Abstract
Altered expression of survivin and leukocyte antigen class I (HLA-I) proteins is associated with tumor progression. This study investigated their expressions in clear cell renal cell carcinoma (ccRCC) tissues for association with a clinical significance of ccRCC patients. Ninety ccRCC and 20 normal tissue samples (i.e., control) were immunohistochemically stained for survivin and HLA-I expression for an association with clinicopathological data and survival of ccRCC patients. Survivin protein was expressed in 82.2 % (74/90) of ccRCC tissue samples compared to 0 % in the normal tissues, and HLA-I protein was expressed in 90 % (18/20) of the normal tissues vs. 67.8 % (61/90) in ccRCC samples. Survivin expression was associated with tumor grade, stage, and lymph node metastasis (p = 0.000, p = 0.016, and p = 0.001, respectively). Conversely, lost HLA-I expression did not have any associations with clinicopathological data (p > 0.05). Survivin-negative patients had a higher tumor-free survival rate than patients with survivin expression (p = 0.037). Patients with normal HLA-I levels had a higher tumor-free survival rate than those with reduced HLA-I levels (p = 0.02). The uni- and multivariate analyses indicated that expression of survivin and HLA-I, individually and in combination, was an independent predictor for survival of ccRCC patients. Overexpression of survivin but reduced HLA-I expression is useful in the prediction of tumor-free survival of ccRCC patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Renal cell carcinoma (RCC) is the most frequently diagnosed primary tumor in the kidney accounting for more than 85 % of all renal malignancies [1], with annual estimations of 209,000 new cases and 102,000 cancer-related deaths in the world [2]. Clear cell renal cell carcinoma (ccRCC) represents the predominant subtype of RCC and constitutes for approximately 75–80 % of all cases [3]. Surgery is the most effective treatment for RCC, while chemo- and radiotherapy are not as successful in controlling RCC. However, approximately a quarter of RCC clinic patients will develop a metastatic disease despite curative surgical removal of the primary tumor [4]. Early diagnosis and reliable prognostic biomarkers are the keys for the improvement of clinical outcome for RCC patients [5–7].
Survivin is a member of the inhibitor of apoptosis (IAP) family. Survivin protein functions to inhibit caspase activation, thereby leading to a negative regulation of apoptosis. Thus, it has been characterized to have a strong antiapoptotic activity [8]. Normally, survivin is not expressed in differentiated tissues or cells except for the testis, thymus, and placenta [9], but it was reported to be significantly upregulated in various cancer tissues, such as bladder [10], cervical [11], colon [12], and lung [13] cancers. Overexpression of survivin could assist in the early diagnosis and prediction of tumor prognosis and could serve as a novel target for cancer therapy [6, 14]. A recent study demonstrated that survivin expression was associated with advanced clinicopathological stages and grades of ccRCC, while ccRCC patients with low survivin levels had a better survival rate compared to patients with high survivin-expressed tumor [15]. Overexpression of survivin protein could inhibit tumor cell apoptosis, promote metastatic ability of tumor cells, and increase genomic instability [16], thereby boosting malignant phenotypes, such as local invasion and distant metastasis [5, 17]. Furthermore, human leukocyte antigen class I (HLA-I) genes provide instructions for making proteins that are present on the surface of almost all cells. HLA-I proteins display these peptides to the immune system, and if the immune system recognizes the peptides as foreign (such as viral or bacterial peptides), it responds by destroying the infected cells. HLA-I can target different types of cells, including CD8+ cytotoxic T lymphocytes, which results in apoptosis [16]. Expression of the HLA-I protein was reduced in a number of cancers and contributed to the host’s inability to effectively manage CD8+ cytotoxic T lymphocyte (CTL) activation and subsequent tumor immune escape [18]. In addition, mature donor T cells, the major cell type facilitating such effects, harbor HLA-I molecules as the dominant antigen-presenting component, indicating that HLA-I molecules play an important role in tumor immune therapy [19, 20].
Both altered expression of survivin and HLA-I proteins could contribute to ccRCC tumor progression, e.g., survivin promotes tumor cell immortalization, and HLA-I is through evasion of the immune system. However, these two proteins preserve tumor cell viability via very different mechanisms; thus, they may have synergistic effects on ccRCC progression. Thus, we explored their clinical significance in ccRCC tumors obtained from 90 surgically treated patients with more than 5 years of median follow-up data.
