Abstract
The toxic metal cadmium is linked to a series of degenerative disorders in humans, in which Cd-induced programmed cell death (apoptosis) may play a role. The yeast, Saccharomyces cerevisiae, provides a valuable model for elucidating apoptosis mechanisms, and this study extends that capability to Cd-induced apoptosis. We demonstrate that S. cerevisiae undergoes a glucose-dependent, programmed cell death in response to low cadmium concentrations, which is initiated within the first hour of Cd exposure. The response was associated with induction of the yeast caspase, Yca1p, and was abolished in a yca1Δ mutant. Cadmium-dependent apoptosis was also suppressed in a gsh1Δ mutant, indicating a requirement for glutathione. Other apoptotic markers, including sub-G1 DNA fragmentation and hyper-polarization of mitochondrial membranes, were also evident among Cd-exposed cells. These responses were not distributed uniformly throughout the cell population, but were restricted to a subset of cells. This apoptotic subpopulation also exhibited markedly elevated levels of intracellular reactive oxygen species (ROS). The heightened ROS levels alone were not sufficient to induce apoptosis. These findings highlight several new perspectives to the mechanism of Cd-dependent apoptosis and its phenotypic heterogeneity, while opening up future analyses to the power of the yeast model system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As a form of programmed cell death (PCD), apoptosis has been shown to provide a critical contribution to metazoan development, homeostasis and cellular differentiation [1, 2]. It has also been implicated as a major element in the response network of cells to external and internal stressors [3], aging [4, 5] and disease control; being involved (for example) in tumor suppression [6, 7]. Unicellular organisms, such as Saccharomyces cerevisiae also undergo PCD [8]. While this finding was initially surprising, some of the potential benefits of selective apoptosis, of a few for the sake of the population as a whole, have now been rationalized [9, 10]. Moreover, the discovery of apoptosis in yeast has proven to be invaluable for the elucidation of several key apoptotic regulators in a variety of other organisms [11–15]. Despite the conservation of only a single caspase-like protein, Yca1p, which regulates caspase-dependent apoptosis in yeast [16, 17], the retention of specific cell death pathways in common with humans [13] has made S. cerevisiae a key model system in which to study the mechanisms that may underlie these shared apoptotic pathways.
The toxic effects of heavy metals, such as cadmium, have been known for a long time. Cadmium itself is a carcinogen, and elicits a number of toxic effects within cells. These range from the more chronic genotoxic effects, such as altering the capacity of cells to mismatch repair DNA [18], to more acute responses involving protein damage and/or lipid peroxidation [19]. The cellular responses to Cd overlap markedly with those attributed to other forms of oxidative stress [20–23]. While cadmium does not catalyze the production of reactive oxygen species (ROS) directly, it has been shown to promote oxidative stress by depleting the cell’s anti-oxidant defences [24, 25], or by displacing redox-active metals from proteins [26]. The sensitivities of individual yeast cells to oxidative stressors such as peroxides, superoxide and cadmium are heterogeneous [27], and have been shown to fluctuate (under varying concentrations of oxidative stressors) during yeast metabolic oscillations (ultradian rhythms; period <24 h) [28–30], which are associated with concerted transcriptomic and metabolomic changes [31–33].
A major consequence of ROS induced oxidative stress in yeast is apoptosis [17], although this has not previously been tested in the case of cadmium-induced ROS. Cadmium, however, has been associated with apoptosis in rat testes [34], human hepatoma cells [35] and cultured renal tissue [36, 37]. Even so, the precise role(s) that cadmium plays in these processes is only now starting to become apparent [36, 38, 39]. In particular, the caspase-dependency of Cd-induced apoptosis remains uncertain [40, 41]. Here, we demonstrate that low levels of cadmium induce apoptotic cell death in yeast, and that this induction is heterogeneous among individual cells within a population. Furthermore, the programmed cellular response within the affected cells requires the resident caspase, Yca1p, and, perhaps more intriguingly, glutathione; a critical component of the cellular response of yeast to oxidative- and cadmium-induced stresses [42, 43].
Materials and methods
Strains and growth conditions
Saccharomyces cerevisiae BY4741 (MATα his3Δ1 leu2Δ0 met15Δ0 ura3Δ0) and the isogenic mutants yca1Δ and gsh1Δ were obtained from Euroscarf (Frankfurt, Germany). Experimental cultures were inoculated from 24 h starter cultures derived from single colonies and grown overnight to exponential phase (OD600 ∼ 2.0) at 30°C with orbital shaking (120 rev min) in YEPD broth [44]. When necessary for experiments, cadmium nitrate [Cd(NO3)2] was added to growing cultures to a final concentration of 30 μM. Again when necessary, N-Acetyl cysteine was added, to the growth medium (to a final concentration of either 4 or 10 mM), as previously described [45].
RNA isolation, microarray analysis and realtime RT-PCR
Total RNA was isolated using Qaigen RNAeasy kit. RNA samples were prepared from BY4741 untreated and samples treated with Cd(NO3)2 for 5, 15, 30 and 60 min. Relative quantification RT-PCR was used to confirm the expression levels of YCA1 with a One Step RT-PCR kit (Qiagen) and the 7500 SDS system (PE Biosystems). Gene specific probes and primers were as follows: YCA1 (forward primer 5′GGATGCGCAACCCAATGA3′; reverse primer 5′AAATCTTCAGTTTGGC-CACCAT3′; probe FAM-TCTTTGTTCCTTCATTATT-CTGGA-TAMRA), ACT1—endogenous control: (forward primer 5′ATGCAAACCGCTGCT-CAA3′; reverse primer 5′AGTTTGGTCAATACCGG-CAGA3′ and probe 6 FAM–TGGTAACGAA-AGATTCAGAGCCC–TAMRA). Fold changes in gene expression were calculated using the comparative CtΔΔCt method [46, 47].
Cell viability and tests for apoptotic markers
The viability of Cd-treated and untreated cells was determined according to colony forming ability. Cultures were diluted and spread plated onto YEPD plates. Colonies were enumerated after incubation for 3 days at 30°C.
