Abstract
Mancozeb (MZ), a mixture of ethylene-bis-dithiocarbamate manganese and zinc salts, is one of the most widely used fungicides in agriculture. Toxicologic studies in mammals and mammalian cells indicate that this fungicide can cause neurological and cytological disorders, putatively associated with pro-oxidant and apoptotic effects. Yeast adaptation to sub-inhibitory concentrations of MZ has been correlated with oxidative response, proteins degradation, and energy metabolism, and its main effect on yeast has been attributed to its high reactivity with thiol groups in proteins. Herein, we show that acute MZ treatments on aerobic exponentially growing yeast of wild type (BY4741) and deletion mutant strains, coupled with multiplex flow cytometry analysis, conclusively demonstrated that MZ displays the typical features of pro-oxidant activity on Saccharomyces, elevating mitochondrial ROS, and causing hyper-polarization of mitochondrial membranes leading to apoptosis. A drastic reduction of cellular viability associated with the maintenance of cell membrane integrity, as well as phosphatidyl serine externalization on yeast cells exposed to MZ, also supports an apoptotic mode of action. Moreover, abrogation of the apoptotic response in yca1 deficient mutants indicates that metacaspase-1 is involved in the programmed cell death mechanism induced by MZ in yeast.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mancozeb (MZ) is an agricultural fungicide formed by a mixture of manganese and zinc salts of the organosulfur compound ethylene-bis-dithiocarbamete (EBDC). The broad spectrum of MZ against phytopathogenic fungi has led to the wide application of this fungicide in agriculture worldwide, particularly on several fruits, including vineyards [1]. Despite its relatively low acute toxicity and limited environmental persistence, acute exposure to MZ and other dithiocarbamates has been putatively linked to adverse effects such as skin diseases, immune disorders, neurotoxicity, and Parkinson’s Disease, as well as several forms of cancer [2–6].
Experimental data using mammalian cell lines indicate that MZ induces mitochondrial dysfunction, DNA damage, and apoptosis in rat cells [5, 6], as well as in human lymphocytes [7]. These effects have been associated with MZ’s pro-oxidant activity [5, 6, 8]. Although not completely understood, the mode of action of MZ on mammalian cells has been attributed to both the EBDC and manganese components. The metal is capable of induction of reactive oxygen species (ROS) via an oxidase-dependent redox cycling [6], while the organosulfur component may inhibit the activity enzymes and introduce unspecific protein damage due to its high reactivity with thiol groups [9].
Beyond plant pathogens, MZ exhibits antifungal activity against single cell yeast Saccharomyces cerevisiae, and this has been known to negatively affect the fermentation of wine [10]. Studies on this model organism have focused on the mechanisms of resistance with the objective to understand the global response and tolerance to MZ in eukaryotic cells. Proteome analysis of S. cerevisiae following adaptation to sub-inhibitory concentrations of MZ showed that upregulated genes were involved with the yeast response to oxidative stress, protein translation, initiation and folding, disassembly of protein aggregates, and degradation of damaged proteins [11]. Subsequently, a chemogenomic approach led to the identification of 286 genes that provided protection against MZ in S. cerevisiae [12]. Gene ontology and genetic interaction analysis of this dataset highlighted the role of oxidative stress response, protein degradation, and carbohydrate/energy metabolism in MZ tolerance. Both studies showed than the vast majority of the up-regulated genes under MZ stress were downstream targets of the major oxidative stress regulator in yeast (Yap1p). Moreover, other studies showed that MZ resistance in yeast also involves up-regulation of FLR1, a proton-driven multidrug antiporter channel, which is under coordinated control by the transcription factors Yap1p, Rpn4p, Pdr3p, and Yrr1p [13, 14].
Despite the well-established consequences of MZ on mitochondrial dysfunction in mammalian cells [5–7], the corresponding experimental measurements in yeast cells did not reveal elevated ROS production in response to this fungicide [12]. The authors attributed this observation to the low biochemical respiration of fermenting yeasts, assuming an absence of mitochondrial electron leakage under these conditions. However, anoxic conditions are not representative for plant pathogens treated with MZ, and a role for mitochondria should be suspected due to the partial tolerance reported for petit mutants compared to wild type S. cerevisiae [15].
