Abstract
The present study was aimed to find out whether an increase of cytosolic free calcium level induces egg apoptosis through mitochondria-caspase mediated pathway. To increase cytosolic free calcium level and morphological apoptotic changes, ovulated eggs were cultured in Ca2+/Mg2+ free media-199 with or without various concentrations of calcium ionophore (0.5, 1, 2, 3, 4 μM) for 3 h in vitro. The morphological apoptotic changes, cytosolic free calcium level, hydrogen peroxide (H2O2) concentration, catalase activity, cytochrome c concentration, caspase-9 and caspase-3 activities and DNA fragmentation were analyzed. Calcium ionophore induced morphological apoptotic features in a concentration-dependent manner followed by degeneration at higher concentrations (3 and 4 μM). Calcium ionophore increased cytosolic free calcium level, induced generation of hydrogen peroxide (H2O2) and inhibited catalase activity in treated eggs. The increased H2O2 concentration was associated with increased cytochrome c concentration, caspase-9 and caspase-3 activities that resulted in the induction of morphological features characteristic of egg apoptosis. The increased caspase-3 activity finally induced DNA fragmentation as evidenced by TUNEL positive staining in calcium ionophore-treated eggs. These findings suggest that high cytosolic free calcium level induces generation of H2O2 that leads to egg apoptosis through mitochondria-caspase mediated pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Calcium is one of the major signaling molecules that regulate various aspects of function in wide variety of cells including oocytes/eggs [1, 2]. Changes in intracellular calcium ([Ca2+]i) level modulate meiotic cell cycle, apoptosis and/or cell death depending upon its concentration in several mammalian species including mouse, rat and bovine eggs [3–6]. A transient increase of ([Ca2+]i) in the presence of calcium ionophore A 23187 (hereafter calcium ionophore) under in vitro culture conditions stimulates meiotic resumption, while high sustained level maintains meiotic arrest and induces apoptosis [6–9]. On the other hand, abnormally high ([Ca2+]i) level results into cell death [10, 11]. The calcium rise in an egg occurs by means of two principal mechanisms: the efflux from the store via ligand-gated channels on organelle membrane, and the entry through ion channels in the plasma membrane [2]. Calcium ionophore increases cytosolic free calcium [12] possibly by mitochondrial remodelling [13] and mitochondria membrane depolarization [14]. Under in vitro culture condition, calcium ionophore induces apoptosis in rat and pig eggs cultured in vitro [5, 9, 15, 16].
The possible mechanism(s) by which high level of intracellular calcium induces apoptosis in mammalian eggs remains to be elucidated. Few studies indicate that calcium ionophore increases ([Ca2+]i) level possibly by depleting essentially all internal calcium pools from endoplasmic reticulum and mitochondria [2, 13–16]. High cytosolic free calcium level induces generation of reactive oxygen species (ROS) in various somatic cells [5, 17–19] as well as in rat eggs [9, 20]. The increased intracellular level of ROS may inhibit catalase activity [21, 22] and induced egg apoptosis [9, 23]. On the other hand, addition of antioxidants to the culture medium enhanced catalase activity and reduced oxidative stress in porcine oocytes in vitro [9, 22].
The high level of ROS can modulate membrane potential of mitochondria [13, 14] and promotes cytochrome c release from mitochondria in somatic cells [24, 25]. This notion is strengthened by the observations that increased level of ROS including H2O2 resulted in the loss of outer mitochondrial membrane integrity leading to cytochrome c release from mitochondria of eggs [26, 27]. The release of cytochrome c from mitochondria initiates the apoptotic signals in wide variety of cells [26, 28–31]. The mechanism by which cytochrome c induces apoptosis in eggs remains to be elucidated. However, few studies using somatic cells indicated that, in the cytosol, cytochrome c binds to apoptotic factor-1 (Apaf-1) leading to the recruitment and activation of procaspase-9 in a large complex termed the apoptosome [32, 33]. As a result, procaspase-9 auto processes and cleaves the effectors procaspase including procaspase-3. The activated procaspase-3 cleaves key structural and regulatory proteins that result in the biochemical and morphological changes associated to apoptosis [34, 35]. Although mechanism by which calcium induces apoptosis in somatic cells is clear to some extent, in germ cells particularly in eggs, it remains unclear whether increase in cytosolic free calcium load induces generation of ROS and if so whether activation of caspase-cascade through mitochondria mediated pathway is involved during calcium- induced egg apoptosis. Therefore, in the present study we used calcium ionophore to induce the endogenous burst of calcium from internal stores and then we analyzed morphological features characteristics of egg apoptosis, cytosolic free calcium and intracellular H2O2 levels, catalase activity, cytochrome c concentration, caspases activities and DNA fragmentation in rat eggs cultured in vitro.