For determination of apoptosis, cell cultures were grown to an OD600 ∼ 2.0 and induced by exposure to 30 μM Cd(NO3)2 for 1 h (unless otherwise specified). Cells were harvested by centrifugation. To test for DNA fragmentation, the cell pellet was resuspended in 300 μl of ddH2O, and 700 μl of 95% ethanol was added before incubation for 3 days at 4°C. Cells were harvested by centrifugation and the pellet resuspended in 1 ml of 50 mM citrate buffer, pH 7.4. Cells were then re-pelleted and resuspended in citrate buffer with 0.25 mg ml−1 RNase, followed by incubation for 2 h at 50°C. Finally, cells were pelleted and resuspended in 1 ml of 16 μg ml−1 propidium iodide (PI) in 50 mM citrate buffer. After incubation for 30 min at room temperature, the PI-stained cells were analyzed (613 nm) by flow cytometry with a FACSCanto (BD Biosciences) [48].
The presence of yeast caspase was detected using an SR_FLICA activated-caspase detection kit (Immunochemistry Technologies, LLC) [49]. Cell pellets, obtained as described above, were resuspended to OD600 ∼ 1.0 in YEPD broth and incubated with SR_FLICA reagent (SR-VAD-FMK/SR-DEVD-FMK) at 30°C for 3 h. The relative intensity of FAM/sulphorhodamine fluorescence in the cells was determined at 530/590 nm emission using a FACSCanto (BD Biosciences). Images were captured using a Zeiss Axioimager fluorescent microscope equipped with Zeiss CP-ACHROMAT 100X/1.25 oil objective, a rhodamine filter and a Zeiss AxioCam MRc5.
Intracellular ROS were detected using the oxidant-sensitive dye dihydro-rhodamine-123 (DHR123, Sigma Aldrich) [50]. DHR123 was added at 5 μg per ml of cell culture, from a 2.5 mg ml−1 stock solution in ethanol, and cells were incubated at 30°C for 3 h. Cells were observed by fluorescence microscopy as described above. Fluorescence was also determined with the FACSCanto at 525 nm to measure the ROS content. To determine whether the same cells that have high intracellular ROS are also caspase-positive, cells were incubated with both DHR123 and SR_FLICA reagent for 3 h. Spectral overlap of the dye emissions at 535 and 590 nm was compensated using FACS Diva software (v 5.0.1), with reference to control cell samples.
To evaluate mitochondrial membrane potential, cells (obtained as described above) were incubated with a final concentration of 2 μM rhodamine-123 and analyzed with the FACSCanto at an emission wavelength of 535 nm.
PI assay of plasma membrane permeabilization
Cells were grown to an OD600 ∼ 2.0 and treated with Cd as described above. Cells were harvested by centrifugation and the pellet was resuspended in YEPD and diluted to OD600 ∼ 1.0. PI was added to 1 ml of the Cd treated and control (untreated) cells to a final concentration of 25 μg ml−1, and cells were incubated for an additional 1–3 h at 30°C. Cells were examined by fluorescence microscopy, and by flow cytometry (emission 613 nm) using a FACSCanto. To determine whether the same cells had high intracellular ROS and high PI, cells were incubated with both DHR123 and PI for 3 h. Compensation for spectral overlap between the two fluorochromes at emission wavelengths of 535 and 613 nm was calculated using Facs Diva software v (5.0.1).
Results and discussion
Cadmium induces the yeast caspase, and a glucose-dependent apoptosis
Previously it was established that 30–50 μM Cd(NO3)2 was sufficient to initiate discernible oxidative stress in yeast, but insufficient to cause acute cellular damage and extensive loss of viability [22, 51]. In preliminary microarray analyses of the transcriptional response of S. cerevisiae to 30 μM Cd (data not shown), we noted that a principal upregulated gene in Cd-treated cultures was YCA1. YCA1 encodes the pivotal apoptotic marker protein, yeast caspase [17, 52]. Consequently, RT-PCR measurements were used to investigate this upregulation of YCA1 more rigorously (Fig. 1A). These analyses revealed a 2-fold increase in the YCA1 mRNA levels of Cd-treated versus control cells, an increase that had started within 2 min and was largely complete within 5 min, and sustained over a 1 h time course of cadmium exposure.
Cadmium induces the yeast caspase, and a glucose-dependent apoptosis. (A) Transcriptional induction of the yeast caspase gene (YCA1) was determined using realtime RT-PCR at various intervals after the exposure of S. cerevisiae (BY4741) to 30 μM Cd(NO3)2 in YEPD medium. Fold changes in YCA1 mRNA were calculated using SDS software through the ΔΔCt method, with ACT1 mRNA as an internal control. The data represent fold-changes (log2) in YCA1 mRNA levels. Each data point (with associated standard deviations) represents the mean of at least 3 independently derived values, with error bars representing standard deviation from the mean. (B) Cells were exposed to 30 μM Cd(NO3)2 for 1 h then ‘recovered’ in fresh YEPD medium for 3 h. Following cell permeabilization with ethanol, cellular DNA was stained with propidium iodide, before the relative DNA content of individual cells were determined with flow cytometry. The presented data are representative of 2 independent experiments. (C) Cells treated as in (B) were tested for caspase activation with SR_FLICA detection chemistry (Immunochemistry, LLC) and analyzed by fluorescence microscopy: the left-most panel is a representative phase contrast image, with the two other panels being fluorescent micrographs of the same cells stained with Hoechst (middle) and SR_FLICA (right). No brightly fluorescent cells were observed within control populations that were not exposed to Cd. (D) Cells prepared as described in (C) were analyzed by flow cytometry. Data in panels C and D are representative of at least 3 independent experiments. The lower panels show the corresponding analyses for cells that were incubated in the absence of glucose during the 3 h recovery period. The red dots define cells or cell clusters that exhibit a higher fluorescence than the cells within the gated control sample. (E) Cells of the yca1Δ mutant were tested for caspase activation (SR_FLICA fluorescence) after incubation, as described in (C). Again, red dots define cells or cell clusters that exhibit a higher fluorescence than the cells within the gated control sample. Data shown are representative of at least 2 independent experimental analyses
YCA1 induction has previously been observed in yeast cells that have been shown to undergo apoptosis [17, 50]. We hypothesized, therefore, that 30 μM Cd(NO3)2 provokes a similar caspase-mediated apoptotic response in S. cerevisiae. In support of this hypothesis, we initially made observations of DNA fragmentation in Cd-exposed cells using the fluorescent DNA stain propidium iodide (PI) [48, 53]. Preliminary experiments indicated that after 3 h Cd exposure, ∼10% of cells had a well-defined, weakly staining “sub-G1” DNA content, indicative of apoptosis (data not shown). Moreover, given that significant YCA1 induction occurred between 2 and 30 min of Cd exposure (Fig. 1A), the possibility that 1 h exposure was sufficient to initiate an apoptotic response was tested. Accordingly, cells were incubated with 30 μM Cd(NO3)2 for 1 h, followed by a 3 h ‘recovery’ period in fresh YEPD growth medium to allow common apoptotic indicators, such as DNA fragmentation, to become apparent. The presence of a tell-tale, distinctive sub-G1 peak, representative of a subpopulation of cells within the cultures, indicated that 1 h exposure to Cd was indeed sufficient to promote an apoptosis-like response (Fig. 1B). This was despite the fact that overt cellular indicators of apoptosis (e.g., enhanced cellular complexity associated with cellular “blebbing”) did not become apparent until much later (data not shown).