In the present work, we show that MZ has a pro-oxidant activity on aerobically grown S. cerevisiae in exponential phase, triggering an apoptotic metacaspase-dependent cell death mechanism, which demonstrates the induction of oxidative stress response is common to yeast and mammalian cell toxicity of MZ.
Materials and methods
Yeast strains and media
Saccharomyces cerevisiae BY4741 (MATa his3∆1 leu2∆0 met15∆0 ura3∆0) and the isogenic mutants Y06233 (yca1::kanMX4), Y0233 (aif1::kanMX4) and Y01217 (nuc1::kanMX4) were obtained from Euroscarf (Frankfurt, Germany).
Yeasts were cultured at 28 °C with orbital shaking (150 rpm) in YEPD broth (2 % yeast extract, 1 % peptone, 2 % glucose, pH 6.5) or SD medium (0.67 % Yeast Nitrogen Base without aminoacids, 2 % glucose, with 20 mg/L histidine, methionine, and uracil, and 60 mg/L leucine, pH 6.5). Mancozeb was purchased from Sigma-Aldrich, and stock solutions (10 mM in dimethylsulfoxide, DMSO) were prepared just before each experiment.
Yeast growth and viability assay
Yeasts from overnight cultures in YEPD broth (OD600 ~ 1.0) were inoculated in the same medium, and grown to exponential phase (OD600 up to 0.7) at 28 °C with orbital shaking (150 rpm). Cells were harvested by centrifugation, washed with 0.9 % NaCl, and cell density adjusted to 107 cells/ml in SD medium. Control cultures (untreated) or those treated with MZ in the indicated concentrations were incubated for 360 min (6 h) at 28 °C with shaking (150 rpm), unless the time/concentration in initial experiments.
The viability of MZ-treated and untreated yeasts was determined by spot assay. Cultures were diluted at tenfold series, and aliquots (10 μl) of each dilution were spotted onto YEPD plates. Colony were enumerated after 48 and 72 h incubation at 28 °C, and expressed as percentage of colony forming units (c.f.u.) compared with the control (untreated cells).
Assays for ROS, apoptosis, and other markers
Phosphatidylserine externalization, cell membrane integrity, ROS production, mitochondrial membrane potential, and cell cycle evaluation were performed by flow cytometry using a FACSCalibur (Becton–Dickinson) instrument equipped with an argon-ion laser emitting at 488 nm.
For flow analysis, MZ-treated (100 μM) and untreated cultures were harvested, washed, suspended in PBS (pH 7.4), and immediately analyzed (except for Annexin V/7-AAD and cell cycle assays). In all the experiments, yeast cells were initially gated using forward- and side-scatter, and 10,000 cells were included for each analysis.
Apoptosis was measured quantifying the levels of detectable phosphatidylserine on the outer membranes of yeast cells using the Annexin V-PE/7-AAD apoptosis detection kit (BD Pharmingen). Briefly, yeast cells were harvested, and washed in sorbitol buffer (1.2 M sorbitol; 0.5 mM MgCl2; 35 mM K2HPO4; pH 6.8), and spheroplasts were obtained by treatment with Zymolyase (5 U/ml) at 30 °C for 1 h. Spheroplasts were collected by centrifugation at low speed (1000 rpm), and suspended in 100 μl of binding buffer (1.2 M sorbitol; 10 mM HEPES/NaOH pH 7.4; 140 mM NaCl; 2.5 mM CaCl2). Staining and flow cytometry analysis were performed following the manufacturer instructions. Cells untreated and treated with H2O2 (50 mM) were used as negative and positive controls, respectively.
Cell membrane integrity was evaluated using the LIFE/DEAD FungaLight Yeast Viability kit (Invitrogen) that includes two nucleic acid dyes: SYTO® 9, a green-fluorescent that stain both integral and damaged cells, and the red-fluorescent propidium iodide, which penetrates only in membrane-damaged cells. Staining and flow cytometry analysis followed kit instructions.
