatory aging-mediated abortive SEA
BHA is a well-known cell permeable free radical scavenger and could reduce ROS load during postovulatory aging and thereby abortive SEA. For this purpose, a group (12–15 eggs/group) of M-II arrested eggs was cultured in Ca2+-free medium containing with or without various concentrations (0, 12.5, 25, 50 and 100 μM) of BHA for 3 h in vitro. The millimolar concentration of BHA did not show any adverse effect under in vitro culture conditions and prevented spontaneous meiotic resumption from diplotene arrest in rat oocytes (Pandey and Chaube 2014). Based on concentration-response study, 100 μM concentration of BHA was used to analyze the time-course effect of BHA. For this purpose, 12–15 eggs were cultured in Ca2+-free medium containing 100 μM concentration of BHA for various times (1, 2 and 3 h) at 37°C in vitro. At the end of each incubation period, eggs were washed three times with PBS and then morphological changes were analyzed using phase-contrast microscope (Nikon Eclipse-E200, Japan).
Effects of pentoxifylline and db-cAMP on postovulatory aging-mediated abortive SEA
To find out in vitro effects of pentoxifylline (a phosphodiestrase inhibitor) and db-cAMP (a non-degradable analogue of cAMP), a group of 12–15 eggs was cultured in medium containing with or without various concentrations of pentoxifylline (0.2, 0.4, 0.6, and 0.8 mM) or db-cAMP (0.25, 0.5, 1, and 2 mM) for 3 h in CO2 incubator (Eppendorf-Galaxy-170R, New Brunswick, Germany) at 37°C for 3 h in vitro. These concentrations of pentoxifylline and db-cAMP did not show any adverse effect under in vitro culture conditions but prevented spontaneous meiotic resumption from diplotene arrest in rat oocytes (Chaube et al. 2000; Pandey and Chaube 2014). At the end of the incubation period, eggs were removed, washed, and then analyzed for their morphological changes using phase-contrast microscope (Nikon-Eclipse-E200, Tokyo, Japan).
Analysis of mitochondrial distribution and membrane potential
Detection of Rho123 fluorescence intensity of mitochondria reveals its quantity and function in terms of trans-membrane potential. The Rho123 fluorescence intensity is stronger in active cells than in quiescent cells, and the intensity decreases in damaged mitochondria (Takahashi et al. 2003). The amount of Rho123 conjugated with mitochondria differs in different types of cells and in different cell functional status (Evan and Littlewood 1998). Rho123 is a membrane-permeable cationic fluorescent dye that accumulates into mitochondria with respect to its negative trans-membrane potential (ΔΨ m). The ΔΨ m was monitored using Rho123 as described previously (Shapiro 2000) with some modifications. Briefly, freshly ovulated M-II arrested eggs (12–15 eggs) were cultured in medium-199 for various times (0, 1, and 3 h) in a humidified incubator at 37°C with 5% CO2 (Galaxy 170R, Eppendorf, New Brunswick, Germany). The ΔΨ m was measured in aged eggs that were cultured in medium supplemented with Rho123 (10 μM) for 10 min for various times (0, 1, and 3 h). At the end of the incubation period, eggs were washed three times with medium. Cellular fluorescence intensity of Rho123 was analyzed using a Nikon fluorescence microscope (Nikon Ni-U, Japan) with ex480 nm and em550 nm. Background fluorescence signals of Rho123 were subtracted from those obtained from the egg’s cytoplasm. Egg’s ΔΨ m was expressed as fold over control eggs at 0 h. Each group had 10–15 eggs and experiment was repeated three times to confirm the results. A total of 30–45 eggs from three independent experiments were used in each group and 5–8 eggs from each experiment were used for CTCF analysis. All parameters were kept constant and for each egg the whole area was selected.
Analysis of p286-CaMK-II and Emi2 levels
The p286-CaMK-II and Emi2levels were detected in eggs using their specific antibodies purchased from SantaCruz Biotechnology, California, USA. For this purpose, a group of eggs was fixed overnight with 3.7% buffered formaldehyde and then permeabilized (0.01% triton X-100 in PBS) for 10 min at 37°C. After washing three times with phosphate buffer saline (PBS), eggs were exposed to blocking buffer (2.5% PBS-BSA solution) for 30 min at 37°C and then exposed to anti-p286-CaMK-II (1:1000 dilution in blocking buffer) oranti-Emi2 antibody (1:500 dilution in blocking buffer) at 37°C in humidified chamber for 2 h. After five washes with PBS, slides were exposed to FITC-labeled secondary antibody (1:1000 dilutions in blocking buffer; SantaCruz Biotechnology, CA) for 1 h at 37°C in humidified chamber. The slides were then washed five times with PBS, mounted with anti-fade mounting media (HiMedia laboratories, Mumbai, India) and observed under epifluorescence microscope (Model, Ni-U, Nikon Eclipse Tokyo, Japan) at 520 nm at ×400 magnifications. Each group had 12–15 eggs and each experiment was repeated three times to confirm results. A total of 36–45 eggs from three independent experiments were used and 5–8 eggs from each experiment were used for CTCF analysis. All parameters were kept constant, and for each egg the whole area was selected.