Materials and methods
Chemicals and reagents
All chemicals used in the present study were purchased from Sigma Chemical Co. (St. Louis, MO) unless stated otherwise. The serum free Ca2+/Mg2+deficient medium-199 (HiMedia Laboratories, Mumbai, India) was prepared as per company manual protocol, pH was adjusted to 7.2 and osmolarity was found to be 290 mOsmol. The culture medium was supplemented with sodium bicarbonate (0.035% w/v), penicillin (100 IU/ml) and streptomycin (100 mg/ml). The stock solution of calcium ionophore (1 μg/μl) was prepared in DMSO, aliquoted and then kept at −20°C until use. The final concentrations of calcium ionophore (0.5, 1, 2, 3 and 4 μM) were prepared by diluting stock solution with serum-free and Ca2+/Mg2+-deficient medium-199 (HiMedia Laboratories, Mumbai, India). Addition of calcium ionophore at final concentrations did not alter the osmolarity (290 mOsmol) and pH (7.2) of the culture media used in the present study.
Experimental animal and collection of eggs
The experimental rats of Charls-Foster (CF) strain were housed in air-conditioned, light controlled room and provided food and water ad libitum. The immature female rats (22–24 days old) were subjected to superovulation induction protocol (20 IU pregnant mare’s serum gonadotropin for 48 h followed by 20 IU human chorionic gonadotropin for 14 h) to collect ovulated eggs. All procedure was in conformation to the stipulations of the University Animal Ethical Committee of Banaras Hindu University, Varanasi and in keeping with the Guidelines for the Care and Use of Laboratory Animals (NIH Publication). Ovulated cumulus-enclosed eggs were isolated in serum-free and Ca2+/Mg2+-deficient medium-199 under a dissecting microscope (Nikon dissecting microscope, model C-DS; Tokyo, Japan) by puncturing oviduct using 26-gauge needle attached to a 1 ml syringe. All ovulated cumulus-enclosed eggs were picked up using microtubing (inner diameter 2 mm) attached with disposable glass micropipette (inner diameter, 100 μm; Clay Adams, NJ) and transferred to culture medium containing 0.01% hyaluronidase at 37°C. After 3 min of treatment, denuded eggs were removed and washed three times with culture medium and cultured in vitro.
Effect of calcium ionophore on morphological apoptotic changes in vitro
A group of 12–14 eggs were cultured in Ca2+/Mg2+-deficient medium-199 with or without various concentrations of calcium ionophore (0.5, 1, 2, 3 and 4 μM) for 3 h. At the end of incubation period, eggs were removed, washed three times with culture medium and transferred on to a grooved slide with 100 μl of culture medium and then examined for morphological changes using a phase contrast microscope (Nicon, Eclipse; E600, Tokyo, Japan) at 400× magnification.
Qualitative analysis of cytosolic free calcium level
The qualitative analysis of cytosolic free calcium was carried out following the protocol published earlier [36]. Eggs were treated with or without 3 μM calcium ionophore and 50 μM Fluo3/AM at 37°C in humidified chamber for 3 h. At the end of incubation period, 10–12 eggs were washed with fresh medium three times and then observed for Fluo3 fluorescence using fluorescence microscope (Nikon Eclips E800 (Nikon, Tokyo Japan). The corrected total cell fluorescence was calculated following the method published earlier [37] using Image J Software (version 1.44 from National Institute of Health, USA). The experiment was repeated three times to confirm results.
Quantitative estimation of intracellular H2O2 concentration
The intracellular H2O2 concentration in egg lysates was analyzed following our previous published protocol [23] using H2O2 assay kit purchased from Northwest Life Science Specialties, LLC, WA. In brief, control and 3 μM calcium ionophore treated eggs that had morphological apoptotic features (100 eggs from each group) were transferred to a microcentrifuge tube containing 100 μl of hypotonic lysis buffer (5 mM Tris, 20 mM EDTA, 0.5% Triton X-100, pH 8) for 1 h on ice for lysis. Lysates were centrifuged at 10,000×g at 4°C for 15 min and clear supernatant was immediately used for the quantitative estimation of H2O2 concentration by colorimetric assay as per company manual protocol. The optical density (OD) was determined using a microplate reader (Micro Scan MS5608A, ECIL, Hyderabad, India) set at 560 nm. All samples (in triplicate) were run in one assay to avoid inter-assay variation and intra-assay variation for H2O2 concentration was 1.5%.
Catalase activity assay
The catalase activity in eggs was analyzed following our previous published protocol [38] using catalase activity assay kit purchased from BioVision, Inc., CA. In brief, egg lysates were prepared as described above for the measurement of H2O2 concentration. Lysates were immediately used for the estimation of catalase activity as per company manual protocol. In brief, 25 μl of H2O2 standards, positive control solution, high control solution and egg lysates were loaded to respective wells in 96 microplate. The high control and standard wells received 10 μl of stop solutions provided with the assay kit. The final volume of each well was made 90 μl with assay buffer provided with the assay kit. 12 μl of 1 mM H2O2 was added in high control as well as in samples wells. The plate was incubated at 25°C for 30 min and then 10 μl of stop solution was added in these wells. The 50 μl of freshly prepared develop mix (50 μl develop mix = 46 μl Assay Buffer, 2 μl OxiRed prob, 2 μl HRP solution) was added to each well and mixed well. The plate was incubated at 25°C for 10 min and then OD was taken at 570 nm using microplate reader. The catalase activity was calculated as amount of H2O2 decomposed/min/ml and represented as μU/mg protein of egg lysate.