As increased expression of YCA1 was observed by both microarray and real time RT-PCR analyses (Fig. 1A), we ascertained whether activated caspase could be used to monitor apoptotic responses of individual cells to cadmium. We employed a fluorescent dye with the caspase inhibitor SR_VAD_FMK (SR_FLICA), which is a strong and selective indicator of the presence of “activated” caspase [49, 54]. Staining of cells that had been exposed to Cd revealed that some cells had markedly increased levels of activated caspase (Fig. 1C, D). Flow cytometric analysis further indicated that this caspase-positive subpopulation comprised approximately 10–15% of the total cell population. Specificity of the fluorescent marker for caspase was confirmed by showing that the SR-FLICA-positive subpopulation was completely abolished in a (Cd-exposed) yca1Δ mutant (Fig. 1E).
Unlike necrotic cell death scenarios, programmed apoptotic responses to cellular stress are known to require ongoing protein synthesis and cellular metabolism [50, 55]. We applied SR_FLICA to determine the requirement for glucose in the response of S. cerevisiae to 30 μM Cd. Cells that were exposed to cadmium (as above), but in the complete absence of glucose, failed to exhibit any signs of caspase-specific apoptosis (data not shown). More specifically, cells that were exposed to cadmium in the presence of glucose for 1 h, but which were subsequently allowed to recover from Cd exposure in the absence of glucose, also failed to exhibit any caspase-specific apoptotic response (Fig. 1D). These results indicated that the apoptotic response to cadmium is dependent on the continued metabolism of glucose, consistent with other types of stress-induced apoptotic responses observed in yeast [50, 53].
Cadmium induces mitochondrial membrane hyper-polarization and enhanced ROS levels in a cell subpopulation
To substantiate an apoptotic response to 30 μM Cd and to examine a potential role for miochondria in the apoptotic process, we tested additional markers of PCD. Mitochondrial dysfunction in yeast has been discovered in a number of apoptotic scenarios, the effects including mitochondrial membrane hyper-polarization, oxidative bursts and, ultimately, breakdown of membrane potential and mitochondrial fragmentation [52, 56–58]. Alterations in the mitochondrial membrane potential (Δψm; an early indicator of apoptosis) were assessed in Cd-treated cells, according to uptake of the cationic, lipophilic dye rhodamine123 (RH123) [59]. In contrast to control (untreated) cells, a sub-population (comprising ∼5–10% of the total population of Cd-exposed cells) exhibited a distinct hyper-polarization of the mitochondrial membrane (Fig. 2A). This fraction of cells corresponded well with that exhibiting high levels of bound SR_FLICA (Fig. 1D), suggesting that these cells may induce a caspase-related, hyper-polarization of their mitochondrial membranes.
Cadmium induces mitochondrial membrane hyperpolarization and enhanced ROS levels in a cell subpopulation. Cells were incubated in the absence or presence of 30 μM Cd(NO3)2 for 1 h then recovered in fresh YEPD medium for 3 h. (A) Changes in the mitochondrial membrane potential as a consequence of Cd treatment were determined following staining with RH123. Fluorescence from RH123 was analyzed using flow cytometry. Control cells were treated in precisely the same way as the +Cd cells except for the absense of Cd. (B) Heterogeneous production of intracellular ROS was detected using the oxidant-sensitive fluorescent dye DHR123, which was added to the cells after incubation in the absence or presence of Cd as above. The left most panel is a representative phase contrast image, with the images to its immediate right being fluorescent micrographs of the same cells stained with Hoechst33342 (middle) and DHR123 (far right). (C) Scatter lots depicting cells treated in the same way as in (B) with DHR123 and analyzed using flow cytometry. (D) Cells were dual-stained with DHR123 (for ROS levels) and SR_FLICA (for caspase activation) prior to flow cytometric analysis. The sector labeled ‘Q2’ contains cells (labeled in red) that are both ROS- and activated caspase-positive. In the figure, data shown are a representative of at least 2 independent analyses. (E) A series of scatter plots, representing fluorescence of cells using DHR123, which were pretreated with indicated concentrations of N-Acetyl cysteine (NAC) prior to the addition of Cd, as in (C). Red dots denote cells or cell clusters that exhibit a higher fluorescence than the cells within the gated control sample. Percentage values in the top right corner of each panel indicate the relative loss in highly fluorescent cells as a consequence of NAC treatment. Data shown are representative of at least 2 independent analyses. (F) A similar series of scatter plots to those shown in (E), representing fluorescence of cells labeled with SR_FLICA, that were pretreated with NAC, as described in text. Again, percentage values indicate the relative loss in highly fluorescent cells, relative to a control, as a consequence of NAC treatment. Data shown are representative of 2 independent analyses
Elevated cellular ROS levels are also a characteristic of apoptotic yeast cells [17, 50]. Furthermore, even though cadmium itself cannot induce ROS directly, it is a potent pro-oxidant and can promote oxidative stress even at the relatively low concentrations used here [22]. Considering also that mitochondria are principal sources of cellular ROS and that Cd-treatment is associated with mitochondrial membrane hyper-polarization (Fig. 2A), we tested for ROS over-production in cells exposed to 30 μM Cd. Dihydrorhodamine123 (DHR123) was used to monitor the presence of ROS in cells, as this dye readily permeates cells and is quantitatively oxidized to its fluorescent product in the presence of ROS [9]. Analysis of ROS levels in this way showed that cadmium treatment did indeed elicit a marked increase in ROS production, and in a similar manner to that observed for caspase and mitochondrial membrane hyper-polarization (Fig. 2B, C). It should be noted that it is possible for dead cell particles and cellular debris to bind fluorescent dyes, such as DHR123, nonspecifically, giving rise to artifactual labeling problems [60, 61]. Such concerns were alleviated here by the use of appropriate controls, and the absence of any apoptotic response (heightened levels of fluorescence; for example SR_VAD_FMK in Fig. 1D) in the absence of glucose. Beyond this, care was taken to confirm that the forward scatter plots of stained cells adequately reflected the typical forward scatter pattern of living cells. In addition, the enhanced fluorescence exhibited by some of the cells in response to Cd, remained responsive to antioxidants, such as N-Acetyl cysteine (NAC: Fig. 2E).