Analysis of MPF level
Thr-161 phosphorylated Cdk1 and cyclin B1 levels were detected in eggs using their specific antibodies purchased from Santa Cruz Biotechnology. For this purpose, a group of 12–15 eggs was fixed overnight with 3.7% buffered formaldehyde and then permeabilized (0.01% triton X-100 in PBS) for 10 min at 37°C. After washing three times with phosphate-buffered saline (PBS), eggs were exposed to blocking buffer (2.5% PBS-BSA solution) for 30 min at 37°C and then exposed to anti-pThr-161 (sc-12341) (1:1000 dilution in blocking buffer) and anti-cyclin B1 antibody (sc-752) (1:1000 dilution in blocking buffer) at 37°C in humidified chamber for 2 h. After five washes with PBS, slides were exposed to FITC or TRITC-labeled secondary antibody (1:1000 dilutions in blocking buffer; Santa Cruz Biotechnology) for 1 h at 37°C in humidified chamber. The slides were then washed five times with PBS, mounted with anti-fade mounting media (Himedia Laboratories, Mumbai, India.) and observed under epifluorescence microscope (Model, Ni-U, Nikon Eclipse Tokyo, Japan) at 520 nm at ×400 magnifications. Each group had 12–15 eggs and each experiment was repeated three times to confirm results. A total of 36–45 eggs from three independent experiments were used and 5–8 eggs from each experiment were used for CTCF analysis. All parameters were kept constant and for each egg the whole area was selected.
Statistical Analysis
Data are mean ± standard error of mean (SEM) of three independent experiments. All percentage data were subjected to arcsine square-root transformation before statistical analysis. Data were analyzed by either Student’s t test or one-way ANOVA using SPSS software, version 17.0 (SPSS, Inc., Chicago, IL). A probability of p < 0.05 was considered significant. Corrected total cell fluorescence (CTCF) was measured using ImageJ software (NIH, Bethesda, MD).
Results
Postovulatory aging drives abortive SEA
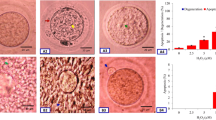
Freshly ovulated eggs collected 14 h post-hCG surge were arrested at M-II stage and showed extrusion of PBI (Fig. 1a ; green arrow). Culture of these eggs for 3 h under in vitro culture conditions induced initiation and extrusion of PB II (Fig. 1b ; red arrow). The M-III like arrest was noticed if the aged eggs were cultured in vitro for 5 or 7 h as evidenced by degenerating PBI (green arrow) and presence of PBII (red arrow) in vitro (Fig. 1c ). The morphological features were further confirmed by their chromosomal status lower panel (Fig. 1a ′–c′). The M-II plate towards the polar body area of the eggs haploid set of chromosomes confirms M-II stage (Fig. 1a ′). The separation of haploid chromosomes suggests anaphase-II stage (Fig. 1b ′), while scattering of chromosomes in the egg cytoplasm confirms M-III like arrest (Fig. 1c ′).
Representative photograph showing postovulatory aging-mediated morphological features and meiotic stages in rat eggs cultured in vitro. (a) M-II arrested egg, (b) Egg showing extrusion of second polar body after exit from M-II arrest (abortive SEA), (c) Aged eggs showing M-III like arrest (c). Lower panel (a′–c′) photographs showing PI stained eggs as shown in upper panel photographs (a–c). (a′) M-II arrested egg showing formation of metaphase plate chromosomes, (b′) Exit from M-II arrest as the egg showing first polar body chromosomes (encircled) as well as second polar body chromosomes during postovulatory aging-mediated abortive SEA, (c′) M-III arrested egg showing scattered haploid set of chromosomes throughout the eggs cytoplasm. Bar 20 μm.