Immunocytochemistry for cytochrome c
Immunostaining for cytochrome c was carried out using anti-cytochrome c antibody following company manual protocol with some modifications. In brief, ten eggs from control and calcium ionophore-treated group were fixed in 3.7% formaldehyde solution in PBS (0.01 M, pH 7.4). After washing twice with PBS for 5 min each time, slides were treated with 0.3% H2O2 in absolute methanol for 15 min to quench endogenous peroxidase activity. The slides were washed twice with PBS and then exposed to PBS containing 0.1% Triton X-100 for permeabilization. Slides were exposed to 100 μl of blocking buffer (0.5% BSA, 0.1% tween-20 in 100 ml PBS) at room temperature for 1 h and then incubated with 25 μl of diluted (1:2000 in PBS) cytochrome c polyclonal rabbit antibody (Santa Cruz Biotechnology Inc., CA, USA) tagged with horseradish peroxidase (HRP) at room temperature for 1 h in a humidified chamber. At the end of the incubation period, slides were washed three times with PBS and then exposed to 25 μl of freshly prepared diaminobenzidine (DAB) solution (1 μl of 30% H2O2 and 5 μl of DAB in 1 ml of PBS; R&D Systems Inc., MN, USA) for 15 min. Thereafter, slides were washed four times in PBS and subsequently counterstained with 25 μl of 1% Methyl Green solution (R&D Systems Inc., MN, USA) for 2 min. The slides were again washed three times with PBS and then mounted in distyrene plasticizer xylene (DPX). The mounted slides were analyzed for DAB-positive staining using a phase-contrast microscope at 400× magnification. The experiment was repeated three times and a representative photograph is shown in the result section.
Quantitative analysis of intracellular cytochrome c concentration
The cytochrome c concentration in egg lysates was analyzed using cytochrome c ELISA kit purchased from R&D Systems MN, USA as per company manual protocol. The egg lysates were prepared as described above for the measurement of H2O2 concentration. A clear supernatant was removed from each sample and stored at −20°C until assay. All egg lysates were quickly thawed and used for the analysis of cytochrome c concentration as per company manual protocol and the plate was read at 450 nm using Microplate Reader. All samples were run in triplicate to avoid inter-assay variation and intra-assay variation was 1.02%.
Caspase activity assays
The intracellular caspase-3 and caspase-9 activities in egg lysates were analyzed following our previous published protocol [39] using caspase-3 and caspase-9 colorimetric assay kit purchased from R&D Systems (MN, USA). In brief, egg lysates were prepared as described above for the measurement of H2O2 concentration. A clear supernatant was removed from each sample and stored at −20°C until assay. Samples were quickly thawed and 50 μl of each sample (in triplicate) was loaded in 96 microwells plate. The 50 μl of 2× reaction buffer (buffer 9 for caspase-9 and buffer 3 for caspase-3 assay provided with the kit) was added in each well. The 5 μl of caspase substrate (LEHO-pNA, caspase-9 substrate; DEVD-pNA, caspase-3 substrate) was added to each well and then plates were incubated at 37°C for 2 h. At the end of incubation period, plates were read at 405 nm using microplate reader for caspase-9 and caspase-3 activities. All samples (in triplicate) were run in one assay to avoid inter-assay variation and OD values are directly used to depict caspase-9 and caspase-3 activities.
TUNEL analysis for DNA fragmentation
The DNA fragmentation was detected following our previous published protocol [38] using terminal deoxynucleotidyl transferase (TDT) nick-end labeling (TUNEL) kit purchased from R&D Systems (MN, USA). In brief, control and calcium ionophore-treated eggs (12–15 eggs in each group) were fixed in 3.7% (v/v) formaldehyde in PBS for 15 min at 18–20°C. After washing, eggs were transferred separately on to poly l-lysine-coated slides and then air-dried. Slides were treated with 50 μl of proteinase K solution for 30 min and then immersed in quenching solution for 3–4 min. Thereafter, slides were immersed in 1× TDT labeling buffer for 5 min and then incubated with 50 μl of labeling reaction at 37°C for 1 h. The slides were immersed in 1× TDT stop buffer to stop the reaction. Washed slides were incubated with 50 μl of diluted (1:500) biotin labeled anti-BrdU at 37°C for 1 h. Slides were washed with PBS containing 0.05% Tween-20 and then treated with 50 μl of Streptavidin-HRP solution for 10 min. Washed slides were immersed in DAB solution for 5 min and then in methyl green solution for 2 min after washing. Slides were mounted in DPX and then analyzed for TUNEL positive staining under phase contrast microscope at 400× magnification. The TUNEL analysis was repeated three times and representative photographs are shown in the result section.