Intracellular ROS were detected using the oxidant-sensitive dyes dihydro-rhodamine-123 (DHR123, Sigma), dihydroethidium (DHE, Sigma), and 2′,7′-dichlorofluorescein diacetate (H2DCFDA, Sigma). Stock solutions were prepared by dissolving DHR123 at 2 mg/ml in ethanol; and DHE and H2DCFDA at 5 mg/ml in DMSO. Staining was performed in 500 µl for MZ-treated and untreated samples using a final concentration of 10, 5 and 5 µg/ml for each dye, respectively. Samples were evaluated by flow cytometry using FL1 channel (488/533) for DHR123 and H2DCFDA, and FL3 (488/670) for DHE.
To evaluate mitochondrial membrane potential, cells obtained as described above were suspended in SD medium, and stained with 175 nM of 3,3′-dihexyloxacarbocyanine iodide (DiOC6) for 30 min at 30 °C in the dark. After staining, cells were analyzed by flow cytometry using FL1 filter.
Cell cycle phase was evaluated by flow cytometry following the procedure reported by Delobel and Tesnière [16].
Results
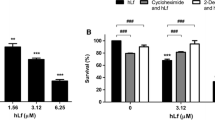
First, we established the required concentration of MZ to attain 5 to 10 % viability for exponentially aerobic growing cells of S. cerevisiae BY4741 under acute exposure conditions (Fig. 1). We observed a dose- and time-dependent reduction on cell viability and identified the concentration of 100 µM (26.6 mg/l MZ), which produced a reduction of 95.2 % c.f.u. after 6 h of MZ treatment, and this condition was selected for further analysis.
Provided that the deleterious effect of MZ and other dithiocarbamates on mammalian cells has been associated to pro-apoptotic activity [5, 6, 8], we compared the effect of MZ on the wild-strain (BY4741) and three isogenic mutants bearing deletions of key genes of the apoptotic pathways of Saccharomyces: ∆yca1, ∆aif1 and ∆nuc1 [17]. The ∆yca1 mutant strain exhibited significantly lower sensitivity to MZ compared to the wild-type yeast, as well as the ∆aif1 or ∆nuc1 mutant strains. These results indicated that a Yca1-dependent pathway may be implicated in yeast death induced by MZ (Fig. 2a, b).
Viability (% c.f.u./cell count) of wild-type BY4741 and mutants exposed to 100 μM MZ for 6 h (a). Results are the average of three independent experiments, standard deviation are indicated as error bars. Inset showing the attenuated loss in cell viability for the metacaspase-1 defective mutant ∆yca1 following MZ treatment compared to wild-type (b)
We performed staining using the dye pair SYTO9/PI for flow cytometry analysis of untreated controls versus MZ-treated yeast of the wild-type and ∆yca1 mutant strains, and we concomitantly analyzed c.f.u. viability (Fig. 3). The data showed that 82 % of wild-type cells treated with MZ (100 µM) retain membrane integrity, even though this population shows compromised ability to multiply and form colonies. Contrastingly, we observed no significant differences between the percentage of PI-negative cells and c.f.u. viability for the yca1 mutant strain (Fig. 3).
Cell membrane integrity (% PI negative) and cell viability (% c.f.u.) of wild-type BY4741 yeast, and ∆yca1 mutant strain treated for 6 h with 100 μM MZ. Values are mean ± standard deviation of three independent experiments. Means comparison: ***significantly different (p < 0.001); ns: non-significant difference
In order to confirm that MZ induces apoptosis in Saccharomyces, we compared wild-type and ∆yca1 mutant yeast before and after MZ exposure, which we evaluated using the stains Annexin V-PE/7-AAD and analyzed by flow cytometry. Annexin V-PE binds to phosphatidylserine, an inner-membrane component externalized in the first steps of the apoptotic process, and 7-AAD is a nucleic acid dye that penetrates only membrane-damaged cells. The combination of these dyes allowed the identification of normal, apoptotic, necrotic, and apoptotic/necrotic cells [18]. Following MZ-treatment in the standard experimental conditions we observed 54 % of cells of the wild-type yeast strain were apoptotic (positive for Annexin V positive and negative for 7AAD), whereas these cells represented only 6.5 % for the ∆yca1 mutant strain. However, the treatment did not produce significant differences in the number of necrotic cells of wild-type and ∆yca1 mutant strains in the presence of MZ (Table 1).