Postovulatory aging mediates generation of H2O2 and total ROS level
As shown in Fig. 2, a significant (p < 0.05) increase of H2O2 level (86.7 ± 1.1 ng/egg) was noticed in eggs that underwent exit from M-II arrest (within 1 h) as compared to M-II arrested control eggs (81.6 ± 0.69 ng/egg). Increased level of H2O2 might have elevated total ROS level in aged eggs cultured in vitro. Hence, we analyzed total ROS level using oxidation-sensitive fluorescent dye H2DCF. As shown in Fig. 3, a high fluorescence intensity of dye was observed in aged eggs that underwent either exit from M-II arrest or M-III like arrest SEA (Fig. 3b–c ) as compared to control eggs (Fig. 3a ) M-II arrested control eggs. Figure 3a’–c’ shows the phase-contrast images of the respective Fig. 3a–c . The CTCF analysis using Image J software further confirms our findings (Fig. 3d ).
Representative photograph showing changes in ROS level postovulatory egg aging in vitro. Postovulatory aging increased ROS level in a time-dependent manner as the meiotic cell cycle progress from M-II arrest (EM-II) and reached to M-III like arrest (a–c) as evidenced by fluorescence intensity of H2DCF. The upper panel shows light microscope photographs (a′–c′) of the lower panel (a–c). The CTCF analysis further confirms our observations that postovulatory egg aging significantly increased total ROS level during meiotic cell cycle progression from M-II arrest to M-III like arrest in aged eggs cultured in vitro (d). Data are mean ± SEM of three independent experiments and analyzed by ANOVA. Significant at p < 0.001 level. Bar 20 μm.
BHA protects postovulatory aging-induced abortive SEA
As shown in Fig. 4a , BHA inhibited abortive SEA in a concentration-dependent manner (one-way ANOVA, F = 198.788; p < 0.001) if the eggs were cultured for 3 h in vitro. Based on this result, we selected 100 μM of BHA for time-course study. Data suggest that 100 μM of BHA protected postovulatory aging-mediated abortive SEA in a time-dependent manner (one-way ANOVA; F = 529.147; p < 0.001; Fig. 4b ).
Pentoxifylline and db-cAMP protect postovulatory aging-mediated abortive SEA
As shown in Fig. 5a , pentoxifylline inhibited abortive SEA in a concentration-dependent manner (one-way ANOVA, F = 198.788; p < 0.001). Similarly, db-cAMP, cell permeable analogue of cAMP, prevented postovulatory aging-mediated abortive SEA in a concentration-dependent manner (one-way ANOVA, F = 198.788; p < 0.001; Fig. 5b ).
In vitro effects of pentoxifylline and db-cAMP on postovulatory aging-mediated abortive SEA in eggs cultured in vitro. Both pentoxifylline (a) and db-cAMP (b) inhibited abortive SEA in concentration-dependent manner. Data are mean ± SEM of three independent experiments and analyzed by one-way ANOVA.
Postovulatory aging alters mitochondria distribution and decreases membrane potential
In the present study, we used Rho123 investigate mitochondrial ΔΨ m, distribution and movement. Data suggest that postovulatory aging-induced exit from M-II arrest is associated with movement of mitochondria towards central part of egg cytoplasm (Fig. 6b ) as compared to M-II arrested control eggs (Fig. 6a ). An accumulation of mitochondria in the center of the cytoplasm was reduced, and clustered mitochondria seen throughout the cytoplasm of eggs were arrested at M-III like stage (Fig. 6c ). Figure 7 shows changes in MMP in aged eggs. Data suggest that an exit for M-II arrest is associated with significant (p < 0.05) decrease (Fig. 7b ) a significant (p < 0.05) increase (Fig. 7c ) of Rho123 fluorescence intensity as compared to M-II arrested control eggs (Fig. 7a ). The upper panel (Fig. 7a ′–c′) shows the phase-contrast images of Fig. 7a–c . The CTCF analysis using Image J software further confirms our findings (Fig. 7d ).
Representative photograph showing postovulatory aging-mediated changes in distribution of mitochondria in eggs cultured in vitro. Postovulatory aging disturbed mitochondrial distribution as evidenced by increased mitochondrial clusters in the central area of aged eggs that underwent abortive SEA (b), which was decreased during M-III arrest (c) as compared to M-II arrested eggs (a). Bar 20 μm.
Representative photograph showing postovulatory aging-induced changes in mitochondria membrane potential ΔΨ m in eggs cultured in vitro. The postovulatory aging triggered disturbed MMP as evidenced by centralized and disturbed fluorescence intensity of Rho123 in aged eggs cultured in vitro (b and c) as compared to M-II arrested control eggs (a). The upper panel shows light microscope photograph (a′–c′) of lower panels photographs (a–c). Data are analyzed by Student’s t-test, * and ** denotes significance at the level of p < 0.05 level (M-II versus EM-II and M-II versus M-III). Bar 20 μm.