Determination of live and dead status of eggs
The live and dead status of control and calcium ionophore-treated eggs that underwent degeneration was examined by means of eosin/nigrosin dye-exclusion test as per published protocol [40]. In brief, control eggs and 4 μM calcium ionophore-treated eggs (3–4 eggs in each group) that underwent degeneration were removed, washed three times with washing media and then stained with 10 μl of 1% eosin (w/v) followed by 10 μl of nigrosin (10%;w/v) for 1 min at room temperature. The stained eggs were observed for live and dead status using a phase-contrast microscope at 400× magnification.
Statistical analysis
Data are expressed as mean ± S.E. of mean (S.E.M.) of triplicate samples. All percentage data were subjected to arcsine square-root transformation before statistical analysis. Data were analyzed by either Student’s t-test or one-way analysis of variance (ANOVA) using SPSS software, version 11.5 (SPSS, Inc. Chicago, IL, USA). A probability of p < 0.05 was considered to be statistically significant.
Results
Calcium ionophore induces morphological apoptotic changes in eggs cultured in vitro
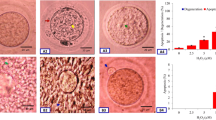
As shown in Fig. 1a, calcium ionophore induced morphological apoptotic features in a concentration-dependent manner (one-way ANOVA, F = 163.79, p < 0.001). Fig. 1b shows calcium ionophore- induced morphological apoptotic changes in treated eggs. Shrinkage (S) was the first morphological feature observed followed by cytoplasmic fragmentation (CF). Cytoplasmic granulation (CG) was the last morphological feature observed at higher concentration of calcium ionophore (4 μM) just prior to degeneration (D). Most of the control eggs (C) were arrested at M-II stage extruded first polar body with normal morphology. However, exit from M-II arrest was observed in few control eggs that showed extrusion of second polar body (data not shown).
Calcium ionophore-induced morphological changes in eggs cultured for 3 h in vitro. a Calcium ionophore-induced morphological apoptotic features and degeneration in concentration-dependent manner. Data are means ± SEM of three replicates. Data were analyzed by one-way ANOVA. b Representative photographs showing calcium ionophore-induced morphologic apoptotic changes in eggs cultured in vitro. c control egg showing first polar body with normal morphology (arrow), S calcium ionophore-treated egg undergoing shrinkage (arrow), CF cytoplasmic fragmentation (arrow), CG cytoplasmic granulation (arrow) and D degeneration (arrow). Bar = 20 μm
Cytosolic free calcium level rises during calcium ionophore-induced egg apoptosis
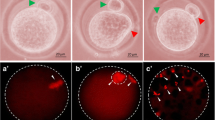
As shown in Fig. 2a, calcium ionophore significantly increased free cytosolic calcium level as evidenced by bright fluo-3 fluorescence in eggs showing either shrinkage (S) or cytoplasmic fragmentation (CF) as compared to control egg that had normal morphology without any apoptotic features (C). The corrected total cell fluorescence (CTCF) analysis using ImageJ software further suggest that calcium ionophore significantly increased cytosolic free calcium level in apoptotic eggs showing either shrinkage (1.70 fold) or cytoplasmic fragmentation (1.50 fold) as compare to control eggs that had normal morphology (Fig. 2b).
Representative photographs showing calcium ionophore-induced release of free calcium in egg cytoplasm. a Calcium ionophore-induced release of cytosolic free calcium as evidenced by more fluorescence intensity of Fluo-3 in apoptotic eggs undergoing either shrinkage (S, arrow) or cytoplasmic fragmentation (CF, arrow) as compared to control egg showing first polar body with normal morphology (C, arrow) (Bar = 20 μm). b Image J analysis of fluorescence intensity of Fluo-3 revealed that calcium ionophore induced increase of cytosolic free calcium in eggs showing shrinkage (1.70 fold) or cytoplasmic fragmentation (1.50 fold) as compared to control egg that had normal morphology without any morphological apoptotic features
Intracellular H2O2 concentration rises during calcium ionophore-induced egg apoptosis
As shown in Fig. 3a, calcium ionophore significantly (p < 0.05) increased intracellular concentration of H2O2 (12.63 ± 0.54 μM) in eggs that showed morphological apoptotic features as compare to control eggs which had normal morphology with first polar body (6.06 ± 0.13 μM).
Effect of calcium ionophore on H2O2 concentration and catalase activity in eggs cultured in vitro. Calcium ionophore induced generation of H2O2 (a) and inhibited catalase activity (b) in eggs cultured in vitro. Data are means ± SEM of three replicates. * Significant (p < 0.05) difference as compared to control eggs (Student’s t-test)
Catalase activity decreases during calcium ionophore-induced egg apoptosis
As shown in Fig. 3b, calcium ionophore significantly (p < 0.05) decreased catalase activity (8.25 ± 0.49 μU/ml) in eggs that showed morphological apoptotic features as compare to control eggs which had normal morphology with first polar body (12.23 ± 0.88 μU/ml).