ROS are one of the mayor factors triggering programmed cell death in different organisms, including yeasts [19, 20]. In order to determine whether ROS could be responsible for the apoptotic behavior of yeast cells treated with MZ, we evaluated ROS accumulation in exponentially growing cells of wild type and ∆yca1 yeast strains, before and after MZ treatment. Three standard redox-sensitive probes were used: DH123, DHE, and H2DCFDA (methods section). The results showed that MZ-treated cells of both strains labeled with DHE and DH123 exhibited increased fluorescence, indicating a high concentration of intracellular ROS (Fig. 4). MZ-treated cells stained with H2DCFDA showed decreased fluorescence intensity compared to control cells, consistent with observations reported by Dias et al. [12], (data not shown). Moreover, a comparison of median fluorescence intensity from stains DHE and DH123 (Fig. 4), showed that MZ triggers higher ROS production in the wild-type than in the Δyca1 strain.
ROS intracellular concentration (fluorescence intensity) in untreated yeast (grey area) versus cells treated with MZ (white area) for 6 h with 100 μM MZ. Left panels, a and c, correspond to the wild-type BY4741 yeast. Right panels b and d, correspond to ∆yca1 mutant yeast. The stains are indicated in the X axis as dihydroethidium (DHE) and dihydro-rhodamine 123 (DHR123). Numbers within figures indicate the median peak fluorescence for 10,000 cells
The relationship between mitochondrial dysfunction and apoptosis is well established in yeast and mammalian cells when submitted to oxidative stress. We measured the effect of MZ on the mitochondrial membrane potential (Δψm) of yeast cells using the mitochondria-specific voltage-dependent dye DiOC6, which aggregates into healthy mitochondria and produces green fluorescence. As shown in Fig. 5, MZ-treated cells of both wild-type and yca1 mutant strains exhibited increased fluorescence compared to controls, indicating that MZ induces hyper-polarization of yeast mitochondrial membranes.
Changes in yeast mitochondrial membrane potential (Δψm) induced by MZ treatment revealed by staining with 3,3′-dihexyloxacarbocyanine iodide (DiOC6). a BY4741 wild-type yeast, and b ∆yca1 mutant strain. Grey area represents untreated control cells, and white area represents cells treated with 100 μM (6 h). Ten thousands cells were computed for each treatment
To exclude the possibility of interfering effects of MZ fungicide with the yeast cell cycle and proliferation we evaluated MZ-treated and untreated wild-type and ∆yca1 yeast strains by flow cytometry using the nucleic acid binding dye SYTO9. The results showed no significant modification of the percentage of cells in G1, S, and G2 stages, indicating that the fungicide did not interfere with cell cycling (Table 2).
Discussion
Based on the results of initial experiments (Fig. 1), we selected a 6 h treatment with 100 µM MZ, which is almost tenfold lower than the recommended field spray application of 200–250 g MZ per 100 l of water. This treatment produced the shortest response time to reduce wild-type yeast viability by more than 90 %. This concentration corresponds to twofold the minimal inhibitory concentration (MIC) for MZ, 50 µM, which was estimated in preliminary experiments (data non shown), and approximately tenfold higher than the sub-inhibitory treatments used in the MZ-resistance studies [11–13].
Considering that dithiocarbamates show pro-apoptotic activity on mammalian cells [5, 6, 8], we evaluated the effect of MZ on a set of Saccharomyces mutant strains lacking specific key genes in apoptotic pathways [17]. Among these strains, the ∆yca1 mutant exhibited significantly lower sensitivity to MZ than the wild-type strain, as well as other mutants, ∆nuc1 and ∆aif1 (Fig. 2). Transcription of yca1 is activated in oxidatively and physiologically stressed cells to trigger the apoptotic cascade, and consequently, knock-out of yca1 results in abrogation of the programmed cell death process and a net increase of viability for this mutant strain following MZ treatment compared with wild-type strains [17, 19]. Moreover, while MZ-treated wild type cells displayed normal plasma membrane integrity (Fig. 3), but lose their culture-ability, the ∆yca1 mutation rescued this phenotype and improved viability (Fig. 3). Our results with the dye pair SYTO9/PI are consistent with previous studies that evaluated the apoptotic response to lead in Saccharomyces [21]. Together, these results strongly suggest that acute treatment with MZ induces an apoptotic metacaspase-dependent programmed cell death in S. cerevisiae.