Material and methods
Patients and specimen source
Ninety primary renal cancer and 20 cases of distant normal tissue samples (at least 1 cm away from a tumor lesion) were obtained from ccRCC patients that underwent radical or partial nephrectomy between August 2004 and September 2006 at the Department of Urology, Xiangya Hospital, The Central South University. None of the patients received any preoperative chemo- and radiotherapy or other medical interventions. The patients consisted 52 men and 38 women (mean age, 52.2 years; ranging between 30 and 80 years). All cases were pathologically diagnosed to have ccRCC and staged in accordance with the latest tumor-node-metastasis (TNM) classification system. The clinicopathological characteristics of the patients were retrieved from the medical records and are summarized in Table 1. Follow-up data were obtained by phone interview, postal letter communication, and outpatient clinical database. All patients were monitored from the date of initial surgery until death or the closing date of this study (November 30, 2011). The mean follow-up time period was 48.7 months (range between 4 and 62 months). This study was reviewed and approved by the Ethics Committee of The Central South University, and an informed consent form was signed by each patient before surgery.
Immunohistochemistry
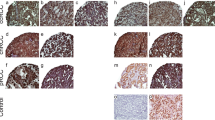
To detect the expression of survivin and HLA-I proteins, formalin-fixed and paraffin-embedded tissue sections were immunostained with a polyclonal antisurvivin antibody (at 1:200 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA,) or an anti-HLA-I antibody (at 1:100 dilution; Abcam, Cambridge, UK) using standard techniques [21, 22]. The immunostained sections were then evaluated in accordance with a previous study [23]. Briefly, all tissue sections were reviewed under a light microscopy and scored for at least five fields at a × 400 magnification independently by two pathologists who were unaware of any clinical or outcome data. Tissue sections were considered to be survivin positive if the cytoplasm and cell membrane stained brown (Fig. 1). In contrast, HLA-I expression was determined by staining on the membrane of tumor cells and graded semiquantitatively according to the following criteria: 0, no visible staining; 1, over 20 % of tumor cells stained weakly or incomplete membrane staining or intermediate staining in the cytoplasm, but none on cell membrane (Fig. 1); and 2, over 80 % of tumor cells presented positive membrane staining (Fig. 1). HLA-I production was classified as reduced immune expression (0 or 1) or normal (2). All images were captured using a Nikon E1000 microscopic imaging system (Tokyo, Japan).
Expression of survivin and HLA-I proteins in clear cell renal carcinoma tissues vs. distant normal tissues. a Survivin expression in normal renal tissue. b Survivin expression in ccRCC tissue (dense tumor cytoplasmic staining for surviving). c HLA-I expression in normal renal tissue. d, e Reduced HLA-I immune expression in ccRCC tissues. f Illustration of proposed survivin and HLA-I interaction. 1 Survivin presented on tumor cell surface as a tumor-specific antigen that is recognized by CTLs, which leads to intense lymphocytic infiltration. 2 CTLs will be activated and produce IFN-γ. 3 IFN-γ further stimulates HLA-I expression in tumor cells. 4 Reduced HLA-I expression induces immune escape of tumor cells. 5 Reduced HLA-I expression does not occur in survivin-expressing cells
Statistical analyses
Statistical analyses were performed using SPSS software version 16.0 for Microsoft Windows (SPSS, Inc., Chicago, IL, USA). Group differences for qualitative variables were analyzed using chi-square test or Fisher’s exact test. Tumor-free survival of patients was stratified using the Kaplan-Meier method and was statistically analyzed using the log-rank statistic. Uni- and multivariate analyses using Cox proportional hazard models were conducted to measure correlations between clinicopathological factors and tumor-free survival. A p < 0.05 was considered statistically significant.