Cytosolic cytochrome c level rises during calcium ionophore-induced egg apoptosis
Calcium ionophore induced release of cytochrome c from mitochondria and increased cytochrome c in egg cytoplasm. As shown in Fig. 4a, calcium ionophore significantly (p < 0.05) increased cytosolic cytochrome c concentration (2.45 ± 0.09 ng/ml) in eggs that showed morphological apoptotic features as compare to control eggs (0.96 ± 0.05 ng/ml). Immunocytochemistry data further supported our data wherein a significant increase in DAB positive staining in egg cytoplasm was observed in calcium ionophore-treated eggs showing cytoplasmic fragmentation (CF) as compare to control egg (Fig. 4b).
Immunocytochemical localization of cytosolic cytochrome c in rat eggs cultured in vitro. a An increase of cytosolic cytochrome c level was observed in apoptotic eggs cultured in vitro. Data are means ± SEM of three replicates. * Significantly (p < 0.05) higher as compared to control eggs (Student’s t-test). b Representative photographs showing increased cytosolic cytochrome c level as evidenced by increased DAB positive staining in apoptotic egg showing cytoplasmic fragmentation (CF, arrow) compared to control egg (C, arrow). Bar = 20 μm
Caspase-9 and caspase-3 activities increase during calcium ionophore-induced egg apoptosis
As shown in Fig. 5a, calcium ionophore significantly (p < 0.05) increased caspase-9 (0.260 ± 0.02 OD) and caspase-3 (0.264 ± 0.009 OD) activities in eggs that showed morphological apoptotic features as compare to their respective controls (caspase-9, 0.11 ± 0.01 OD: caspase-3, 0.125 ± 0.004 OD).
Calcium ionophore-induced increase of caspases activities and DNA fragmentation in eggs cultured in vitro. a Calcium ionophore-induced increase of caspase-9 and caspase-3 activities. Data are means ± SEM of three replicates. * Significantly (p < 0.05) higher as compare to their respective controls (Student’s t-test). b Representative photographs showing calcium ionophore-induced DNA fragmentation in apoptotic egg (S) compare to control egg (C) as evidenced by TUNEL positive (dark brown color) staining. c Representative photograph showing live oocyte with eosin negative staining (arrow) and calcium ionophore (4 μM)-induced degeneration of oocyte (D) as evidenced by eosin positive staining (arrow). (Bar = 20 μm) (Color figure online)
DNA fragmentation is induced during calcium ionophore-induced egg apoptosis
Calcium ionophore-induced DNA fragmentation was confirmed by TUNEL analysis. As shown in Fig. 5b, control egg shows TUNEL negative staining (C). Conversely, calcium ionophore-treated eggs that had morphological apoptotic feature showed TUNEL positive staining (S).
Degeneration occurs after calcium ionophore-induced egg apoptosis
As shown in Fig. 5c, control eggs that had normal morphology with first polar body did not take nigrosin positive stain (C). On the other hand, degenerated eggs after calcium ionophore treatment showed nigrosin positive staining (D) further supporting our results that higher concentrations of calcium ionophore (4 μM) induced degeneration of eggs after 3 h of in vitro culture.
Discussion
Intracellular calcium levels have been implicated in regulating various aspects of cell functions in wide variety of cells. Elevated ([Ca2 +]i) has been reported to induce apoptosis in aged mouse eggs cultured in vitro [20]. Calcium ionophore increases cytosolic free calcium level in somatic cells [2, 13–16] and induces egg apoptosis in vitro [9, 20]. A possibility exists that the calcium ionophore may induce calcium release from internal stores that requires ROS production by mitochondria to enhance the apoptotic signal in eggs cultured in vitro. Therefore in this study, calcium ionophore was induced to increase ([Ca2 +]i) in order to investigate the possible mechanism by which high cytosolic free calcium induces apoptosis in eggs cultured in vitro. Data of the present study suggest that calcium ionophore induced morphological apoptotic features in eggs in a concentration-dependent manner and degeneration was observed at higher concentrations (3 and 4 μM). These data reconfirm our previous observations that calcium ionophore induces egg apoptosis in vitro [9, 20]. The possibility exist that the calcium ionophore might have induced endogenous burst of calcium from internal stores such as mitochondria and increased cytosolic free calcium load in eggs cultured in vitro. This hypothesis is further strengthened by data of the present study that calcium ionophore increased cytosolic free calcium (≥1.50 fold) in apoptotic eggs as compare to control eggs. Although reports are not available to support our findings, it has been reported that calcium ionophore induced increase of cytosolic free calcium level in somatic cells [12, 13]. These results together with previous observations in somatic cells suggest that calcium ionophore depletes calcium internal stores and induces cytosolic free calcium load that results in apoptotic cell death.
Studies using somatic cells suggest that the depletion of calcium stores and/or increase of cytosolic free calcium load generate ROS [19, 41]. Similarly, a possibility exist that calcium ionophore-induced increase of cytosolic free calcium level may generate ROS in apoptotic eggs. This possibility is supported by data of the present study that calcium ionophore induced H2O2 accumulation in treated eggs. The increased intracellular H2O2 may inhibit catalase activity, an enzyme responsible for the conversion of H2O2 into water. Our results indicate that the catalase activity was significantly decreased in calcium ionophore-treated eggs that had morphological apoptotic features. These finding are in agreement with the previous observations that calcium ionophore induces generation of ROS [9, 19, 42] and that increased level of ROS inhibits catalase activity in eggs [21, 22].
