Abstract
Apoptosis causes elimination of more than 99 % of germ cells from cohort of ovary through follicular atresia. Less than 1 % of germ cells, which are culminated in oocytes further undergo apoptosis during last phases of oogenesis and depletes ovarian reserve in most of the mammalian species including human. There are several players that induce apoptosis directly or indirectly in oocytes at various stages of meiotic cell cycle. Premature removal of encircling granulosa cells from immature oocytes, reduced levels of adenosine 3′,5′-cyclic monophosphate and guanosine 3′,5′-cyclic monophosphate, increased levels of calcium (Ca2+) and oxidants, sustained reduced level of maturation promoting factor, depletion of survival factors, nutrients and cell cycle proteins, reduced meiotic competency, increased levels of proapoptotic as well as apoptotic factors lead to oocyte apoptosis. The BH3-only proteins also act as key regulators of apoptosis in oocyte within the ovary. Both intrinsic (mitochondria-mediated) as well as extrinsic (cell surface death receptor-mediated) pathways are involved in oocyte apoptosis. BID, a BH3-only protein act as a bridge between both apoptotic pathways and its cleavage activates cell death machinery of both the pathways inside the follicular microenvironment. Oocyte apoptosis leads to the depletion of ovarian reserve that directly affects reproductive outcome of various mammals including human. In this review article, we highlight some of the important players and describe the pathways involved during oocyte apoptosis in mammals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mammalian ovary is responsible for generating competent oocytes required for the successful fertilization and early embryonic development. Apoptosis, a programmed cell death, plays a major role in the elimination of germ cells at all the stages of oogenesis and even after ovulation [1, 2]. More than 99 % of germ cells are eliminated from ovary via apoptosis through follicular atresia, while less than 1 % are culminated into oogonia [1, 3]. These oogonia enter into meiosis to give rise to primary oocytes [4, 5]. Primary oocytes are arrested at diplotene stage for several months to several years depending upon the mammalian species [6, 7]. These diplotene-arrested oocytes are encircled by several layers of granulosa cells inside the follicular microenvironment.
A cross-talk between encircling granulosa cells and diplotene-arrested oocytes is important for the survival of both cell types [8, 9]. The granulosa cell apoptosis and/or premature removal of encircling granulosa cells deprive oocyte from growth factors, nutrients and survival factors that may lead to apoptosis in diplotene-arrested oocytes cultured in vitro [10–14]. Our studies suggest that granulosa cell apoptosis inside the follicular microenvironment leads to oocyte apoptosis in rat [14–16]. The granulosa cell intactness protects oocytes from oxidative stress damage in vitro [17–19]. Generation of reactive oxygen species (ROS) or depletion of antioxidant system leads to oocyte apoptosis [12–14, 20]. However, granulosa cell apoptosis in ovulated cumulus oocyte complexes can be used as predictors of oocyte quality [21–23].
A small number of follicles containing primary oocytes are selectively recruited during entire reproductive life of mammalian female including human. Follicular oocytes resume meiosis in response to pituitary gonadotropins surge or if removed from ovary and cultured in vitro [6, 7, 10, 11, 24, 25]. Although these diplotene-arrested oocytes (Fig. 1a) frequently undergo spontaneous meiotic resumption from diplotene arrest and further get arrested at metaphase-I (M-I) stage (Fig. 1b) but they are more susceptible to in vitro culture conditions and frequently die via apoptosis [15, 16, 18, 19].
Representative photograph showing morphological features characteristics of a diplotene arrest (green arrow showing germinal vesicle), b M-I arrest (green arrow showing germinal vesicle breakdown), c M-II arrest (black arrow showing first polar body), d M-III like arrest (black arrow showing first polar body red arrow showing second polar body extrusion) and e apoptosis in mammalian oocytes. Several factors could induce apoptosis in oocytes at various stages of meiotic cell cycle and reduces ovarian reserve (Color figure online)
At the time of ovulation, graafian follicles rupture and give rise to competent oocytes arrested at metaphase-II (M-II) stage. Once ovulated, these oocytes possess first polar body and waits for fertilization (Fig. 1c). If the fertilization does not occur, postovulatory aging results either spontaneous activation followed by metaphase-III (M-III) like arrest (Fig. 1d) [7, 26–29] or apoptosis (Fig. 1e) in oocytes [15, 16, 20, 30–34]. Studies suggest that good quality oocytes are ovulated first during early reproductive life. As the maternal aging occurs, oocyte becomes more susceptible towards apoptosis and limits reproductive outcome in human [35–37]. Thus, apoptosis plays a major role in eliminating majority of germ cells at all the stages of oogenesis and depletes ovarian reserve in various mammalian species including human.