Several markers have been used to differentiate necrotic, apoptotic and autophagic cell death in yeasts and other organisms [18]. The externalization of phosphatidylserine evaluated by Annexin V binding, in particular when combined with a dye like 7AAD that cannot penetrate intact cells, is considered the method of choice for the quantification of early and late stages of the apoptotic process [22]. Annexin V-positive and 7AAD-negative cells (early apoptotic cells) increased from 3.9 to 54 % after treatment of wild type strain with MZ, whereas the ∆yca1 mutant strain exhibited a modest change from 3.4 to 6.5 % (Table 2). Conversely, the number of necrotic cells (Annexin V- and 7AAD-positive) showed little increase in either of the strains, which is consistent and confirm an apoptotic cell death mechanism induced by the acute treatment with MZ in Saccharomyces.
Apoptosis, and in particular the metacaspase-dependent pathway, is triggered by oxidative stress, by elevated mitochondrial membrane potential, and by cytochrome C leakage into the cytoplasm [18–20]. MZ-treated cells of both wild-type and yca1 mutant strains exhibited a significant elevation of ROS compared to controls, as evidenced by DHE and DH123 fluorescence (Fig. 4). However, as previously reported by Santos et al. [11], we observed an unexpected reduction of fluorescence using the H2DCFDA dye. This apparent discrepancy may potentially reveal the sub-cellular location of ROS produced by MZ, provided that DHE and DH123 are considered mitochondrial dyes, whereas H2DCFDA is mainly distributed throughout the cytoplasm. These conclusions are consistent with programmed cell death observations in mammalian cell culture [5–7].
The higher ROS production exhibited by wild type treated cell compared with the Δyca1 strain (Fig. 4), can be attributed to a reduction of respiration rate and heme synthesis in yca1 mutants [23].
ROS effects on mitochondria induce a sequence of events that include mitochondrial membrane hyper-polarization, oxidative burst, followed by breakdown of membrane potential, and mitochondrial fragmentation [19, 24]. Both wild-type and yca1 mutant cells treated with 100 µM MZ for 6 h displayed a similar level hyper-polarization of mitochondrial membranes (Fig. 5). This effect has been reported on early-apoptotic yeast cells treated with pheromone and amiodarone [25], cadmium [26], among other stress factors [27].
Our data suggest that the cell cycle was not modified by MZ treatment, indicating that the fungicide did not interfere with the mitotic division. Our measurements did not reveal a sub-G0 population, which was previously observed in yeast apoptotic cells after treatment with cadmium [26].
The pro-oxidant and pro-apoptotic activities of MZ are consistent with the up-regulation of the resistance factor FLR1, a plasma membrane transporter induced by oxidants or pro-oxidants [13, 14], as well as the involvement of the transcription factor Yap1p, one of the key regulators of the oxidative stress response during yeast adaptation to sub-inhibitory concentrations of MZ [11]. Using sub-inhibitory treatment of MZ for a chemogenomic study, Dias et al. [12] concluded that MZ acted as a thiol-reactive compound in yeast, rather than a ROS inducer. This mechanistic discrepancy can be attributed to the experimental design using a sub-inhibitory and low-oxygen fermentation growth conditions. However, herein we focused on aerobic growth conditions and treatment with high MZ concentrations that are relevant to the agricultural applications. Other than the dithiocarbamate moiety of the molecule, high MZ concentrations elevate Mn and Zn ions within cells, and like other metals, these are known to cause cellular dysfunctions, increase ROS and induce apoptosis [6, 21, 26, 28]. Both ROS and thiol-reactivity can in fact be involved in MZ action on yeasts, explaining the “multi-site” description of MZ fungicide action.
Taken together, our experimental results provide strong evidence that MZ has pro-oxidant action in Saccharomyces growing under exponential aerobic conditions. The specific mechanism involves mitochondrial ROS accumulation, and hyper-polarization of mitochondrial membranes, a well-documented effect on mammalian cells [5, 6]. The drastic reduction of viability despite of intact cell membrane permeability, and the simultaneous externalization of phosphatidylserine by yeast cells treated with MZ also support an apoptotic mechanism. Moreover, the abrogation of this apoptotic response in yca1 deficient mutant strain indicates that metacaspase-1 is involved in the programmed cell death induced by MZ in yeast.