To determine whether the cells generating increased ROS, following Cd treatment (Fig. 2B, C), constitute the same apoptotic sub-population of cells as those showing increased activated caspase (Fig. 1D), cells were simultaneously labeled with caspase inhibitor SR_VAD_FMK and DHR123. These analyses required compensation for the spectral overlap of the fluorochromes (see Materials and Methods section). The resultant dot-plots showed that the cells, which had accumulated high ROS were the same cells that exhibited high levels of caspase-induced apoptosis (Fig. 2D).
As ROS have been shown to be key regulators of apoptosis in yeast [50], and knowing that there are marked overlaps in the responses of cells to cadmium and hydrogen peroxide [21, 62], it could be argued that it is the heightened production of ROS that is responsible for the caspase-dependent apoptotic response of yeast to cadmium.
To test this idea, first the effect of adding the antioxidant, N-acetylcysteine (NAC) was determined. N-Acetylcysteine is a thiol containing antioxidant that is commonly used as a ROS scavenger [63]. At low concentrations (4 mM), NAC had little effect (∼3%) on either the levels of ROS in the cells, or their apoptotic response (Fig. 2E and F). Addition of 10 mM NAC, however, significantly decreased the proportion of cells that exhibited exaggerated ROS levels and caspase activation by 43 and 54%, respectively (Fig. 2E and F).
The NAC doses required to suppress apoptosis here were higher than those typically used elsewhere [64]. Consequently, to test further any role for heightened ROS in Cd-induced apoptosis, apoptotic indicators seen above in cadmium-treated cells were tested in cells exposed to a range of H2O2 concentrations, under otherwise similar conditions. Even at the highest concentration of H2O2 tested (5 mM), the presence of H2O2 in the growth medium for 1 h failed to cause any perceptible apoptotic response within the exposed cells, according to an absence of caspase activation or mitochondrial membrane hyper-polarization (Fig. 3A). H2O2-induced caspase activation and apoptosis evidently requires prolonged exposures such as those used previously [17]. Nonetheless, relatively high concentrations of H2O2 (1–5 mM) did cause elevated levels of ROS in cells after a 1 h exposure (Fig. 3B). These levels were at least as great as those observed with 30 μM Cd(NO3)2 (Fig. 2B) without, however, eliciting any concomitant apoptotic cell death. Consequently, the levels of ROS in Cd-exposed cells, alone, are not sufficient to explain the apoptotic response to Cd seen after 1 h exposure.
Hydrogen peroxide exposure causes a homogeneous increase in ROS levels in the absence of apoptosis. Cells were incubated in the absence or presence of H2O2 for 1 h then allowed to recover in fresh YEPD medium for 3 h. (A) The cells were stained for caspase activation with SR_FLICA (upper panels) or for mitochondrial membrane hyperpolarization with RH123 (lower panels), and analyzed by flow cytometry. Control cells (left panels) were not incubated with H2O2, whereas the test cells (right panels) were treated with 5 mM H2O2. Similar data as for 5 mM H2O2 were obtained for cells treated with 0.5 mM or 1 mM H2O2 (not shown). Data are representative of at least 2 independent analyses. (B) Cells treated with the indicated concentrations of H2O2 were analyzed for ROS levels (using DHR123 fluorescence) by flow cytometry. Red dots denote cells or cell clusters that exhibit a higher fluorescence than the cells within the gated control sample (0 mM H2O2). Data are representative of 2 independent analyses. (C) Cells treated with the indicated concentrations of H2O2 were analyzed for plasma membrane permeabilization (PI fluorescence) using flow cytometry. The data shown are representative of data from at least 3 independent analyses. Similar data as for 5 mM H2O2 were obtained for cells treated with 0.5 or 1 mM H2O2 (not shown)
An additional observation made during the above series of experiments was that cellular ROS levels increased gradually with increasing H2O2 concentration (Fig. 3B), and in a much more homogeneous fashion than those seen with Cd (Fig. 2B). Thus, Cd exposed cell populations, stained with DHR123, exhibited a clear binary phenotype (i.e., individual cells had either low or high ROS), whereas ROS levels were relatively uniform across the population after 1 h exposure to any concentration of H2O2. Binary phenotypes commonly stem from bi-stable gene expression patterns upstream. In turn, these can arise from positive auto-regulatory feedback and similar regulatory mechanisms, increased input of noise in regulatory cascades, or changes in the rates of promoter state transitions [27]. The binary nature of ROS generation among Cd-exposed cells sets this apoptotic response apart from that of cells exposed to H2O2, and reinforces the value of Cd-induced stress as a model heterogeneous phenotype [30].