Players of oocyte apoptosis
There are several players responsible for oocyte apoptosis in mammals (Fig. 2). Encircling granulosa cells decide the fate of an oocyte inside the follicular microenvironment [8]. Deprivation of oocytes from various signal molecules, survival factors and growth factors from encircling granulosa cells trigger susceptibility of oocytes towards apoptosis [15, 16]. This is supported by the observations that premature removal of granulosa cells from oocyte or granulosa cell apoptosis reduce meiotic as well as developmental competence [38–41] and increase susceptibility of follicular oocyte towards apoptosis [12, 13, 33, 34, 42].
Schematic representation showing various players of oocyte apoptosis such as premature disruption of gap junctions, Signal molecules (Ca2+, cAMP and cGMP), Oxidants (NO, H2O2 and OH−), MPF Destabilization, Meiotic competency, Oocyte aging, Survival factors, Proapoptotic factors (Bax, cytochrome c, caspases 8 and 9), BH3-only proteins and apoptotic factors (caspase 3 and DNA fragmentation). Casp 3 Caspase 3, DNA Frag DNA Fragmentation, Cyto c Cytochrome c, Casps 8, 9 Caspases 8 and 9
Reduced granulosa cell-oocyte communication interrupts the transfer of adenosine 3′,5′-cyclic monophosphate (cAMP) [43], guanosine 3′,5′-cyclic monophosphate (cGMP) [44, 45] and nitric oxide (NO) [46] levels to the follicular oocyte. Reduction of these signal molecules may trigger the generation of reactive oxygen species (ROS) in diplotene-arrested oocytes [11, 24, 25, 47]. These findings are further supported by our previous studies that diplotene-arrested oocytes are more susceptible to hydrogen peroxide (H2O2)-induced apoptosis as compare to M-II arrested oocytes [12–14, 30]. Increased inducible nitric oxide synthase expression and thereby NO level induce oocyte apoptosis [16, 18, 19, 32].
Calcium (Ca2+) is one of the major signal molecules that regulate oocyte physiology [48–50]. The high sustained level of intracellular calcium ([Ca2+]i) induces meiotic cell cycle arrest and apoptosis [26, 51, 52]. On the other hand, abnormally high ([Ca2+]i) results in cell death [53, 54]. Calcium ionophore (CI) increases cytosolic free Ca2+ possibly by mitochondrial remodelling [55] and mitochondria membrane depolarization [56] leading to apoptosis in rat [30], pig [57] and bovine oocytes [58, 59] cultured in vitro. Studies from our laboratory suggest that CI increases cytosolic free Ca2+ level, induces generation of ROS and thereby apoptosis in rat oocytes cultured in vitro [30, 32, 60]. Sustained reduced levels of cAMP and cGMP as well as increased level of Ca2+ induce generation of ROS [24, 25]. This notion is further supported by our studies that increased levels of ROS with reduced catalase activity induce morphological apoptotic features in rat oocytes [15, 16, 18, 19].
Increased generation of ROS may lead to oxidative stress [6, 24, 25, 61, 62]. The oxidative stress reduces survival factors and induces destabilization of maturation promoting factor (MPF) in diplotene as well as M-II arrested oocytes. MPF stabilisation requires a series of phosphorylation/dephosphorylation of Cdk1, dissociation and degradation of cyclin B1 in oocytes [7]. Studies from our laboratory suggest that the inhibition of Cdk1 activity using roscovitine induces meiotic cell cycle arrest and apoptosis [33, 34, 63–65] probably by modulating the level of MPF heterodimer. Although MPF destabilization triggers spontaneous exit from M-II arrest [27–29], sustained decrease of destabilised MPF level triggers oocyte apoptosis [33, 34].