References
Gullino ML, Tinivella F, Garibaldi A, Kemmitt GM, Bacci L, Sheppard B (2010) Mancozeb: past, present, and future. Plant Dis 94:1076–1087. doi:10.1094/PDIS-94-9-1076
Zhou Y, Shei FS, Piccardo P, Montine TJ, Zhang J (2004) Proteosomal inhibition induced by manganese ethylene-bis-dithiocarbamate: relevance to Parkinson’s disease. Neuroscience 128:281–291. doi:10.1016/j.neuroscience.2004.06.048
Corsini E, Birindelli S, Fustinoni S, De Paschale G, Mammone T, Visentin S, Galli CL, Marinovich M, Colosio C (2005) Immunomodulatory effects of the fungicide Mancozeb in agricultural workers. Toxicol Appl Pharmacol 208:178–185. doi:10.1016/j.taap.2005.02.011
Kamel F, Engel LS, Gladen BC, Hoppin JA, Alavanja MC, Sandler DP (2005) Neurologic symptoms in licensed private pesticide applications in the agricultural health study. Environ Health Perspect 113:877–882. doi:10.1289/ehp.7645
Calviello G, Piccioni E, Boninsegna A, Tedesco B, Maggiano N, Serini S, Wolf FI, Palozza P (2006) DNA damage and apoptosis induction by the pesticide Mancozeb in rat cells: involvement of the oxidative mechanism. Toxicol Appl Pharmacol 211:87–96. doi:10.1016/j.taap.2005.06.001
Domico LM, Cooper KR, Bernard LP, Zeevalk GD (2007) Reactive oxygen species generation by the ethylene-bis-dithiocarbamate (EBDC) fungicide mancozeb and its contribution to neuronal toxicity in mesencephalic cells. Neurotoxicology 28:1079–1091. doi:10.1016/j.neuro.2007.04.008
Srivastava AK, Ali W, Singh R, Bhui K, Tyagi S, Al-Khedhairy AA, Srivastava PK, Musarrat J, Shukla Y (2012) Mancozeb-induces genotoxicity and apoptosis in cultured human lymphocytes. Life Sci 90:815–824. doi:10.1016/j.lfs.2011.12.013
Fitsanakis VA, Amarnath V, Moore JT, Montine KS, Zhang J, Montine TJ (2002) Catalysis of catechol oxidation by metal-dithiocarbamate complexes in pesticides. Free Radical Bio Med 33:1714–1723. doi:10.1016/S0891-5849(02)01169-3
Xie J, Potter A, Xie W, Lynch C, Seefeldt T (2014) Evaluation of a dithiocarbamate derivative as a model of thiol oxidative stress in H9c2 rat cardiomyocytes. Free Radical Biol Med 70:214–222. doi:10.1016/j.freeradbiomed.2014.02.022
Cabras P, Angioni A (2000) Pesticide residues in grapes, wine, and their processing products. J Agric Food Chem 48:967–973. doi:10.1021/jf990727a
Santos PM, Simões T, Sá-Correia I (2009) Insights into yeast adaptive response to the agricultural fungicide mancozeb: a toxicoproteomics approach. Proteomics 9:657–670. doi:10.1002/pmic.200800452
Dias PJ, Teixeira MC, Telo JP, Sá-Correia I (2010) Insights into the mechanisms of toxicity and tolerance to the agricultural fungicide Mancozeb in yeast, as suggested by a chemogenomic approach. OMICS 14:211–227. doi:10.1089/omi.2009.0134
Teixeira MC, Dia PJ, Simões T, Sá-Correia I (2008) Yeast adaptation to mancozeb involves the up-regulation of FLR1 under the coordinate control of Yap1, Rpn4, Pdr3, and Yrr1. Biochem Biophys Res 367:249–255. doi:10.1016/j.bbrc.2007.12.056
Monteiro PT, Dias PJ, Ropers D, Oliveira AL, Sá-Correia I, Teixeira MC, Freitas AT (2011) Qualitative modelling and formal verification of the FLR1 gene mancozeb response in Saccharomyces cerevisiae. IET Syst Biol 5:308–316. doi:10.1049/iet-syb.2011.0001