Cadmium causes membrane damage
In an attempt to obtain additional labels for differentiating apoptotic and non-apoptotic cells within the Cd-exposed population, propidium iodide (PI) staining was employed. The application of PI as a DNA stain (Fig. 1B) relies on a membrane-permeabilization step to allow entry of the dye. The PI-impermeability of live (or apoptotic) yeast cells is commonly exploited to distinguish these from necrotic cells, in which the cellular entry of PI indicates that the membranes have been severely compromised [65]. As even low Cd concentrations cause peroxidation of membrane lipids in yeast [20], we speculated that cadmium could be causing some cellular necrosis, in addition to the observed apoptosis; a possibility that we sought to test with PI (Fig. 4). Unexpectedly, the proportion of Cd-treated cells that we found to be PI-positive (∼10%) approximated the proportion which we had shown earlier to be undergoing apoptotic, PCD. To ascertain whether the cells that were undergoing apoptosis were the same cells that had become PI-permeant, Cd-exposed cells were dual-labeled. Owing to a significant overlap in the fluorescence properties of PI and most of the other dyes used in this study, only DHR123 could be used in combination with PI here (with appropriate compensation). Dual-labeling with PI and DHR123 demonstrated that the cells which accumulated high ROS (which were also those with activated caspase; Fig. 2D) were the same cells that had become sufficiently permeabilized for PI to gain entry (Fig 4B). Permeabilization of plasma membranes is normally associated with late apoptosis, and it is unusual for it to be evident so early after the onset of apoptosis. Moreover, permeabilization of the plasma membrane did not occur in the yca1Δ mutant (Fig. 4C), nor did a similarly timed exposure to high levels of H2O2 (Fig. 3C), indicating that this, seemingly Cd-specific, membrane damage requires an active caspase. In this regard it is interesting to note that even prolonged exposure exposure to H2O2, which does initiate a caspase dependent apoptosis [17], failed to elicit an associated permeability to PI (data not shown). One common cause of membrane permeabilization in cells is lipid peroxidation, which is known to be a principal mechanism of Cd toxicity [19, 20]. Lipid peroxidation has previously been implicated in the apoptotic cell death of yeast, mediated by the heterologous action of human Bax [15]. Furthermore, fatty acid induced lipoapoptosis in the fission yeast, Schizosaccharomyces pombe, has also been shown to have an essential ROS component [66]. Thus, it is possible that Cd-induced lipid peroxidation (and associated plasma membrane permeabilization) provides additional cellular cues that enhance the ability of this metal to elicit an apoptotic response in yeast.
Cadmium-induced apoptosis in yeast is characterized by plasma membrane permeabilization that requires YCA1. Cells were incubated in the absence or presence of 30 μM Cd(NO3)2 for 1 h then recovered in fresh YEPD medium for 3 h, before staining for loss of plasma membrane integrity with propidium iodide (PI). (A) The upper panel is a representative phase contrast image, and the lower panel a fluorescence micrograph of the same PI-stained cells. (B) Cells were dual-stained with DHR123 (for ROS levels) and PI, before flow cytometric analysis. The sector labeled ‘Q2’ contains cells (red dots) that are both PI- and ROS-positive. (C) Cells of the yca1Δ mutant were tested for plasma membrane integrity with PI after incubation, as described previously. All data presented here, represent at least 2 independent sets of analyses
The apoptotic response to cadmium is abrogated in caspase- and glutathione-deficient mutants
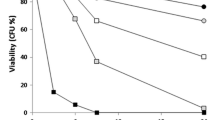
Having demonstrated the apparent involvement of caspase in Cd-induced membrane damage and apoptosis (Figs. 1, 4C), a yca1Δ mutant was further used to investigate whether this caspase is required for the apoptotic response and associated cell death. Cell viability was determined according to colony forming ability on YEPD agar after incubation in broth with 30 μM Cd(NO3)2. These analyses showed that there was a progressive loss in viability (from ∼20 to 40% cell death) of wild type cells with time of incubation in the presence of Cd (Fig. 5A). This progression was mirrored by the proportions of cells undergoing caspase-induced apoptosis under similar conditions of prolonged exposure to cadmium (Fig. 5A). In corroboration of the suggestion that the viability loss was due to a caspase-mediated response, ∼100% of yca1Δ cells retained their viability under identical conditions of Cd exposure (Fig. 5A). As indicated above, the yca1Δ mutant does not exhibit any upregulation of activated caspase within the first hour of Cd exposure (Fig. 1E). Moreover, these cells also failed to show any overproduction of intracellular ROS (Fig. 5B, left panels) or mitochondrial membrane hyper-polarization (Fig. 5B, right panels), which all occur in wild type cells (Fig. 2). This indicates not only that caspase production is required for Cd-induced apoptosis, but also that the activation of caspase precedes (and is potentially a prerequisite for) the stages of the programmed cascade in this cell death response that involve mitochondrial hyper-polarization and increased ROS production [67]. The latter conclusion is consistent with the observation (discussed above) that elevated levels of ROS are not sufficient, in themselves, to initiate apoptosis within 1 h.
Cadmium-induced apoptosis is caspase- and glutathione-dependent. S. cerevisiae BY4741 (wild type) or the isogenic mutants yca1Δ and gsh1Δ were incubated in the presence of 30 μM Cd(NO3)2 for up to 3 h. (A) Viability was determined at intervals after the addition of Cd by spread plating aliquots from dilutions (OD600 = 1.0) of the wild type (○), yca1Δ (•) and gsh1Δ (□) cultures onto YEPD agar. Colonies were enumerated after incubation for 3 days at 30°C. Percentage viability was calculated with reference to the number of colonies formed by untreated cells at zero time. The histogram shows the proportion of apoptotic cells within wild type cultures at intervals after Cd addition-determined using SR_FLICA staining. No SR_FLICA-positive cells were detected in yca1Δ cultures. Each data point represents the average viability of 3 independent experiments. Error bars denote standard deviation from the mean. (B) Cells of the yca1Δ mutant were tested for ROS levels (DHR123 fluorescence) and mitochondrial membrane hyper-polarization (RH123 fluorescence), after incubation for 1 h in the absence (top panels) or presence of 30 μM Cd(NO3)2 (lower panels), followed by 3 h recovery in fresh YEPD. The corresponding analyses with wild type cells are shown in Fig. 2B. (C) Cells of the gsh1Δ mutant were tested for caspase activation (SR_FLICA fluorescence) and ROS levels (DHR123 fluorescence) after incubation, as described in (B). All data shown represent data sets from at least 3 independent sets of analyses
Glutathione (GSH) plays a major role in the cellular regulation of redox homeostasis, being directly (and indirectly) involved in the sequestration of oxidatively damaged proteins [42, 68]. Glutathione is also a key component in the cellular response to cadmium stress as, in addition to its role in relieving oxidative stress, it is associated with the direct chelation and vacuolar detoxification of cadmium [43, 69, 70]. Consequently, cells lacking the ability to synthesize glutathione were tested to see if the absence of glutathione might precipitate an exaggerated response to cadmium. Intriguingly, gsh1Δ cells lacking γ-glutamyl synthetase, which catalyzes the rate-limiting step of glutathione (GSH) synthesis, retained ∼100% viability following incubation in the presence of 30 μM Cd (Fig. 5A). In addition, this glutathione-deficient mutant failed to show any discernible induction of activated caspase, or heightened intracellular ROS (Fig. 5C).