Oocyte after ovulation, either in vivo or under in vitro culture conditions, has limited number of adenosine triphosphate (ATP) [66] that results in the generation of ROS and thereby downregulation of anti-apoptotic factor such as Bcl2 [16]. The reduced anti-apoptotic factor leads to increased proapoptotic as well as apoptotic factors results in oocyte apoptosis [12–16, 33, 34, 60]. Factors that push oocytes to initiate apoptotic cell death indirectly are termed as proapoptotic factors. Apoptotic factors are directly involved in the disruption of histoarchitecture of a cell leading to appearance of morphological apoptotic features. BH3-only proteins act as proapoptotic factors and are essential mediators of apoptosis within ovary in several mammalian species [2, 5]. Apoptosis in the follicular oocytes results in the depletion of germ cells from ovarian reserve [67]. The ratio of apoptotic promoter (such as Bax expression) to suppressor (such as Bcl2 expression) within a cell determines whether cell will undergo apoptosis or survive [12–16, 68]. The involvement of Bax protein and caspase-3 activation during oocytes apoptosis has been reported in mouse and rat oocytes [12, 13, 69].
It has been generally accepted that an increased level of cytochrome c initiates apoptosis in oocytes [34, 60, 70]. The release of cytochrome c from internal stores activates upstream and downstream caspases in a cell leading to oocyte apoptosis [12–16, 32, 34, 60]. Caspases are a family of cysteine-dependent aspartate-directed proteases that cleave intracellular polypeptides resulting into disruption of cellular architecture that leads to morphological changes characteristics of apoptosis [69]. Caspase-3 has substrate specificity for destruction of structural and specific proteins that leads to DNA damage in multiples of 180–200 base-pair, a hallmark feature of oocyte apoptosis [12–16, 18, 19, 30, 33, 34, 57, 60, 69, 71]. Although we have described major players responsible for depleting ovarian reserve by inducing oocyte apoptosis, there are several other equally important players involved in this process, which are not discussed herewith.
Underlying pathways in oocyte apoptosis
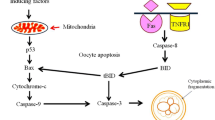
Oocyte apoptosis in mammals involves both mitochondria-mediated (intrinsic) [72] as well as cell surface death receptors-mediated (extrinsic) pathways (Fig. 3). Increased oxidative stress is one of the major factors that induce oocyte apoptosis [18, 19]. Various players, as described above, follow either mitochondria-mediated or death receptors-mediated pathways and some of them links these two pathways to induce oocyte apoptosis [2]. Players that induce generation of ROS follow mitochondria-mediated oocyte apoptosis [18, 19]. The proapoptotic ligands (FASL and TNFα) bind to their respective receptor and activate cell surface death receptors. Activation of death-receptors followed by caspases leads to death-receptor mediated apoptosis [73].
Increased levels of ROS due to decreased levels of cAMP as well as cGMP in oocytes [24, 47] and increased level of cytosolic free Ca2+ drive mitochondria-mediated apoptosis in follicular oocytes in mammals [60]. Studies suggest that increased cytosolic free Ca2+ level in response to CI induces generation of H2O2 [74]. The increased level of ROS can modulate expressions of Bax/Bcl2 ratio in mitochondria membrane and thereby membrane potential [69, 74]. Change in the mitochondria membrane potential triggers cytochrome c release in the cytoplasm of a cell [34, 60, 70], which activate upstream and downstream caspases in oocytes [74].
The proapoptotic BH3-only proteins act as key regulators of apoptosis within the ovary [2]. BID, a BH3-only protein, acts as a bridge between mitochondria-mediated and death receptor-mediated pathways. A truncated BID (tBID) induces overexpression of Bax, which then modulates mitochondria membrane potential that results in the release of cytochrome c. Cytochrome c binds to apoptotic protease activating factor 1 in the cytoplasm that activates caspase-9 as well as caspase-3. The caspase-3 cleaves key structural and regulatory proteins leading to several biochemical and morphological changes associated with oocyte apoptosis [60, 69, 75–77].
The extrinsic apoptotic pathway is initiated by activation of tumour necrosis factor receptor family (FAS and TNFR1), which bind to their ligands (FASL and TNFα) [2, 33]. Recent studies from our laboratory suggest that reduced Thr-161 phosphorylated Cdk1 as well as cyclin B1 levels destabilize MPF and push FasL-mediated oocyte apoptosis [33]. The increased FasL concentration results in Fas receptor trimerization and recruitment of the adaptor molecule Fas-associated death domain-containing protein (FADD) through interaction between its own and clustered receptor death domains [33, 78]. On recruitment by FADD, procaspase-8 gets oligomerized and activated via autocatalysis. Active caspase-8 stimulates apoptosis by cleaving and activating caspase-3 [73]. This notion is further strengthened by our observations that roscovitine increases caspases-8 as well as caspases-3 activities in treated oocytes that showed cytoplasmic fragmentation (Fig. 1e). The activated caspase-3 cleaves key structural and regulatory proteins that result in DNA fragmentation, a hallmark feature of apoptosis [69]. These fragmented DNA are detected in a single oocyte using TUNEL assay [12–16, 33, 34, 69, 78–80].