It has been generally accepted that an increased level of ROS can modulate mitochondria membrane potential and trigger cytochrome c release in cytoplasm to initiate apoptotic signaling pathway [26, 28–30, 43]. Hence, we hypothesize that calcium ionophore -induced increase of intracellular H2O2 may trigger cytochrome c release from mitochondria of the eggs. Our results suggest that calcium ionophore significantly induced cytochrome c release from mitochondria of the treated eggs. These results are in agreement with previous findings that calcium ionophore-induces generation of ROS and cytochrome c release in eggs cultured in vitro [8, 26, 27, 44]. Taken together, these findings suggest that an increased level of ROS trigger cytochrome c release from mitochondria that may initiate apoptotic signaling pathway in calcium ionophore-treated eggs.
The release of cytochrome c from mitochondria activates upstream and downstream caspases in a cell. In the cytosol, cytochrome c binds to Apaf-1 leading to the activation of upstream and downstream caspases including procaspase-3. The activated procaspase-3 cleaves key structural and regulatory proteins that result in the biochemical and morphological changes associated with apoptosis [35]. Similarly, we hypothesize that an increase of cytochrome c concentration can stimulate upstream and downstream caspases in eggs treated with calcium ionophore in vitro. Our results suggest that calcium ionophore induced both caspase-9 and caspase-3 activities in eggs that had morphological apoptotic features. Similarly, an increased caspase-3 activity during egg apoptosis has been reported in rat [6, 9]. These data together with our previous findings suggest that calcium ionophore increases cytosolic calcium load, induces generation of ROS and thereby cytochrome c release from mitochondria of the egg. The increased level of cytochrome c induces caspase-9 followed by caspase-3 activities that ultimately leads to the appearance of morphological apoptotic features in eggs.
The fragmentation of DNA in multiples of 180–200 base-pair is hallmark feature of egg apoptosis [44]. The fragmented DNA can be detected in a single cell using in situ technique such as TUNEL [34, 39, 45–48]. Data of the present study suggest that calcium ionophore induced DNA fragmentation as evidenced by DAB positive staining in eggs that had morphological apoptotic features. These results reconfirm our previous finding that calcium ionophore induces DNA fragmentation in rat eggs [9] suggesting the involvement of mitochondria-caspase mediated pathway during calcium ionophore-induced apoptosis in rat eggs cultured in vitro. The physiological relevance of the present study is to explain why ovulated eggs undergo apoptosis if not fertilized in the oviduct of several mammalian species. As the time passes after ovulation, endogenous burst of calcium from internal stores and inability of these stores to replenish calcium result in high sustained cytosolic free calcium level and apoptotic cell death in ovulated eggs.
In summary, data of the present study suggest that the increased cytosolic free calcium level in response to calcium ionophore induced generation of H2O2 and inhibited catalase activity. The high concentration of H2O2 induced release of cytochrome c from mitochondria and increased cytosolic cytochrome c level. The increased cytosolic cytochrome c load stimulated caspase-9 and then caspase-3 activities. Caspase-3 finally cleaved structural and regulatory proteins and induced DNA fragmentation that resulted in the morphological changes associated with egg apoptosis. Based on these results, we suggest that high cytosolic free calcium level increases ROS generation and thereby egg apoptosis through mitochondria-caspase mediated pathway in rat eggs cultured in vitro. Although, involvement of extrinsic pathway during calcium-induced apoptosis in rat eggs cannot be ruled out, our findings suggest that mitochondria-mediated pathway is one of the pathways by which high cytosolic free calcium level induces apoptosis in rat eggs cultured in vitro.