Results
Expression of survivin and HLA-I proteins in ccRCC tissue specimens
In this study, we first assessed survivin expression in 20 pairs of normal and tumor tissue samples using immunohistochemistry and found that none of 20 normal tissues expressed the survivin protein. Thus, we continued analyzing survivin expression in these 90 cases of tumor samples. Our data showed that survivin protein was undetectable in 20 cases of the normal kidney tissues but was expressed at various levels in 74 of the ccRCC tissues (Fig. 1a) and produced a survivin-positive rate of 82.2 %. Survivin protein was mainly expressed in the cytoplasm and sometimes on the membrane of tumor cells. In contrast, the HLA-I protein was clearly expressed in 18 of 20 distant normal kidney tissues (Fig. 1c) but was reduced in the ccRCC tissues. In particular, 31.1 % (28/90) of the cases expressed low levels of HLA-I protein (score 0), 36.7 % (33/90) moderately expressed the HLA-I protein (score 1), and 32.2 % (29/90) highly expressed the HLA-I protein (score 2). Thus, the HLA-I protein was reduced in 67.8 % (61/90) ccRCC cases. Altogether, 58 % (52/90) of the cases of ccRCC tissues expressed high levels of survivin but reduced HLA-I expression, while 8 % (7/90) of the cases of ccRCC tissues expressed high levels of HLA-I, but without an increase in survivin expression. Furthermore, 24 % (22/90) of the cases of ccRCC tissues expressed both normal levels of HLA-I and survivin proteins, and 10 % (9/90) of the cases of ccRCC tissues expressed low levels of HLA-I and survivin proteins.
Association of survivin and HLA-I expression with clinicopathological features from ccRCC patients
Survivin expression was associated with advanced tumor grade/stage and lymph node metastasis (p = 0.001, p = 0.016, and p = 0.001, respectively; Table 1). Conversely, there were no significant differences observed between survivin expression and other patient data, such as age or gender (p > 0.05; Table 1). Furthermore, HLA-I expression exhibited no significant association with age, gender, clinical stage, tumor grade, or lymph node metastasis (p > 0.05; Table 2).
Association of survivin and HLA-I expression with survival of ccRCC patients
ccRCC patients were monitored for up to 62 months with a median of 48.7 months (ranging between 4 and 62 months). MRI, CT, or B-mode ultrasound was performed every 6 months following surgery. There were 28 patients who experienced relapse in this cohort of patients. Patients with survivin-negative expression had a higher tumor-free survival rate than those survivin-positive counterpart patients (p = 0.037, Fig. 2a); this figure also demonstrated that the subgroup of patients with high survivin and low HLA-1 expression had an extremely poor prognosis (median survival of 15 months). However, patients with ccRCC who expressed normal HLA-I levels displayed a higher tumor-free survival rate than those with reduced HLA-I levels (p = 0.020, Fig. 2b). Tumor-free survival of ccRCC patients with survivin and HLA-I expression was significantly greater than that of patients with survivin expression, but reduced HLA-I levels (p = 0.002, Fig. 2c). Tumor-free survival of ccRCC patients with survivin-negative and HLA-I expression was significantly greater than that of patients with survivin-negative expression, but reduced HLA-I expression (p = 0.022, Fig. 2d). Similarly, ccRCC patients with high levels of HLA-I expression, but with low levels of survivin expression, had much better tumor-free survival than ccRCC patients with high levels of survivin, but reduced HLA-I expression (Fig. 3). The uni- and multivariate analyses indicated that expression of survivin and HLA-I individually and in combination was independent predictors for tumor-free survival of ccRCC patients (Table 3). The univariate analysis showed that tumor stage, grade, lymph node metastasis, and survivin or HLA-I expression were associated with poor survival of ccRCC patients, while the multivariate analysis showed that tumor grade and survivin or HLA-I expression were independent predicators for survival of ccRCC patients (Table 3).
Kaplan-Meier curves for tumor-free survival of ccRCC patients. a Tumor-free survival of survivin-positive tumors with normal and low immune expression of HLA-I. b Tumor-free survival of survivin-positive vs. survivin-negative patients. c Tumor-free survival of patients with normal and low levels of HLA-I expression. d Tumor-free survival of survivin-negative tumors with normal and low immune expression of HLA-I
Discussion
In this study, we detected an expression in survivin and HLA-I proteins in ccRCC vs. normal kidney tissues for biomarker discovery. Survivin protein was upregulated, but the HLA-I protein was downregulated in ccRCC tissues. Overexpression of the survivin protein was associated with advanced ccRCC grade/stage and lymph node metastasis, but downregulation of the HLA-I protein was not associated with all the clinicopathological data from the patients. Moreover, overexpression of survivin but a reduction in the HLA-I protein was associated with poor tumor-free survival of ccRCC patients. When these two proteins were combined, the prediction value of tumor-free survival was far more dramatic. The uni- and multivariate analyses demonstrated that expression of survivin and HLA individually and in combination is an independent predictor for tumor-free survival of ccRCC patients. The results indicated that the detection of these two proteins may be further evaluated as a biomarker for the prediction of ccRCC progression and tumor-free survival.