Postovulatory aging decreases Emi2 level by increasing p286-CaMK-II
As shown in Fig. 8, p286-CaMK-II level was significantly increased during postovulatory aging induces exit from M-II arrest (Fig. 8b ) and M-III-like arrest (Fig. 8c ) as compared to M-II arrested control eggs (Fig. 8a ). The CTCF analysis further confirms above observations (Fig. 8d ). Figure 9 shows the changes in Emi2 level during postovulatory egg aging. Postovulatory aging significantly reduced Emi2 level in eggs that underwent either exit from M-II arrest (Fig. 9b ) or M-III-like arrest as compare to M-II arrested eggs (Fig. 9a ). The CTCF analysis of fluorescence intensity of Emi2 confirms our observations (Fig. 9d ).
Representative photograph showing postovulatory aging-induced changes in p286-CaMK-II in rat eggs cultured in vitro. Postovulatory aging increased fluorescence intensity of p286-CaMK-II in aged eggs that underwent abortive SEA (b) and M-III arrest (c) as compared to control eggs (a). Data are mean ± SEM of fluorescence intensity three independent experiments and analyzed by Student’s t test. * denotes significantly higher at p < 0.05 level. Bar 20 μm.
Representative photograph showing postovulatory aging-induced changes in Emi2 level in eggs cultured in vitro. Postovulatory aging increased fluorescence intensity of Emi2 in aged eggs that underwent abortive SEA (b) and M-III arrest (c) as compared to control eggs (a). Data are mean ± SEM of fluorescence intensity three independent experiments and analyzed by Student’s t test. * denotes significantly different at p < 0.05 level. Bar 20 μm
Postovulatory aging decreases MPF level
As shown in Fig. 10, pThr-161 phosphorylated Cdk1 level significantly decreased in eggs that underwent postovulatory aging abortive SEA (Fig. 10b ) as compared to control eggs (Fig. 10a ). Similarly, a significant reduction of cyclin-B1 level was noticed during postovulatory egg aging (Fig. 11b ) as compared to control eggs (Fig. 11a ). The CTCF analysis further suggests that reduced level of pThr-161 phosphorylated Cdk1 (Fig. 10d ) and cyclin-B1 (Fig. 11d ) induced postovulatory aging-induced abortive SEA.
Representative photograph showing postovulatory aging-induced changes in Cdk1 level in eggs cultured in vitro. Postovulatory aging increased fluorescence intensity of Cdk1 in aged eggs that underwent abortive SEA (b) and M-III arrest (c) as compared to control eggs (a). Data are mean ± SEM of fluorescence intensity three independent experiments and analyzed by Student’s t-test. * denotes significantly lower at p < 0.001 level. Bar 20 μm.
Representative photograph showing postovulatory aging-induced changes in cyclin B1 level in eggs cultured in vitro. Postovulatory aging increased fluorescence intensity of cyclin B1 in aged eggs that underwent abortive SEA (b) and M-III arrest (c) as compared to control eggs (a). Data are mean ± SEM of fluorescence intensity three independent experiments and analyzed by Student’s t test. * denotes significantly lower at p < 0.001 level. Bar 20 μm.
Discussion
Recent studies from our laboratory suggest that the postovulatory aging mediates spontaneous exit from M-II arrest (Premkumar and Chaube 2013, 2014, 2015). Meiotic cell cycle proceeds further and gets arrested at M-III like stage, which is characterized by scattered chromosomes in the cytoplasm of aged eggs and extrusion of second polar body without forming pronuclei (Ross et al. 2006; Premkumar and Chaube 2013). Data of the present study further support our previous observations that postovulatory aging drives freshly ovulated M-II arrested eggs towards meiotic exit from M-II arrest as evidenced by initiation of extrusion of second polar body, a first sign of abortive spontaneous egg activation. As the time passes, in vitro aging triggers meiotic cell cycle progression towards M-III like arrest. However, pronuclei formation has not been observed in any aged eggs cultured in vitro for 3 h. These changes were further confirmed by their chromosomal status. The chromosomal plate towards polar body forming area of an egg confirms M-II stage. The separation of haploid set of chromosomes confirms anaphase-II stage, while scattering of chromosomes in egg cytoplasm confirms M-III like arrest. The similar chromosomal status has been reported in eggs that underwent postovulatory aging-mediated SEA (Kubiak 1989; Zernika-Goetz 1991; Ross et al. 2006).