References
Berridge MJ, Bootman MD, Lipp P (1998) Calcium: a life and death signal. Nature 395:645–648
Tosti E (2006) Calcium ion currents mediating oocyte maturation events. Reprod Biol Endocrinol 4:26–34
Miyazaki S, Shirakawa H, Nakada K (1993) Essential role of inositol 1,4,5-trisphosphate receptor/Ca2+ release channel in Ca2+ waves and Ca2+ oscillations at fertilization of mammalian eggs. Dev Biol 158:62–78
Xu Z, Abbott A, Kopf GS, Schultz RM, Ducibella T (1997) Spontaneous activation of ovulated mouse eggs: time-dependent effects on M-phase exit, cortical granule exocytosis, maternal messenger ribonucleic acid recruitment, and Inositol 1,4,5-trisphosphate sensitivity. Biol Reprod 57:743–750
Sergeev IN, Norman AV (2003) Calcium as a mediator of apoptosis in bovine oocytes and preimplantation embryos. Endocrine 22:169–176
Chaube SK, Dubey PK, Mishra SK, Shrivastav TG (2007) Verapamil inhibits spontaneous parthenogenetic activation in aged rat eggs cultured in vitro. Cloning Stem Cells 9:15–624
Vincent C, Cheek TR, Johnson MH (1992) Cell cycle progression of parthenogenetically activated mouse oocytes to interphase is dependent on the level of internal calcium. J Cell Sci 103:389–396
Lu Q, Chen ZJ, Gao X, Ma SY, Li M, Hu JM, Li Y (2006) Oocyte activation with calcium ionophore A23187 and puromycin on human oocytes that failed to fertilize after intracytoplasmic sperm injection. Zhonghua Fu Chan Ke Za Zhi 41:182–185
Chaube SK, Khatun S, Mishra SK, Shrivastav TG (2008) Calcium ionophore-induced egg activation or apoptosis is associated with the generation of intracellular hydrogen peroxide. Free Radic Res 42:212–220
Ruddock NT, Machaty Z, Cabot RA, Prather RS (2001) Porcine oocyte activation: roles of calcium and pH. Mol Reprod Dev 59:227–234
McConkey DJ, Orrenius S (1997) The role of calcium in the regulation of apoptosis. Biochem Biophys Res Commun 239:357–366
Penzo D, Petronilli V, Angelin A, Cusan C, Colonna R, Scorrano L, Pagano F, Prato M, Di-Lisa F, Bernardi P (2004) Arachidonic acid released by phospholipase A(2) activation triggers Ca(2+)-dependent apoptosis through the mitochondrial pathway. J Biol Chem 279:25219–25225
Tan AR, Cai AY, Deheshi S, Rintoul GL (2011) Elevated intracellular calcium causes distinct mitochondrial remodelling and calcineurin-dependent fission in astrocytes. Cell Calcium 49:108–114
Cho SY, Lee JH, Bae HD, Jeong EM, Jang GY, Kim CW, Shin DM, Jeon JH, Kim IG (2010) Transglutaminase 2 inhibits apoptosis induced by calcium-overload through down-regulation of Bax. Exp Mol Med 42:639–650
Wang ZG, Wang W, Yu SD, Xu ZR (2008) Effects of different activation protocols of preimplantation development, apoptosis and ploidy of bovine parthenogenetic embryos. Anim Reprod Sci 105:292–301
Ma W, Zhang D, Hou Y, Li Y-H, Sun Q-Y, Sun X-F, Wang W-H (2005) Reduced expression of MAD2, BCL2 and MAP Kinase activity in pig oocytes after in vitro aging are associated with defects in sister chromatid segregation during meiosis II and embryo fragmentation after activation. Biol Reprod 72:373–383
Takasu N, Yamada T, Shimizu Y (1987) Generation of H2O2 is regulated by cytoplasmic free calcium in cultured porcine thyroid cells. Biochem Biophys Res Commun 148:1527–1532
Zoccarato F, Valente M, Alexandre A (1993) Identification of an NADH plus iron dependent, Ca2+ activated hydrogen peroxide production in synaptosomes. Biochem Biophys Acta 1176:208–214
Przygodzki T, Sokal A, Bryszewska M (2005) Calcium ionophore A23187 action on cardiac myocytes is accompanied by enhanced production of reactive oxygen species. Biochem Biophys Acta 1740:481–488
Gordo AC, Rodrigues P, Kurokawa M, Jellerette T, Exley GE, Warner C, Fissore R (2002) Intracellular calcium oscillations signal apoptosis rather than activation in vitro aged mouse eggs. Biol Reprod 66:1828–1837
Cetica PD, Pintos LN, Dalvit GC, Beconi MT (2001) Antioxidant enzyme activity and oxidative stress in bovine oocyte in vitro maturation. IUBMB Life 51:57–64
Whitaker BD, Knight JW (2008) Mechanisms of oxidative stress in porcine oocytes and the role of anti-oxidants. Reprod Fertil Dev 20:694–702
Tripathi A, Khatun S, Pandey AN, Mishra SK, Chaube R, Shrivastav TG, Chaube SK (2009) Intracellular levels of hydrogen peroxide and nitric oxide in oocytes at various stages of meiotic cell cycle and apoptosis. Free Radic Res 43:287–294
Qian T, Herman B, Lemasters JJ (1999) The mitochondrial permeability transition mediates both necrotic and apoptotic death of hepatocytes exposed to Br-A23187. Toxicol Appl Pharmacol 154:117–125
Petrocillo G, Ruggiero FM, Pistolese M, Paradies G (2004) Ca2+-induced reactive oxygen species production promotes cytochrome c release from rat liver mitochondria via mitochondrial permeability transition (MPT)-dependent and MPT-independent mechanisms. J Biol Chem 279:53103–53108