Commonly, survivin is expressed in embryonic and fetal organs, but not in most terminally differentiated normal cells [9]. It functions as a suppressor of cell death, and the mechanism of its action involves a physical association with initiator and effector caspases, preventing the proteolytic maturation and enzyme activities of the caspases [5, 6, 24]. Indeed, Mahalingam et al. [25] reported that the effectiveness of combined histone deacetylase (HDAC) inhibitor vorinostat and the mammalian target of rapamycin (mTOR) inhibitor temsirolimus on a panel of RCC cell lines in vitro and in two xenograft models in vivo was due to the suppression of survivin expression and corresponding induction of apoptosis and enhanced inhibition of angiogenesis. Our data demonstrated that 82.2 % of the ccRCC tissue samples increased levels of survivin expression, but none of the normal kidney tissues had a detectable level of survivin protein. Thus, targeting survivin could be a promising therapeutic strategy to improve RCC therapy [24].
Furthermore, previous studies demonstrated that survivin expression was associated with a clinical significance of several types of cancer, such as bladder [10], cervical [11], colon [12], and lung cancers [13]. In this study, survivin expression was associated with advanced pathological stage and tumor differentiation in ccRCC patients, i.e., a poorly differentiated ccRCC and lymph node metastasized ccRCC expressed a higher level of the survivin protein. This finding was consistent with previous studies [10–13], which suggests that survivin expression is associated with ccRCC progression. Consequently, survivin expression could also be associated with tumor-free survival of ccRCC patients. This hypothesis was supported by the follow-up data from the cohort of patients used in this study, which demonstrated that patients with survivin-expressed ccRCC generally displayed a poorer prognosis.
Recently, it was shown that survivin-specific CTLs can be detected in patients with CLL or malignant melanoma and that these CTLs (when isolated by magnetic beads) were able to lyse HLA-matched allogeneic breast cancer and malignant melanoma cells. This finding indicated that survivin-derived epitopes could be applied for immunotherapy of human cancers [26]. This assumption was strengthened by identification of a natural processed HLA-A2 binding peptide that could be used for generation of survivin-specific CTLs when pulsed on dendritic cells [27]. Indeed, cell-specific immune responses play a key role in antitumor immunity of the host against human tumors [28–30]. As an important part of such responses, CTL-facilitated cytotoxic killing of tumor cells is mediated by CTL recognition of tumor surface HLA-I antigens. Hence, CTLs and HLA-I molecules are both indispensible for the eradication of tumor cells [31]. Downregulation of the HLA-I protein occurred more frequently in breast and prostate cancer, but less frequently in melanoma, head and neck, lung, colorectal, and cervical carcinomas [32]. HLA-I protein was expressed in 90 % of distant normal kidney tissues but downregulated in 67.8 % of the ccRCC tissues. Although detection of the reduced HLA-I protein alone in ccRCC tissues was not associated with any clinicopathological data from ccRCC patients (e.g., tumor stage, grade, or lymph node metastasis), the combination of survivin and HLA-I expression was able to predict ccRCC progression and survival of patients. Moreover, reduced HLA-I protein was an early event in ccRCC and may interfere with presentation of tumor-associated antigens to CTLs and compromise cellular antigenicity for reducing host antitumor activity. Thus, the tumor cells might not be recognizable by CTLs and result in immune escape. However, reduced HLA-I alone may not be sufficient, and the combination with other gene alterations, such as survivin, may synergistically advance ccRCC and results in poor tumor-free survival. Indeed, this hypothesis was supported by follow-up data of ccRCC patients: reduced HLA-I expression plus overexpression of survivin protein had much poorer tumor-free survival rate than ccRCC with normal levels of HLA-I plus negative survivin expression. In recent years, infusion of in vitro-induced specific T lymphocytes into cancer patients has become a focus of cancer immune therapy [33]. Our data for reduced expression of HLA-I protein in ccRCC tissues may indicate that ccRCC is less immunogenic and, therefore, is not effective to respond to T cell-dependent immune therapy. Conversely, since patients with survivin-overexpressed ccRCC and reduced HLA-I ccRCC had much poor tumor-free survival rate, it is suggested that targeting survivin could be a promising therapeutic strategy to improve ccRCC therapy. Our current study implicates an expression of survivin and HLA-I as biomarkers to identify the most aggressive ccRCC tumors. However, it remains unclear how these two molecules work together to confer such a poor prognosis in ccRCC. One model suggests that the combined effect of survivin and HLA-I on ccRCC tumor aggressiveness occurs at the cellular level (Fig. 1f). Specifically, it has been shown that tumor cell survivin can be recognized by CTLs [26]. However, this study was just a proof of concept, and further studies using larger sample sizes are needed to confirm our data. Future studies should also unearth the underlying molecular mechanisms responsible for alterations of these two genes in ccRCC tissues.