Future prospectus
Several players are involved in inducing oocyte apoptosis either following mitochondria- or death-receptor mediated pathway or both. Based on the available literature, we propose major players and pathways involved in oocyte apoptosis. However, furthermore studies are required to delineate the stage specific involvement of these players and pathways inducing apoptosis in diplotene-, M-I, M-II and M-III arrested oocytes in mammals. Oocyte apoptosis is one of the major causes for the depletion of germ cells from ovary and has direct negative impact on female fertility in various mammalian species including human. Discovery of very small embryonic-like cells opens exciting new perspectives for neo-oogenesis but the source of new oocytes is still unclear and under debate [81, 82]. Although a ray of light is coming from ovarian stem cells to increase the number of oocytes, emphasis must be given to prevent oocyte loss via apoptosis from the ovary due to environmental changes, pathological conditions or drugs treatment so that the early depletion of ovarian reserve can be protected. The availability of good quality and number of oocytes could improve reproductive outcome in several mammalian species including human.
References
Tilly JL (2001) Commuting the death sentence: how oocytes strive to survive. Nat Rev Mol Cell Biol 2:838–848
Hutt KJ (2015) The role of BH3-only proteins in apoptosis within the ovary. Reproduction 149:R81–R89
Matsuda F, Inoue N, Manabe N et al (2012) Follicular growth and atresia in mammalian ovaries: regulation by survival and death of granulosa cells. J Reprod Dev 58:44–50
Morita Y, Tilly JL (1999) Oocyte apoptosis: like sand through an hourglass. Dev Biol 213:1–17
Liew SH, Vaithiyanathan K, Cook M et al (2014) Loss of the proapoptotic BH3-only protein BCL-2 modifying factor prolongs the fertile life span in female mice. Biol Reprod 90:77
Pandey AN, Tripathi A, Premkumar KV et al (2010) Reactive oxygen and nitrogen species during meiotic resumption from diplotene arrest in mammalian oocytes. J Cell Biochem 111:521–528
Tripathi A, Prem Kumar KV, Chaube SK (2010) Meiotic cell cycle arrest in mammalian oocytes. J Cell Physiol 223:592–600
Barrett SL, Albertini DF (2010) Cumulus cell contact during oocyte maturation in mice regulates meiotic spindle positioning and enhances developmental competence. J Assist Reprod Genet 27:29–39
Albertini DF (2011) A cell for every reason: the ovarian granulosa cell. J Assist Reprod Genet 28:877–878
Chaube SK (2001) Role of meiotic maturation regulatory factors in the developmental competence of mammalian oocytes. HPPI 24:218–231
Chaube SK (2002) Does cyclic adenosine 3’5’ monophosphate act as a regulator for oocyte meiotic resumption in mammal? HPPI 25:74–85
Chaube SK, Prasad PV, Thakur SC et al (2005) Hydrogen peroxide modulates meiotic cell cycle and induces morphological features characteristic of apoptosis in rat oocytes cultured in vitro. Apoptosis 10:863–874
Chaube SK, Prasad PV, Thakur SC et al (2005) Estradiol protects clomiphene citrate-induced apoptosis in ovarian follicular cells and ovulated cumulus-oocyte complexes. Fertil Steril 84:1163–1172
Chaube SK, Shrivastav TG, Prasad S et al (2014) Clomiphene citrate induces ROS-mediated apoptosis in mammalian oocytes. Open J Apoptosis 3:52–58
Chaube SK, Shrivastav TG, Tiwari M et al (2014) Neem leaf extract deteriorates oocyte quality by inducing ROS-mediated apoptosis in mammals. SpringerPlus 3:464–468
Tripathi A, Shrivastav TG, Chaube SK (2013) An increase of granulosa cell apoptosis mediates aqueous neem (Azadirachta indica) leaf extract-induced oocyte apoptosis in rat. Intl J Appl Basic Med Res 3:27–36
Tatemoto H, Sakurai N, Muto N (2000) Protection of porcine oocytes against apoptotic cell death caused by oxidative stress during in vitro maturation: role of cumulus cells. Biol Reprod 63:805–810