Given the detoxification role of glutathione against cadmium toxicity (see above), the finding that a gsh1Δ mutant effectively resists Cd-induced apoptotic cell death is seemingly contradictory to the known glutathione function. Furthermore, glutathione is reported to suppress apoptosis in a number of other systems [9, 50, 71–73], although there are also examples of glutathione-stimulated apoptosis similar to our findings [38, 39]. Evidently, in yeast there is a specific requirement for glutathione in the process of apoptotic cell death that is triggered by low concentrations of Cd. Glutathione is known to play a critical role in a number of other cellular processes, including FeS-cluster biosynthesis and metabolic oscillations (ultradian rhythms; period <24 h) [28–30]. These oscillations are associated with concerted transcriptomic and metabolomic changes [31–33], and are susceptible to particular perturbations, such as altered levels of glutathione [74–76]. Indeed, rhythm-dependent oscillations in cellular levels of reduced-glutathione (GSH) have recently been shown to underpin the variable Cd resistances of individual yeast cells [30]. It is possible, therefore, that such variation in GSH could also play a role in the marked cell-to-cell heterogeneity in Cd-induced apoptosis which we describe. That apoptosis only occurs within some cells of a population (i.e., is heterogeneous) is a key feature of the anticipated benefits to unicellular organisms of apoptotic cell death [9, 10].
Conclusions
This paper provides the first demonstration that yeast cells mount an apoptotic response to cadmium. Moreover, there are a number of features of this response that distinguish it from other apoptotic scenarios. The apoptotic response to Cd was highly heterogeneous, even when examined with markers such as DHR123 that, under similar growth conditions, produced a homogeneous response to another apoptotic agent, H2O2. These differences reflect a more complex and rapid cellular response to the presence of Cd than that described for H2O2; potentially mediated by a caspase-dependent mitochondrial pathway. In addition, the plasma membranes of apoptotic cells were permeabilized to an extent that allowed entry of PI. This raises some questions about the general applicability of PI as a common marker of necrosis, at least with regard to toxins that are known to induce membrane damage directly. Furthermore, the caspase- and glutathione-dependency of Cd-induced apoptosis provide further key insights into the nature of this response. Common apoptotic mechanisms are now known to exist in yeast and mammalian cells, and new tools for apoptosis research are being developed with yeast. Given the absence of a number of key mammalian apoptotic regulators in yeast, however, it would be premature (at this stage) to extrapolate these results too far beyond the confines of the yeast system. Even so, the discovery of this novel apoptotic phenotype in Saccharomyces cerevisiae should prove a timely stimulus to progress in our understanding of Cd-induced apoptosis.
References
Duke RC, Ojcius DM, Young JD (1996) Cell suicide in health and disease. Sci Am 275:80–87
Shivapurkar N, Reddy J, Chaudhary PM, Gazdar AF (2003) Apoptosis and lung cancer: a review. J Cell Biochem 88:885–898
Hess EV (2002) Environmental chemicals and autoimmune disease: cause and effect. Toxicology 181–182:65–70
Phaneuf S, Leeuwenburgh C (2002) Cytochrome c release from mitochondria in the aging heart: a possible mechanism for apoptosis with age. Am J Physiol Regul Integr Comp Physiol 282:R423–R430
Brunet A, Sweeney LB, Sturgill JF et al (2004) Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 303:2011–2015
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100:57–70
Lowe SW, Lin AW (2000) Apoptosis in cancer. Carcinogenesis 21:485–495
Madeo F, Frohlich E, Frohlich KU (1997) A yeast mutant showing diagnostic markers of early and late apoptosis. J Cell Biol 139:729–734
Reiter J, Herker E, Madeo F, Schmitt MJ (2005) Viral killer toxins induce caspase-mediated apoptosis in yeast. J Cell Biol 168:353–358
Gourlay CW, Du W, Ayscough KR (2006) Apoptosis in yeast—mechanisms and benefits to a unicellular organism. Mol Microbiol 62:1515–1521
Manon S, Chaudhuri B, Guerin M (1997) Release of cytochrome c and decrease of cytochrome c oxidase in Bax-expressing yeast cells, and prevention of these effects by coexpression of Bcl-xL. FEBS Lett 415:29–32
Ligr M, Madeo F, Frohlich E, Hilt W, Frohlich KU, Wolf DH (1998) Mammalian Bax triggers apoptotic changes in yeast. FEBS Lett 438:61–65
Blanchard F, Rusiniak ME, Sharma K et al (2002) Targeted destruction of DNA replication protein Cdc6 by cell death pathways in mammals and yeast. Mol Biol Cell 13:1536–1549
Chae HJ, Ke N, Kim HR et al (2003) Evolutionarily conserved cytoprotection provided by Bax Inhibitor-1 homologs from animals, plants, and yeast. Gene 323:101–113
Manon S (2004) Utilization of yeast to investigate the role of lipid oxidation in cell death. Antioxid Redox Signal 6:259–267
Uren AG, O’Rourke K, Aravind LA et al (2000) Identification of paracaspases and metacaspases: two ancient families of caspase-like proteins, one of which plays a key role in MALT lymphoma. Mol Cell 6:961–967