Zhang X, Li XH, Ma X, Wang ZH, Lu S, Guo YL (2006) Redox-induced apoptosis of human oocytes in resting follicles in vitro. J Soc Gynecol Investig 13:451–458
Ramalho-Santos J, Varum S, Amaral S, Mota PC, Sousa AP, Amaral A (2009) Mitochondrial functionality in reproduction: from gonads and gametes to embryos and embryonic stem cells. Hum Reprod Update 15:553–572
Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD (1997) The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science 275:1132–1136
Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng TI, Jones DP, Wang X (1997) Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science 275:1129–1132
Braun T, Dar S, Vorobiov D, Lindenboim L, Dascal N, Stein R (2003) Expression of Bcl-x(S) in Xenopus oocytes induces BH3-dependent and caspase-dependent cytochrome c release and apoptosis. Mol Cancer Res 1:186–194
Wang Q, Frolova AI, Purcell S, Adastra K, Schoeller E (2010) Mitochondrial dysfunction and apoptosis in cumulus cells of type I diabetic mice. PLoS One 5:e15901–e15911
Zou H, Li Y, Liu X, Wang X (1999) An APAF-1 cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J Biol Chem 274:11549–11556
Acehan D, Jiang X, Morgan DG, Heuser JE, Wang X, Akey CW (2002) Three-dimensional structure of the apoptosome: implications for assembly, procaspase-9 binding, and activation. Mol Cell 9:423–432
Fuentes-Prior P, Salvesen GS (2004) The protein structures that shape caspase activity, specificity, activation and inhibition. Biochem J 384:201–232
Martin MC, Allan LA, Lickrish M, Sampson C, Morrice N, Clarke PR (2005) Protein kinase A regulates caspase-9 activation by Apaf-1 downstream of cytochrome c. J Biol Chem 280:15449–15455
Carroll J, Swann K (1992) Spontaneous cytosolic calcium oscillations driven by inositol trisphosphate occur during in vitro maturation of mouse oocytes. J Biol Chem 267:11196–11201
Gavet O, Pines J (2010) Progressive activation of cyclinB1-cdk1 coordinates entry to mitosis. Dev Cell 18:533–543
Tripathi A, Premkumar KV, Pandey AN, Khatun S, Mishra SK, Shrivastav TG, Chaube SK (2011) Melatonin protect against clomiphene citrate-induced generation of free radicals and egg apoptosis in rat. Eur J Pharmacol 667:419–424
Chaube SK, Prasad PV, Thakur SC, Shrivastav TG (2005) Hydrogen peroxide modulates meiotic cell cycle and induces morphological features characteristic of apoptosis in rat oocytes cultured in vitro. Apoptosis 10:863–874
Chaube SK, Prasad PV, Khillare B, Shrivastav TG (2006) Extract of Azadirachta indica (Neem) leaf induces apoptosis in rat oocytes cultured in vitro. Fertl Sterl 85(suppl 1):1223–1231
Kajitani N, Kobuchi H, Fujita H, Yano H, Fujiwara T, Yasuda T, Utsumi K (2007) Mechanism of A23187-induced apoptosis in HL-60 cells: dependency on mitochondrial permeability transition but not on NADPH oxidase. Biosci Biotechnol Biochem 71:2701–2711
Pepperell JR, Porterfield DM, Keefe DL, Behrman HR, Smith PJ (2003) Control of ascorbic acid efflux in rat luteal cells: role of intracellular calcium and oxygen radicals. Am J Physiol Cell Physiol 285:C642–C651
Priyadarsini RV, Murugan RS, Maitreyi S, Ramalingam K, Karunagaran D, Nagini S (2010) The flavonoid quercetin induces cell cycle arrest and mitochondria-mediated apoptosis in human cervical cancer (HeLa) cells through p53 induction and NF-κB inhibition. Eur J Pharmacol 649:84–91
Jurisicova A, Acton BM (2004) Deadly decisions: the role of genes regulating programmed cell death in human preimplantation embryo development. Reproduction 128:281–291
Ansari B, Coates PJ, Greenstein BD, Hall PA (1993) In situ end-labelling detects DNA strand breaks in apoptosis and other physiological and pathological states. J Pathol 170:1–8
Roth Z, Hansen PJ (2004) Involvement of apoptosis in disruption of developmental competency of bovine oocytes by heat shock during maturation. Biol Reprod 71:1898–1906
Henkel R, Hajimohammad M, Stalf T, Hoogendijk C, Mehnert C, Menkveld R, Gips H, Schill WB, Kruger TF (2004) Influence of deoxyribonucleic acid damage on fertilization and pregnancy. Fertil Steril 81:965–972
Hao Y, Lai L, Mao J, Im GS, Bonk A, Prather RS (2004) Apoptosis in parthenogenetic preimplantation porcine embryos. Biol Reprod 70:1644–1649
Acknowledgments
The authors are very much thankful to Prof. T.G. Shrivastav, Department of Reproductive Biomedicine, National Institute of Health and Family Welfare, Baba Gang Nath Marg, New Delhi 110067, India for providing fluorescence microscope facility.
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tripathi, A., Chaube, S.K. High cytosolic free calcium level signals apoptosis through mitochondria-caspase mediated pathway in rat eggs cultured in vitro. Apoptosis 17, 439–448 (2012). https://doi.org/10.1007/s10495-012-0702-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-012-0702-9