References
Gupta K, Miller JD, Li JZ, Russell MW, Charbonneau C. Epidemiologic and socioeconomic burden of metastatic renal cell carcinoma (mRCC): a literature review. Cancer Treat Rev. 2008;34:193–205.
Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet. 2009;373:1119–32.
Leibovich BC, Lohse CM, Crispen PL, Boorjian SA, Thompson RH, Blute ML, et al. Histological subtype is an independent predictor of outcome for patients with renal cell carcinoma. J Urol. 2010;183:1309–15.
Grimm MO, Wolff I, Zastrow S, Fröhner M, Wirth M. Advances in renal cell carcinoma treatment. Ther Adv Urol. 2010;2:11–7.
Wang GC, Hsieh PS, Hsu HH, Sun GH, Nieh S, Yu CP, et al. Expression of cortactin and survivin in renal cell carcinoma associated with tumor aggressiveness. World J Urol. 2009;27:557–63.
Mita AC, Mita MM, Nawrocki ST, Giles FJ. Survivin: key regulator of mitosis and apoptosis and novel target for cancer therapeutics. Clin Cancer Res. 2008;14:5000–5.
Parker AS, Leibovich BC, Lohse CM, Sheinin Y, Kuntz SM, Eckel-Passow JE, et al. Development and evaluation of BioScore: a biomarker panel to enhance prognostic algorithms for clear cell renal cell carcinoma. Cancer. 2009;115:2092–103.
Duffy MJ, O’Donovan N, Brennan DJ, Gallagher WM, Ryan BM. Survivin: a promising tumor biomarker. Cancer Lett. 2007;249:49–60.
Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3:917–21.
Sharp JD, Hausladen DA, Maher MG, Wheeler MA, Altieri DC, Weiss RM. Bladder cancer detection with urinary survivin, an inhibitor of apoptosis. Front Biosci. 2002;7:e36–41.
Branca M, Giorgi C, Santini D, Di Bonito L, Ciotti M, Costa S, et al. Survivin as a marker of cervical intraepithelial neoplasia and high-risk human papillomavirus and a predictor of virus clearance and prognosis in cervical cancer. Am J Clin Pathol. 2005;124:113–21.
Xiaoyuan C, Longbang C, Jinghua W, Xiaoxiang G, Huaicheng G, Qun Z, et al. Survivin: a potential prognostic marker and chemoradiotherapeutic target for colorectal cancer. Ir J Med Sci. 2010;179:327–35.
Yamashita S, Chujo M, Miyawaki M, Tokuishi K, Anami K, Yamamoto S, et al. Combination of p53AIP1 and survivin expression is a powerful prognostic marker in non-small cell lung cancer. J Exp Clin Cancer Res. 2009;28:22.
Yamamoto H, Ngan CY, Monden M. Cancer cells survive with survivin. Cancer Sci. 2008;99:1709–14.
Zamparese R, Pannone G, Santoro A, Lo Muzio L, Corsi F, Pedicillo MC, et al. Survivin expression in renal cell carcinoma. Cancer Investig. 2008;26:929–35.
Castedo M, Perfettini JL, Roumier T, Andreau K, Medema R, Kroemer G. Cell death by mitotic catastrophe: a molecular definition. Oncogene. 2004;23:2825–37.
Parker AS, Lohse CM, Leibovich BC, Cheville JC, Sheinin YM, Kwon ED. Comparison of digital image analysis versus visual assessment to assess survivin expression as an independent predictor of survival for patients with clear cell renal cell carcinoma. Hum Pathol. 2008;39:1176–84.
Garcia-Lora A, Algarra I, Garrido F. MHC class I antigens, immune surveillance, and tumor immune escape. J Cell Physiol. 2003;195:346–55.