Chaube SK, Prasad PV, Thakur SC et al (2005) Hydrogen peroxide modulates meiotic cell cycle and induces morphological features characteristic of apoptosis in rat oocytes cultured in vitro. Apoptosis 10:863–874
Chaube SK, Prasad PV, Thakur SC et al (2005) Estradiol protects clomiphene citrate-induced apoptosis in ovarian follicular cells and ovulated cumulus-oocyte complexes. Fertil Steril 84:1163–1172
Tripathi A, Premkumar KV, Pandey AN et al (2011) Melatonin protect against clomiphene citrate-induced generation of free radicals and egg apoptosis in rat. Eur J Pharmacol 667:419–424
Li Q, McKenzie LJ, Matzuk MM (2008) Revisiting oocyte-somatic cell interactions: in search of novel intrafollicular predictors and regulators of oocyte developmental competence. Mol Hum Reprod 14:673–678
Uyar A, Torrealday S, Seli E (2013) Cumulus and granulosa cell markers of oocyte and embryo quality. Fertil Steril 99:979–997
Vigone G, Merico V, Prigione A et al (2013) Transcriptome based identification of mouse cumulus cell markers that predict the developmental competence of their enclosed antral oocytes. BMC Genom 14:380
Pandey AN, Chaube SK (2014) A moderate increase of hydrogen peroxide level is beneficial for spontaneous resumption of meiosis from diplotene arrest in rat oocytes cultured in vitro. BioRes Open Access 3:183–191
Pandey AN, Chaube SK (2015) Reduction of nitric oxide level leads to spontaneous resumption of meiosis in diplotene-arrested rat oocytes cultured in vitro. Exp Biol Med (Maywood) 240:15–25
Chaube SK, Dubey PK, Mishra SK et al (2007) Verapamil inhibits spontaneous parthenogenetic activation in aged rat eggs cultured in vitro. Cloning Stem Cells 9:608–617
Premkumar KV, Chaube SK (2013) An insufficient increase of cytosolic free calcium level results postovulatory aging-induced abortive spontaneous egg activation in rat. J Asst Reprod Genet 30:117–123
Premkumar KV, Chaube SK (2014) RyR channel-mediated increase of cytosolic free calcium level signals cyclin B1 degradation during abortive spontaneous egg activation in rat. In Vitro Cell Dev Biol Anim 50:640–647
Prasad S, Premkumar KV, Koch B et al (2014) Abortive spontaneous egg activation: a pathological condition in mammalian egg. ISSRF News Lett 14:25–27
Chaube SK, Khatun S, Mishra SK et al (2008) Calcium ionophore-induced egg activation and apoptosis are associated with the generation of intracellular hydrogen peroxide. Free Radic Res 42:212–220
Chaube SK, Tripathi A, Khatun S et al (2009) Extracellular calcium protects against verapamil-induced metaphase-II arrest and initiation of apoptosis in aged rat eggs. Cell Biol Int 33:337–343
Tripathi A, Khatun S, Pandey AN et al (2009) Intracellular levels of hydrogen peroxide and nitric oxide in oocytes at various stages of meiotic cell cycle and apoptosis. Free Radic Res 43:287–294
Tripathi A, Chaube SK (2015) Roscovitine induces metaphase-II arrest and apoptosis through FasL-mediated pathway in rat eggs cultured in vitro. In Vitro Cell Dev Biol Anim 51:174–182
Tripathi A, Chaube SK (2015b) Roscovitine inhibits extrusion of second polar body and induces apoptosis in rat eggs cultured in vitro. Pharmacol Rep. doi:10.1016/j.pharep.2015.01.011
Wu J, Zhang L, Wang X (2000) Maturation and apoptosis of human oocytes in vitro are age-related. Fertil Steril 74:1137–1141
Santonocito M, Guglielmino MR, Vento M et al (2013) The apoptotic transcriptome of the human MII oocyte: characterization and age-related changes. Apoptosis 18:201–211
Tsutsumi M, Fujiwara R, Nishizawa H et al (2014) Age-related decrease of meiotic cohesins in human oocytes. PLoS One 9:e96710