Moderate increase of cytosolic free Ca2+is one of the players for postovulatory aging-mediated abortive exit from M-II arrest in rat (Premkumar and Chaube 2013, 2014). We have recently reported that the release of Ca2+ through RyR channels on the plasma membrane of internal stores, and sustained high level of cytosolic free Ca2+ in the egg is one of the driving forces during postovulatory-mediated SEA under in vitro culture conditions (Premkumar and Chaube 2014). Data of the present study suggest that the increased level of H2O2 was associated with exit from M-II arrest. Further, total ROS level was also increased in a similar fashion in aged eggs cultured in vitro. These results suggest that H2O2 could be one of the causes for postovulatory aging-mediated increase in ROS level in aged eggs cultured in vitro. These findings corroborate with previous studies that the generation of ROS associates with postovulatory aging mediates abortive SEA (Takahashi et al. 2003; Chaube et al. 2008).
The increased ROS level may lead to mobilization of Ca2+ from internal stores in aged eggs (Okada et al. 2003; Zamay and Zamay 2006). Further, it was reported that aged eggs are more sensitive to H2O2 and increased cytosolic free Ca2+ levels (Takahashi et al. 2003). ROS are involved in elevating cytosolic free Ca2+ level (Tang et al. 2013) and thereby activation of calcium regulatory protein, CaMK-II (Tang et al. 2013; Zhang et al. 2014). Based on these studies, we propose that increased level of ROS could increase both p268-CaMK-II and cytosolic free Ca2+ level in aged eggs cultured in vitro. The active CaMK-II may increase Wee1 level during postovulatory aging-mediated abortive SEA. Data of the present study suggest that p268-CaMK-II level was significantly increased during postovulatory egg aging in vitro. The increased p268-CaMK-II could lead to release of Ca2+ from internal stores. Although we did not measure the cytosolic free Ca2+ level in the present study, our recent studies suggest that a moderate increase of cytosolic free Ca2+ level mediates postovulatory aging-mediated abortive SEA (Premkumar and Chaube 2014, 2015). The increased cytosolic free Ca2+ level could modulate Emi2 level in aged eggs cultured in vitro. Our results suggest that Emi2 level was significantly reduced in eggs that underwent either meiotic exit from M-II arrest or M-III like arrest. Taking together these observations with our previous findings suggest the role of ROS in the mobilization of Ca2+ and increase of p268-CaMK-II in aged eggs cultured in vitro.
Mitochondria generate superoxide (O2˙¯), which is subsequently converted into hydroxyl radicals and H2O2 (McCord 1969). Superoxide has been reported to accelerate the aging process (Abu-Soud and Stuehr 1993; Rosen et al. 2002). Distribution of mitochondria in egg cytoplasm and its dysfunction affect egg quality during egg aging (Van Blerkom et al. 1995). Data of the present study reveal that postovulatory aging disturbed mitochondria distribution and induced its clustering in the cytoplasm of aging eggs. Both mitochondria distribution and its dysfunction are correlated with the decline in ΔΨ m (Zhang et al. 2011). The higher physiological levels of H2O2 have intense effect on ΔΨ m that affect mitochondrial function (Chakraborti et al. 1999; Cadenas and Davies 2000). Our results suggest that postovulatory aging significantly reduced ΔΨ m in eggs that underwent abortive SEA. Taken together, these results advocate a link between ROS and mitochondrial dysfunction in aged eggs. These findings are further supported by our observations that a cell-permeable antioxidant such as BHA prevented postovulatory aging-induced abortive SEA. Further, pentoxifylline and db-cAMP inhibited postovulatory aging-mediated abortive SEA in a concentration- and time-dependent manner. Although we did not measure ROS during the treatment, our results suggest that supplementation of BHA, and pentoxifylline and/or db-cAMP prevented eggs from undergoing abortive SEA probably by reducing level of ROS in aged eggs. Taken together, these observations strengthen our previous finding that BHA and db-cAMP inhibits ROS-mediated spontaneous resumption of meiosis from diplotene arrest in rat oocytes cultured in vitro (Cheon et al. 2000; Bellomo et al. 2006; Piccoli et al. 2006; Pandey and Chaube 2014).