Madeo F, Herker E, Maldener C et al (2002) A caspase-related protease regulates apoptosis in yeast. Mol Cell 9:911–917
Jin YH, Clark AB, Slebos RJ et al (2003) Cadmium is a mutagen that acts by inhibiting mismatch repair. Nat Genet 34:326–329
Avery AM, Willetts SA, Avery SV (2004) Genetic dissection of the phospholipid hydroperoxidase activity of yeast gpx3 reveals its functional importance. J Biol Chem 279:46652–46658
Howlett NG, Avery SV (1997) Relationship between cadmium sensitivity and degree of plasma membrane fatty acid unsaturation in Saccharomyces cerevisiae. Appl Microbiol Biotechnol 48:539–545
Lee J, Godon C, Lagniel G et al (1999) Yap1 and Skn7 control two specialized oxidative stress response regulons in yeast. J Biol Chem 274:16040–16046
Vido K, Spector D, Lagniel G, Lopez S, Toledano MB, Labarre J (2001) A proteome analysis of the cadmium response in Saccharomyces cerevisiae. J Biol Chem 276:8469–8474
Filipic M, Fatur T, Vudrag M (2006) Molecular mechanisms of cadmium induced mutagenicity. Hum Exp Toxicol 25:67–77
Fortuniak A, Zadzinski R, Bilinski T, Bartosz G (1996) Glutathione depletion in the yeast Saccharomyces cerevisiae. Biochem Mol Biol Int 38:901–910
Stohs SJ, Bagchi D, Hassoun E, Bagchi M (2001) Oxidative mechanisms in the toxicity of chromium and cadmium ions. J Environ Pathol Toxicol Oncol 20:77–88
Stohs SJ, Bagchi D (1995) Oxidative mechanisms in the toxicity of metal ions. Free Radic Biol Med 18:321–336
Avery SV (2006) Microbial cell individuality and the underlying sources of heterogeneity. Nat Rev Microbiol 4:577–587
Wang J, Liu W, Uno T, Tonozuka H, Mitsui K, Tsurugi K (2000) Cellular stress responses oscillate in synchronization with the ultradian oscillation of energy metabolism in the yeast Saccharomyces cerevisiae. FEMS Microbiol Lett 189:9–13
Wang J, Liu W, Mitsui K, Tsurugi K (2001) Evidence for the involvement of the GTS1 gene product in the regulation of biological rhythms in the continuous culture of the yeast Saccharomyces cerevisiae. FEBS Lett 489:81–86
Smith MC, Sumner ER, Avery SV (2007) Glutathione and Gts1p drive beneficial variability in the cadmium resistances of individual yeast cells. Mol Microbiol 66:699–712
Klevecz RR, Bolen J, Forrest G, Murray DB (2004) A genomewide oscillation in transcription gates DNA replication and cell cycle. Proc Natl Acad Sci USA 101:1200–1205
Tu BP, Kudlicki A, Rowicka M, McKnight SL (2005) Logic of the yeast metabolic cycle: temporal compartmentalization of cellular processes. Science 310:1152–1158
Murray DB, Beckmann M, Kitano H (2007) Regulation of yeast oscillatory dynamics. Proc Natl Acad Sci USA 104:2241–2246
Xu C, Johnson JE, Singh PK, Jones MM, Yan H, Carter CE (1996) In vivo studies of cadmium-induced apoptosis in testicular tissue of the rat and its modulation by a chelating agent. Toxicology 107:1–8
Aydin HH, Celik HA, Deveci R et al (2003) Characterization of the cellular response during apoptosis induction in cadmium-treated Hep G2 human hepatoma cells. Biol Trace Elem Res 95:139–153
Lee WK, Abouhamed M, Thevenod F (2006) Caspase-dependent and -independent pathways for cadmium-induced apoptosis in cultured kidney proximal tubule cells. Am J Physiol Renal Physiol 291:F823–F832
Yokouchi M, Hiramatsu N, Hayakawa K et al (2007) Atypical, bidirectional regulation of cadmium-induced apoptosis via distinct signaling of unfolded protein response. Cell Death Differ 14:1467–1474
Kim SC, Cho MK, Kim SG (2003) Cadmium-induced non-apoptotic cell death mediated by oxidative stress under the condition of sulfhydryl deficiency. Toxicol Lett 144:325–336
Sancho P, Fernandez C, Yuste VJ et al (2006) Regulation of apoptosis/necrosis execution in cadmium-treated human promonocytic cells under different forms of oxidative stress. Apoptosis 11:673–686
Kim MS, Kim BJ, Woo HN et al (2000) Cadmium induces caspase-mediated cell death: suppression by Bcl-2. Toxicology 145:27–37
Shih CM, Wu JS, Ko WC et al (2003) Mitochondria-mediated caspase-independent apoptosis induced by cadmium in normal human lung cells. J Cell Biochem 89:335–347
Grant CM, MacIver FH, Dawes IW (1996) Glutathione is an essential metabolite required for resistance to oxidative stress in the yeast Saccharomyces cerevisiae. Curr Genet 29:511–515
Li ZS, Lu YP, Zhen RG, Szczypka M, Thiele DJ, Rea PA (1997) A new pathway for vacuolar cadmium sequestration in Saccharomyces cerevisiae: YCF1-catalyzed transport of bis(glutathionato)cadmium. Proc Natl Acad Sci USA 94:42–47
Shanmuganathan A, Avery SV, Willetts SA, Houghton JE (2004) Copper-induced oxidative stress in Saccharomyces cerevisiae targets enzymes of the glycolytic pathway. FEBS Lett 556:253–259
Brennan RJ, Schiestl RH (1996) Cadmium is an inducer of oxidative stress in yeast. Mutat Res 356:171–178
Chung J, Bachelder RE, Lipscomb EA, Shaw LM, Mercurio AM (2002) Integrin (alpha 6 beta 4) regulation of eIF-4E activity and VEGF translation: a survival mechanism for carcinoma cells. J Cell Biol 158:165–174
Kapitanovic S, Cacev T, Antica M et al (2006) Effect of indomethacin on E-cadherin and beta-catenin expression in HT-29 colon cancer cells. Exp Mol Pathol 80:91–96
Howlett NG, Avery SV (1999) Flow cytometric investigation of heterogeneous copper-sensitivity in asynchronously grown Saccharomyces cerevisiae. FEMS Microbiol Lett 176:379–386