Modina S, Luciano AM, Vassena R et al (2001) Oocyte developmental competence after in vitro maturation depends on the persistence of cumulus-oocyte communications which are linked to the intracellular concentration of cAMP. Ital J Anat Embryol 106:241–248
Li Q, McKenzie LJ, Matzuk MM (2008) Revisiting oocyte-somatic cell interactions: in search of novel intrafollicular predictors and regulators of oocyte developmental competence. Mol Hum Reprod 14:673–678
Yuan Y, Hao ZD, Liu J et al (2008) Heat shock at the germinal vesicle breakdown stage induces apoptosis in surrounding cumulus cells and reduces maturation rates of porcine oocytes in vitro. Theriogenology 70:168–178
Assidi M, Dieleman SJ, Sirard MA (2010) Cumulus cell gene expression following the LH surge in bovine preovulatory follicles: potential early markers of oocyte competence. Reproduction 140:835–852
Wu Y, Wang XL, Liu JH et al (2011) BIM EL-mediated apoptosis in cumulus cells contributes to degenerative changes in aged porcine oocytes via a paracrine action. Theriogenology 76:1487–1495
Rose RD, Gilchrist RB, Kelly JM et al (2013) Regulation of sheep oocyte maturation using cAMP modulators. Theriogenology 79:142–148
Vaccari S, Weeks JL II, Hsieh M et al (2009) Cyclic GMP signaling is involved in the luteinizing hormone-dependent meiotic maturation of mouse oocytes. Biol Reprod 81:595–604
Norris RP, Ratzan WJ, Freudzon M et al (2009) Cyclic GMP from the surrounding somatic cells regulates cyclic AMP and meiosis in the mouse oocyte. Development 136:1869–1878
Jablonka-Shariff A, Olson L (2000) Nitric oxide is essential for optimal meiotic maturation of murine cumulus-oocyte complexes in vitro. Mol Reprod Dev 55:412–421
Cheon YP, Kim SW, Kim SJ et al (2000) The role of RhoA in the germinal vesicle breakdown of mouse oocytes. Biochem Biophys Res Commun 273:997–1002
Berridge MJ, Bootman MD, Lipp P (1998) Calcium- a life and death signal. Nature 395:645–648
Gordo AC, Rodrigues P, Kurokawa M et al (2002) Intracellular calcium oscillations signal apoptosis rather than activation in in vitro aged mouse eggs. Biol Reprod 66:1828–1837
Tosti E (2006) Calcium ion currents mediating oocyte maturation events. Reprod Biol Endocrinol 4:26–34
Vincent C, Cheek TR, Johnson MH (1992) Cell cycle progression of parthenogenetically activated mouse oocytes to interphase is dependent on the level of internal calcium. J Cell Sci 103:389–396
Lu Q, Chen ZJ, Gao X et al (2006) Oocyte activation with calcium ionophore A23187 and puromycin on human oocytes that failed to fertilize after intracyplasmic sperm injection. Zhonghua Fu Chan Ke Za Zhi 41:182–185
McConkey DJ, Orrenius S (1997) The role of calcium in the regulation of apoptosis. Biochem Biophys Res Commun 239:357–366
Ruddock NT, Machaty Z, Cabot RA et al (2001) Porcine oocyte activation: roles of calcium and pH. Mol Reprod Dev 59:227–234
Tan AR, Cai AY, Deheshi S et al (2011) Elevated intracellular calcium causes distinct mitochondrial remodelling and calcineurin-dependent fission in astrocytes. Cell Calcium 49:108–114
Cho SY, Lee JH, Bae HD et al (2010) Transglutaminase 2 inhibits apoptosis induced by calcium-overload through down-regulation of Bax. Exp Mol Med 42:639–650
Ma W, Zhang D, Hou Y et al (2005) Reduced expression of MAD2, BCL2 and MAP Kinase activity in pig oocytes after in vitro aging are associated with defects in sister chromatid segregation during meiosis II and embryo fragmentation after activation. Biol Reprod 72:373–383
Sergeev IN, Norman AV (2003) Calcium as a mediator of apoptosis in bovine oocytes and preimplantation embryos. Endocrine 22:169–176
Wang ZG, Wang W, Yu SD et al (2008) Effects of different activation protocols on preimplantation development, apoptosis and ploidy of bovine parthenogenetic embryos. Anim Reprod Sci 105:292–301
Tripathi A, Chaube SK (2012) High level of cytosolic free calcium signals apoptosis through the mitochondria-caspase mediated pathway in rat eggs cultured in vitro. Apoptosis 17:439–448