The increased ROS level and reduced level of Emi2 in aged eggs may inhibit tyrosine phosphatases (Monteiro et al. 1991; Sullivan et al. 1994) as well as tyrosine kinases (Chan et al. 1986) and thereby decline MPF level in porcine oocytes (Kikuchi et al. 2002). Based on these studies, we hypothesized that increased level of ROS may reduce MPF level by reducing Thr-161 phosphorylation status of Cdk1 as well as cyclin B1 level in aged eggs cultured in vitro. Our results suggest that postovulatory aging significantly reduced MPF level as evidenced by reduced Thr-161 phosphorylation status of Cdk1 as well as cyclin B1 level in aged eggs cultured in vitro. Similarly, reduced MPF level has been reported to induce meiotic resumption in rat eggs cultured in vitro (Monteiro et al. 1991; Premkumar and Chaube 2013, 2015). The impact of reduced level of MPF on spontaneous exit from M-II arrest could be modulated by mitogen-activated protein kinase (MAPK) in aged eggs cultured in vitro. Although we did not analyze MAPK activity in the present study, previous studies suggest that MAPK activity is required for the microtubule assembly and spindle organization in rat eggs (Sun et al. 2008). A reduction in MAPK activity leads to disturbed spindle integrity in rat eggs cultured in vitro (Cui et al. 2013). Taking together our data with previous observations suggest that high MPF level and MAPK activity are essential for the maintenance of the M-II arrest in ovulated rat eggs and reduced MPF level and/or MAPK activity could result in postovulatory aging-mediated abortive SEA.
The postovulatory aging-mediated abortive SEA deteriorates eggs quality since these eggs cannot be used for in vitro fertilization (IVF) or any other ART programs. Hence, protection of postovulatory aging-mediated abortive SEA must be given prime importance to increase IVF rate as well as various ART outcomes. The rat is an interesting model to study the role-abortive SEA, since it begins as quick as 15 min after ovulation under in vivo as well as in vitro culture conditions (Ross et al. 2006; Premkumar and Chaube 2013, 2014, 2015; Prasad et al. 2015). Data of the present study indicate that supplementation of db-cAMP or BHA in culture medium could prevent abortive SEA and extend the maintenance of M-II arrest in ovulated eggs, which may allow more time available for handling of eggs under in vitro culture conditions during various ART programs.
Conclusions
In summary, data of the present study suggest that increased level of ROS disturbed mitochondrial distribution and decreased MMP, which increased of cytosolic free Ca2+ level in aged eggs cultured in vitro (Fig. 12). The increased cytosolic free Ca2+ level reduced Emi2 level and increased p268-CaMK-II level. The reduced Emi2 level was associated with reduced Thr-161 phosphorylated Cdk1 as well as cyclin B1 levels leading to reduced MPF level. The reduced MPF level finally triggers postovulatory aging-mediated abortive SEA. Our results suggest that use of BHA, pentoxyfilline and db-cAMP could prevent postovulatory aging-mediated abortive SEA. Thus, the present study provides an insight to exploit the possibilities to lengthen the time of maintenance of M-II arrest during the handling of eggs for various ART programs.
Schematic chart showing possible involvement of ROS during postovulatory aging-induced abortive SEA in rat. A transient increase in ROS level possibly results in decrease of cAMP as well as mitochondrial membrane potential. Decreased cAMP level results abortive SEA, while decrease of mitochondrial membrane potential results in increase of cytosolic free Ca2+ level. High cytosolic free Ca2+ level increases CaMK-II level that decreases Emi2 level. A decrease in Emi2 level results in the degradation of Cyclin B1 through APC/C-mediated pathway, which finally triggers MPF destabilization. The destabilized MPF results in aging eggs undergoing abortive SEA in rat.
References
Abu-Soud HM, Stuehr DJ (1993) Nitric oxide synthases reveal a role for calmodulin in controlling electron transfer. Proc Natl Acad Sci U S A 90:10769–10772
Bellomo F, Piccoli C, Cocco T, Scacco S, Papa F, Gaballo A, Boffoli D, Signorile A, D’Aprile A, Scrima R, Sardanelli AM, Capitanio N, Papa S (2006) Regulation by the cAMP cascade of oxygen free radical balance in mammalian cells. Antioxid Redox Signal 8:495–502