Dey BR, McAfee S, Colby C, Sackstein R, Saidman S, Tarbell N, et al. Impact of prophylactic donor leukocyte infusions on mixed chimerism, graft-versus-host disease, and antitumor response in patients with advanced hematologic malignancies treated with nonmyeloablative conditioning and allogeneic bone marrow transplantation. Biol Blood Marrow Transplant. 2003;9:320–9.
Su X, Guo S, Zhou C, Wang D, Ma W, Zhang S. A simple and effective method for cancer immunotherapy by inactivated allogeneic leukocytes infusion. Int J Cancer. 2009;124:1142–51.
Parker AS, Kosari F, Lohse CM, Houston Thompson R, Kwon ED, Murphy L, et al. High expression levels of survivin protein independently predict a poor outcome for patients who undergo surgery for clear cell renal cell carcinoma. Cancer. 2006;107:37–45.
Yuan J, Liu S, Yu Q, Lin Y, Bi Y, Wang Y, et al. Down-regulation of human leukocyte antigen class I (HLA-I) is associated with poor prognosis in patients with clear cell renal cell carcinoma. Acta Histochem. 2013;115:470–4.
Kitamura H, Honma I, Torigoe T, Asanuma H, Sato N, Tsukamoto T. Down-regulation of HLA class I antigen is an independent prognostic factor for clear cell renal cell carcinoma. J Urol. 2007;177:1269–72. discussion 72.
Lu J, Tan M, Huang WC, Li P, Guo H, Tseng LM, et al. Mitotic deregulation by survivin in ErbB2-overexpressing breast cancer cells contributes to Taxol resistance. Clin Cancer Res. 2009;15:1326–34.
Mahalingam D, Medina EC, Esquivel JA 2nd, Espitia CM, Smith S, Oberheu K, et al. Vorinostat enhances the activity of temsirolimus in renal cell carcinoma through suppression of survivin levels. Clin Cancer Res. 2010;16(1):141–53.
Andersen MH, Pedersen LO, Capeller B, Brocker EB, Becker JC. thor Straten P. Spontaneous cytotoxic T-cell responses against survivin-derived MHC class I-restricted T-cell epitopes in situ as well as ex vivo in cancer patients. Cancer Res. 2001;61:5964–8.
Schmitz M, Diestelkoetter P, Weigle B, Schmachtenberg F, Stevanovic S, Ockert D, et al. Generation of survivin-specific CD8+ T effector cells by dendritic cells pulsed with protein or selected peptides. Cancer Res. 2000;60:4845–9.
Andersson E, Villabona L, Bergfeldt K, Carlson JW, Ferrone S, Kiessling R, et al. Correlation of HLA-A02* genotype and HLA class I antigen down-regulation with the prognosis of epithelial ovarian cancer. Cancer Immunol Immunother. 2012;61:1243–53.
Tanaka K, Tsuchikawa T, Miyamoto M, Maki T, Ichinokawa M, Kubota KC, et al. Down-regulation of Human Leukocyte Antigen class I heavy chain in tumors is associated with a poor prognosis in advanced esophageal cancer patients. Int J Oncol. 2012;40:965–74.
Ouyang W, Liao W, Luo CT, Yin N, Huse M, Kim MV, et al. Novel Foxo1-dependent transcriptional programs control T(reg) cell function. Nature. 2012;491:554–9.
Stickel JS, Stickel N, Hennenlotter J, Klingel K, Stenzl A, Rammensee HG, et al. Quantification of HLA class I molecules on renal cell carcinoma using Edman degradation. BMC Urol. 2011;11:1.
Seliger B, Cabrera T, Garrido F, Ferrone S. HLA class I antigen abnormalities and immune escape by malignant cells. Semin Cancer Biol. 2002;12:3–13.
Montagna D, Turin I, Schiavo R, Montini E, Zaffaroni N, Villa R, et al. Feasibility and safety of adoptive immunotherapy with ex vivo-generated autologous, cytotoxic T lymphocytes in patients with solid tumor. Cytotherapy. 2012;14:80–90.
Acknowledgments
This work were supported by Hunan Provincial Innovation Foundation for Postgraduate (NO. 250171380100015) and supported by the Fundamental Research Funds for the Central Universities of Central South University (NO. 2014zzts079).
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, S., Qi, L., Yu, Q. et al. Survivin and HLA-I expression predicts survival of patients with clear cell renal cell carcinoma. Tumor Biol. 35, 8281–8288 (2014). https://doi.org/10.1007/s13277-014-2058-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2058-y