Agarwal A, Gupta S, Sharma R (2005) Oxidative stress and its implications in female infertility—a clinician’s perspective. Reprod Biomed Online 11:641–650
Fujii J, Iuchi Y, Okada F (2005) Fundamental roles of reactive oxygen species and protective mechanisms in the female reproductive system. Reprod Biol Endocrinol 3:43–52
Kikuchi K, Naito K, Noguchi J et al (2002) Maturation/M-phase promoting factor regulates aging of porcine oocytes matured in vitro. Cloning Stem Cells 4:211–222
Tatone C, Carbone MC, Gallo R et al (2006) Age-associated changes in mouse oocytes during postovulatory in vitro culture: possible role for meiotic kinases and survival factor Bcl2. Biol Reprod 74:395–402
Suzukamo C, Hoshina M, Moriya H et al (2009) Kinetics of nuclear status and kinase activities during in vitro maturation of canine oocytes. J Reprod Dev 55:116–120
Lord T, Aitken RJ (2013) Oxidative stress and ageing of the post-ovulatory oocyte. Reproduction 146:R217–R227
Myers M, Morgan FH, Liew SH et al (2014) PUMA regulates germ cell loss and primordial follicle endowment in mice. Reproduction 148:211–219
Terranova PF, Tayler CC (1999) Apoptosis (cell death). In: Neil JD, Knobil E (eds) Encyclopedia of reproduction. Academic Press, New York, pp 261–273
Jurisicova A, Acton BM (2004) Deadly decisions: the role of genes regulating programmed cell death in human preimplantation embryo development. Reproduction 128:281–291
Zhang X, Li XH, Ma X et al (2006) Redox-induced apoptosis of human oocytes in resting follicles in vitro. J Soc Gynecol Investig 13:451–458
Perez GI, Tao XJ, Tilly JL (1999) Fragmentation and death (a.k.a. apoptosis) of ovulated oocytes. Mol Hum Reprod 5:414–420
Aitken RJ, Findlay JK, Hutt KJ et al (2011) Apoptosis in the germ line. Reproduction 141:139–150
Kelkar RL, Dharma SJ, Nandedkar TD (2003) Research expression of Fas and Fas ligand protein and mRNA in mouse oocytes and embryos. Reproduction 126:791–799
Liu L, Trimarchi JR, Keefe DL (2000) Involvement of mitochondria in oxidative stress-induced cell death in mouse zygotes. Biol Reprod 62:1745–1753
Martin MC, Allan LA, Lickrish M et al (2005) Protein kinase A regulates caspase-9 activation by Apaf-1 downstream of cytochrome c. J Biol Chem 280:15449–15455
Roth Z, Hansen PJ (2004) Involvement of apoptosis in disruption of developmental competency of bovine oocytes by heat shock during maturation. Biol Reprod 71:1898–1906
Hao Y, Lai L, Mao J et al (2004) Apoptosis in parthenogenetic preimplantation porcine embryos. Biol Reprod 70:1644–1649
Li HJ, Wang CY, Mi Y et al (2013) FasL-induced apoptosis in bovine oocytes via the Bax signal. Theriogenology 80:248–255
Chaube SK, Prasad PV, Khillare B et al (2006) Extract of Azadirachta indica (Neem) leaf induces apoptosis in rat oocytes cultured in vitro. Fertil Steril 85:1223–1231
Tripathi A, Shrivastav TG, Chaube SK (2012) Aqueous extract of Azadirachta indica (Neem) leaf induces generation of reactive oxygen species and mitochondria-mediated apoptosis in rat oocytes. J Asst Reprod Genet 29:15–23
Mooyottu S, Anees C, Cherian S (2011) Ovarian stem cells and neo-oogenesis: a breakthrough in reproductive biology research. Vet World 4:89–91
Gheorghisan-Galateanu AA, Hinescu ME, Enciu AM (2014) Ovarian adult stem cells: hope or pitfall? J Ovarian Res 7:71
Acknowledgments
The part of this study was funded by Department of Science and Technology, Ministry of Science and Technology, Government of India.
Conflict of interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tiwari, M., Prasad, S., Tripathi, A. et al. Apoptosis in mammalian oocytes: a review. Apoptosis 20, 1019–1025 (2015). https://doi.org/10.1007/s10495-015-1136-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-015-1136-y