Cadenas E, Davies KJ (2000) Mitochondrial free radical generation, oxidative stress, and aging. Free Rad Biol Med 29:222–230
Chakraborti T, Das S, Mondal M, Roychoudhury S, Chakraborti S (1999) Oxidation, mitochondria, and calcium; an overview. Cell Signaling 11:77–85
Chan CP, Gallis B, Blumenthal DK, Pallen CJ, Wang JH, Krebs EG (1986) Characterization of the phosphotyrosyl protein phosphatase activity of calmodulin-dependent protein phosphatase. J Biol Chem 261:9890–9895
Chaube SK, Chaki SP, Misro MM (2000) Effect of pentoxifylline and caffeine on spontaneous maturation of rat oocytes. Health Population 23:177–189
Chaube SK, Dubey PK, Mishra SK, Shrivastav TG (2007) Verapamil reversibly inhibits spontaneous parthenogenetic activation in aged rat eggs cultured in vitro. Cloning Stem Cells 9:608–617
Chaube SK, Khatun S, Mishra SK, Shrivastav TG (2008) Calcium ionophore-induced egg activation and apoptosis are associated with the generation of intracellular hydrogen peroxide. Free Rad Res 42:212–220
Chebotareva T, Taylor J, Mullins JJ, Wilmut I (2011) Rat eggs cannot wait: spontaneous exit from meiotic metaphase-II arrest. Mol Reprod Dev 78:795–807
Cheon YP, Kim SW, Kim SJ, Yeom YI, Cheong C, Ha KS (2000) The role of Rho A in the germinal vesicle breakdown of mouse oocytes. Biochem Biophy Res Commun 273:997–1002
Cui W, Zhang J, Zhang CX, Jiao GZ, Zhang M, Wang TY, Luo MJ, Tan JH (2013) Control of spontaneous activation of rat oocytes by regulating plasma membrane Na+/Ca2+ exchanger activities. Biol Reprod 88(160):1–9
Evan G, Littlewood T (1998) A matter of life and cell death. Science 281:1317–1320
Ito J, Shimada T (2003) Effect of protein kinase C inhibitor on mitogen-activated protein kinase and p34cdc2 kinase activity during parthenogenetic activation of porcine oocytes by calcium ionophore. Biol Reprod 69:1675–1682
Ito J, Kaneko R, Hirabayashi M (2006) The regulation of calcium/calmodulin-dependent protein kinase II during oocyte activation in the rat. J Reprod Dev 52:439–447
Keefer CL, Schuetz AW (1982) Spontaneous activation of ovulated rat oocytes during in vitro culture. J Exp Zool 224:371–377
Kikuchi K, Naito K, Noguchi J, Kaneko H, Tojo H (2002) Maturation/M-phase promoting factor regulates aging of porcine oocytes matured in vitro. Cloning Stem Cells 4:211–222
Kubiak JZ (1989) Mouse oocytes gradually develop the capacity for activation during the metaphase II arrest. Dev Biol 136:537–545
Kubiak JZ, Ciemerych MA, Hupalowska A, Sikora-Polaczek M, Polanski Z (2008) On the transition from the meiotic to mitotic cell cycle during early mouse development. Int J Dev Biol 52:201–217
Lu Q, Chen ZJ, Gao X, Ma SY, Li M, Hu JM, Li Y (2006) Oocyte activation with calcium ionophore A23187 and puromycin on human oocytes that failed to fertilize after intra cytoplasmic sperm injection. Zhonghua Fu Chan KeZaZhi 41:182–185
Madgwick S, Jones KT (2007) How eggs arrest at metaphase II: MPF stabilization plus APC/C inhibition equals cytostatic factor. Cell Div 2:4–11
McCord JM (1969) Superoxide dismutase an enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244:6049–6055
Miao YL, Kikuchi K, Sun QY, Schatten H (2009) Oocyte aging: cellular and molecular changes, developmental potential and reversal possibility. Hum Reprod Update 15:573–585
Monteiro HP, Ivaschenko Y, Fischer R, Stern A (1991) Inhibition of protein tyrosine phosphatase activity by diamide is reversed by epidermal growth factor in fibroblasts. FEBS Lett 295:146–148
Oh JS, Susor A, Conti M (2011) Protein tyrosine kinase Wee1B is essential for metaphase II exit in mouse oocytes. Science 332:462–465
Okada S, Li Q, Whitin JC, Clayberger C, Krensky AM (2003) Intracellular mediators of granulysin-induced cell death. J Immunol 171:2556–2562
Ozil JP (1998) Role of calcium oscillations in mammalian egg activation: experimental approach. Biophys Chem 72:141–152
Pandey AN, Chaube SK (2014) Increase of hydrogen peroxide level is beneficial for spontaneous resumption of meiosis from diplotene arrest in rat oocytes cultured in vitro. Bio Reseach Open Access 4:183–191
Piccoli C, Scacco S, Bellomo F, Signorile A, Iuso A, Boffoli D, Scrima R, Capitanio N, Papa S (2006) cAMP controls oxygen metabolism in mammalian cells. FEBS Lett 580:4539–4543
Prasad S, Tiwari M, Koch B, Chaube SK (2015) Morphological, cellular and molecular changes during postovulatory egg aging in mammals. J Bio Med Sci 22:36