Grabarek J, Amstad P, Darzynkiewicz Z (2002) Use of fluorescently labeled caspase inhibitors as affinity labels to detect activated caspases. Hum Cell 15:1–12
Madeo F, Frohlich E, Ligr M et al (1999) Oxygen stress: a regulator of apoptosis in yeast. J Cell Biol 145:757–767
Strouhal M, Kizek R, Vacek J, Trnkova L, Nemec M (2003) Electrochemical study of heavy metals and metallothionein in yeast Yarrowia lipolytica. Bioelectrochemistry 60:29–36
Madeo F, Herker E, Wissing S, Jungwirth H, Eisenberg T, Frohlich KU (2004) Apoptosis in yeast. Curr Opin Microbiol 7:655–660
Balzan R, Sapienza K, Galea DR, Vassallo N, Frey H, Bannister WH (2004) Aspirin commits yeast cells to apoptosis depending on carbon source. Microbiology 150:109–115
Wolbers F, Buijtenhuijs P, Haanen C, Vermes I (2004) Apoptotic cell death kinetics in vitro depend on the cell types and the inducers used. Apoptosis 9:385–392
Hauptmann P, Riel C, Kunz-Schughart LA, Frohlich KU, Madeo F, Lehle L (2006) Defects in N-glycosylation induce apoptosis in yeast. Mol Microbiol 59:765–778
Hall AG (1999) Review: the role of glutathione in the regulation of apoptosis. Eur J Clin Invest 29:238–245
Ludovico P, Rodrigues F, Almeida A, Silva MT, Barrientos A, Corte-Real M (2002) Cytochrome c release and mitochondria involvement in programmed cell death induced by acetic acid in Saccharomyces cerevisiae. Mol Biol Cell 13:2598–2606
Drakulic T, Temple MD, Guido R et al (2005) Involvement of oxidative stress response genes in redox homeostasis, the level of reactive oxygen species, and ageing in Saccharomyces cerevisiae. FEMS Yeast Res 5:1215–1228
Gross A, Pilcher K, Blachly-Dyson E et al (2000) Biochemical and genetic analysis of the mitochondrial response of yeast to BAX and BCL-X(L). Mol Cell Biol 20:3125–3136
Poliakova D, Sokolikova B, Kolarov J, Sabova L (2002) The antiapoptotic protein Bcl-x(L) prevents the cytotoxic effect of Bax, but not Bax-induced formation of reactive oxygen species, in Kluyveromyces lactis. Microbiology 148:2789–2795
Wysocki R, Kron SJ (2004) Yeast cell death during DNA damage arrest is independent of caspase or reactive oxygen species. J Cell Biol 166:311–316
Wemmie JA, Steggerda SM, MoyeRowley WS (1997) The Saccharomyces cerevisiae AP-1 protein discriminates between oxidative stress elicited by the oxidants H2O2 and diamide. J Biol Chem 272:7908–7914
Fleury C, Mignotte B, Vayssiere JL (2002) Mitochondrial reactive oxygen species in cell death signaling. Biochimie 84:131–141
Brace JL, Vanderweele DJ, Rudin CM (2005) Svf1 inhibits reactive oxygen species generation and promotes survival under conditions of oxidative stress in Saccharomyces cerevisiae. Yeast 22:641–652
Waring P, Lambert D, Sjaarda A, Hurne A, Beaver J (1999) Increased cell surface exposure of phosphatidylserine on propidium iodide negative thymocytes undergoing death by necrosis. Cell Death Differ 6:624–637
Zhang Q, Chieu HK, Low CP, Zhang S, Heng CK, Yang H (2003) Schizosaccharomyces pombe cells deficient in triacylglycerols synthesis undergo apoptosis upon entry into the stationary phase. J Biol Chem 278:47145–47155
Silva RD, Sotoca R, Johansson B et al (2005) Hyperosmotic stress induces metacaspase- and mitochondria-dependent apoptosis in Saccharomyces cerevisiae. Mol Microbiol 58:824–834
Wheeler GL, Grant CM (2004) Regulation of redox homeostasis in the yeast Saccharomyces cerevisiae. Physiol Plant 120:12–20
Dormer UH, Westwater J, McLaren NF, Kent NA, Mellor J, Jamieson DJ (2000) Cadmium-inducible expression of the yeast GSH1 gene requires a functional sulfur-amino acid regulatory network. J Biol Chem 275:32611–32616
Fauchon M, Lagniel G, Aude JC et al (2002) Sulfur sparing in the yeast proteome in response to sulfur demand. Molecular Cell 9:713–723
Sugiyama K, Izawa S, Inoue Y (2000) The Yap1p-dependent induction of glutathione synthesis in heat shock response of Saccharomyces cerevisiae. J Biol Chem 275:15535–15540
Watjen W, Beyersmann D (2004) Cadmium-induced apoptosis in C6 glioma cells: influence of oxidative stress. Biometals 17:65–78
Flower TR, Chesnokova LS, Froelich CA, Dixon C, Witt SN (2005) Heat shock prevents alpha-synuclein-induced apoptosis in a yeast model of Parkinson’s disease. J Mol Biol 351:1081–1100
Murray DB, Engelen F, Lloyd D, Kuriyama H (1999) Involvement of glutathione in the regulation of respiratory oscillation during a continuous culture of Saccharomyces cerevisiae. Microbiology 145(Pt 10):2739–2745
Adams CA, Kuriyama H, Lloyd D, Murray DB (2003) The Gts1 protein stabilizes the autonomous oscillator in yeast. Yeast 20:463–470
Sohn HY, Kum EJ, Kwon GS, Jin I, Adams CA, Kuriyama H (2005) GLR1 plays an essential role in the homeodynamics of glutathione and the regulation of H2S production during respiratory oscillation of Saccharomyces cerevisiae. Biosci Biotechnol Biochem 69:2450–2454
Acknowledgments
This work was supported by a grant from the NIH (R01 GM57945). The support of the Georgia Research Alliance is also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nargund, A.M., Avery, S.V. & Houghton, J.E. Cadmium induces a heterogeneous and caspase-dependent apoptotic response in Saccharomyces cerevisiae . Apoptosis 13, 811–821 (2008). https://doi.org/10.1007/s10495-008-0215-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-008-0215-8