Casalone E, Bonelli E, Polsinelli M (2010) Effects of Mancozeb and other dithiocarbamate fungicides on Saccharomyces cerevisiae: the role of mitochondrial petite mutants in dithiocarbamate tolerance. Folia Microbiol 55:593–597. doi:10.1007/s12223-010-0095-5
Delobel P, Tesnière C (2014) A simple FCM method to avoid misinterpretation of Saccharomyces cerevisiae cell cycle assessment between G0 and sub-G1. PLoS One 9:e84645. doi:10.1371/journal.pone.0084645
Carmona-Gutierrez D, Eisenberg T, Büttner S, Meisinger C, Kroemer G, Madeo F (2010) Apoptosis in yeast: triggers, pathways, subroutines. Cell Death Differ 17:763–773. doi:10.1038/cdd.2009.219
Wloch-Salomon DM, Bem AE (2012) Types of cell death and methods of their detection in yeast Saccharomyces cerevisiae. J Appl Microbiol 114:287–298. doi:10.1111/jam.12024
Perrone GG, Tan SX, Dawes IW (2008) Reactive oxygen species and yeast apoptosis. Biochem Biophys Acta 1783:1354–1368. doi:10.1016/j.bbamcr.2008.01.023
Farrugia G, Balzan R (2012) Oxidative stress and programmed cell death in yeast. Front Oncol 2:1–21. doi:10.3389/fonc.2012.00064
Bussche JV, Soares EV (2011) Lead induces oxidative stress and phenotypic markers of apoptosis in Saccharomyces. Appl Microbiol Biotechnol 90:679–687. doi:10.1007/s00253-010-3056-7
Madeo F, Frohlich E, Frohlich KU (1997) A yeast mutant showing diagnostic markers of early and late apoptosis. J Cell Biol 139:729–734. doi:10.1083/jcb.139.3.729
Lefevre S, Sliwa D, Auchère F, Brossas C, Ruckenstuhl C, Boggetto N, Lesuisse E, Madeo F, Camadro JM, Santos R (2012) The yeast metacaspase is implicated in oxidative stress response in frataxin-deficient cells. FEBS Lett 586:143–148. doi:10.1016/j.febslet.2011.12.002
Zdralevic M, Guaragnella N, Antonacci L, Marra E, Giannattasio S (2012) Yeast as a tool to study signaling pathways in mitochondrial stress response and cytoprotection. ScientificWorldJournal. doi:10.1100/2012/912147
Pozniakovsky AI, Knorre DA, Markova OV, Hyman AA, Skulachev VP, Severin F (2005) Role of mitochondria in the pheromone- and amiodarone-induced programmed death of yeast. J Cell Biol 168:257–269. doi:10.1083/jcb.200408145
Nargund AM, Avery SV, Houghton JE (2008) Cadmium induces a heterogeneous and caspase-dependent apoptotic response in Saccharomyces cerevisiae. Apoptosis 13:811–821. doi:10.1007/s10495-008-0215-8
Pereira C, Silva RD, Saraiva L, Johansson B, Sousa MJ, Côrte-Real M (2008) Mitochondria-dependent apoptosis in yeast. Biochim Biophys Acta 1783:1286–1302. doi:10.1016/j.bbamcr.2008.03.010
Pagani MA, Casamayor A, Serrano R, Atrian S, Ariño J (2007) Disruption of iron homeostasis in Saccharomyces cerevisiae by high zinc levels: a genome-wide study. Mol Microbiol 65:521–537. doi:10.1016/j.bbamcr.2008.03.010
Acknowledgments
The authors acknowledge Cytogene Diagnósticos Moleculares Ltda. for access to flow cytometry equipment. F. J. Scariot thanks Coordenação de Aperfeiçoamento de Pessoal de Nivel Superior for fellowship support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Scariot, F.J., Jahn, L.M., Maianti, J.P. et al. The fungicide Mancozeb induces metacaspase-dependent apoptotic cell death in Saccharomyces cerevisiae BY4741. Apoptosis 21, 866–872 (2016). https://doi.org/10.1007/s10495-016-1251-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-016-1251-4