Premkumar KV, Chaube SK (2013) An insufficient increase of cytosolic free calcium level results postovulatory aging-induced abortive spontaneous egg activation in rat. J Asst Reprod Genet 30:117–123
Premkumar KV, Chaube SK (2014) RyR channel-mediated increase of cytosolic free calcium level signals cyclin B1 degradation during abortive spontaneous egg activation in rat. In Vitro Cell Dev Biol Anim 50:640–647
Premkumar KV, Chaube SK (2015) Nitric oxide signals postovulatory aging induced abortive spontaneous egg activation in rats. Redox Rep 20:184–192
Rosen GM, Tsai P, Weaver J, Porasuphatana S, Roman LJ, Starkov AA, Fiskum G (2002) The role of tetrahydrobiopterin in the regulation of neuronal nitric-oxide synthase-generated superoxide. J Biol Chem 277:40275–40280
Ross PJ, Yabuuch A, Cibelli JB (2006) Oocyte spontaneous activation in different rat strains. Cloning Stem Cells 8:275–282
Sergeev IN, Norman AV (2003) Calcium as a mediator of apoptosis in bovine oocytes and preimplantation embryos. Endocrine 22:169–176
Shapiro HM (2000) Membrane potential estimation by flow cytometer. Methods 21:271–279
Sullivan SG, Chiu DT, Errasfa M, Wang JM, Qi JS, Stern A (1994) Effects of H2O2 on protein tyrosine phosphatase activity in HER14 cells. Free Rad Biol Med 16:399–403
Sun SC, Xiong B, Lu SS, Sun QY (2008) MEK1/2 is a critical regulator of microtubule assembly and spindle organization during rat oocyte meiotic maturation. Mol Reprod Dev 75:1542–1548
Takahashi T, Takahashi E, Igarashi H, Tezuka N, Kurachi H (2003) Impact of oxidative stress in aged mouse oocytes on calcium oscillations at fertilization. Mol Reprod Dev 66:143–152
Tang DW, Fang Y, Liu ZX, Wu Y, Wang XL, Zhao S, Han GC, Zeng SM (2013) The disturbances of endoplasmic reticulum calcium homeostasis caused by increased intracellular reactive oxygen species contributes to fragmentation in aged porcine oocytes. Biol Reprod 89:1–9
Tarin JJ, Perez-Albala S, Cano A (2000) Consequences on offspring of abnormal function in ageing gametes. Hum Reprod Update 6:532–549
Van Blerkom J, Davis PW, Lee J (1995) ATP content of human oocytes and developmental potential and outcome after in-vitro fertilization and embryo transfer. Hum Reprod 10:415–424
Verlhac MH, Lefebvre C, Kubiak JZ, Umbhauer M, Rassinier P, Colledge W, Maro B (2002) Mos activates MAP kinase in mouse oocytes through two opposite pathways. EMBO J 19:6065–6074
Wang H, Joseph JA (1999) Quantifying cellular oxidative stress by dichloro fluorescein assay using microplate reader. Free Rad Biol Med 27:612–616
Yanagimachi R (1988) Mammalian fertilization. In: Knobi E, Nei J (eds) The physiology of reproduction. Raven, New York, pp 135–185
Yoo JC, Smith LC (2007) Extracellular calcium induces activation of Ca2+/calmodulin dependent protein kinase II and mediates spontaneous activation in rat oocytes. Biochem Biophys Res Commun 359:854–859
Zamay TN, Zamay AS (2006) Influence of ATP on Ehrlich ascites carcinoma cell free cytoplasmic calcium concentration in the course of tumor growth. Biochemistry (Mosc) 71:1090–1095
Zernika-Goetz M (1991) Spontaneous and induced activation of rat oocytes. Mol Reprod Dev 28:169–176
Zhang N, Wakai T, Fissore RA (2011) Caffeine alleviates the deterioration of Ca(2+) release mechanisms and fragmentation of in vitro-aged mouse eggs. Mol Reprod Dev 78:684–701
Zhang CX, Cui W, Zhang M, Zhang J, Wang TY, Zhu J, Jiao GZ, Tan JH (2014) Role of Na+/Ca2+ exchanger (NCX) in modulating postovulatory aging of mouse and rat oocytes. PLoS One 9, e93446
Acknowledgments
This study was financially supported by Department of Biotechnology, Ministry of Science and Technology, Government of India.
Authors’ contributions
KVP conducted all the experiments and wrote the manuscript under the direction of SKC. Both the authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no competing interests.
Additional information
Editor: Tetsuji Okamoto
Rights and permissions
About this article
Cite this article
Premkumar, K.V., Chaube, S.K. Increased level of reactive oxygen species persuades postovulatory aging-mediated spontaneous egg activation in rat eggs cultured in vitro. In Vitro Cell.Dev.Biol.-Animal 52, 576–588 (2016). https://doi.org/10.1007/s11626-016-0007-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-016-0007-3