Abstract
In recent years, monitoring of airborne bacteria and fungi concentrations has obtained increasing universal attraction not only for influences on ecological balance but also for evaluating their public health consequences. In this study, we aimed to investigate culturable airborne bacteria and fungi levels in different sites of Abadan, and their association with meteorological parameters and PM2.5 levels. Abadan is one of the most industrialized cities in the southwest of Iran where over the current decade has experienced lots of dust storm episodes. In total, 400 air samples were collected in 6 months (autumn and winter) using a single-stage viable Andersen cascade impactor for sampling airborne bacteria and fungi and portable DustTrak Aerosol Monitor 8520 for measuring PM2.5 concentrations and meteorological parameters. Microbial concentrations showed a significant difference between various sites over the study period with averages of 569.57 ± 312.64 and 482.73 ± 242.86 CFU/M3 for bacteria and fungi, respectively. The air temperature had a significant effect on the concentration of both airborne bacteria and fungi. A significant positive correlation between relative humidity and fungi but no correlation between relative humidity and bacteria concentrations were observed. The average airborne PM2.5 concentrations of all sites among the study period was 93.24 ± 116.72 μg/m3. The atmospheric bacterial and fungal communities were strongly positively correlated with the ambient PM2.5 level. The levels of airborne bacteria and fungi along with PM2.5 in the air of the city were relatively higher than the recommended levels. Therefore, the best course of action is needed to control emission sources. Further studies are also needed to evaluate the clinical analysis of the health effects of exposure to these pollutants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In recent years with the development of industrialization and increasing environmental problems such as dust storms that occurred frequently over southwestern Iran, air pollution has become more serious in this area and has raised government and public concerns (Soleimani et al., 2013). A huge mass of dust can move long distances and carry away many airborne pollutants (Kellogg & Griffin, 2006). Among them, suspended particulate matter plays an important role in the air pollution of this area and has adverse effects on human health (Niu et al., 2010; Shahsavani et al., 2012). Fine particulate matters (PM2.5; particulate matters with an aerodynamic diameter ≤ of 2.5 µm), due to their fine size and leaning to stay for a long time in the air, have a big chance to penetrate deep into the lungs and enter into the bloodstream and tissues, causing respiratory and vascular diseases (Franzetti et al., 2011; Liu et al., 2018). Along with physical and chemical components, biological elements also exist in particulate matter that are defined as bioaerosols (). Against the vast amount of information about chemical components of atmospheric particulate matter, few studies focused on the biological composition of ambient air. Over the past decades, an increase in awareness about their hazards has promoted an increasing interest in knowing more about the bioaerosols (Genitsaris et al., 2017; Xu et al., 2017).
Bioaerosols are omnipresent all over the world, either as an individual organism or attached to the surface of other non-biological particles (Innocente et al., 2017; Zhen et al., 2017). These atmospheric aerosols largely drive from natural sources, such as soils, water, plants, leaf surface, and animal and human faeces. The main composition of them are bacteria, fungi, viruses and pollen (Brągoszewska & Pastuszka, 2018; Du et al., 2018a, 2018b, 2018c) that ranges from 0.1 to 100 µm in diameter and thousands to millions of cells per cubic meter of air (Brągoszewska & Pastuszka, 2018; Innocente et al., 2017; Xu et al., 2017; Zhen et al., 2017). Some investigations reported that bioaerosols comprise up to 25 weight per cent of the total atmospheric aerosols, and another study reported that 5–10% of the total suspended particulate mass is bioaerosols (Cao et al., 2014; Du et al., 2018a, 2018b, 2018c; Franzetti et al., 2011; Li et al., 2017).
Studies have shown that bioaerosols are prominent in the surrounding environment, can influence ecological balance, atmospheric processes, and contribute to the formation of clouds and condensation nuclei (Du et al., 2018a, 2018b, 2018c; Hurtado et al., 2014). On the other hand, bioaerosols can put human health in danger through the release of some pathogenic bacteria and fungi from different sources. Exposure to the bioaerosols, depending on their biological and physical properties, chemical composition and size distribution, deposition site and inhaled quantity in the respiratory tract, can lead to some health effects such as eye, skin and urinary tract infection, respiratory diseases, and increased allergen burden leading to an increased incidence of asthma and rust disease in plants (Maki et al., 2013; Maron et al., 2005). Bioaerosols not only affect the community living nearby their sources of emission but also can move farther through air transport and influence other populations (Brodie et al., 2007; Degobbi et al., 2011; Gao et al., 2016). However, considering their potential risks, there is no formally regulated standard for bioaerosols levels in the ambient air by now. Most of the investigations have focused on specific sources such as occupational environment, wastewater treatment plants, and dust events, but less is known about the ambient natural microbial community in urban areas (Chen et al., 2012; Du et al., 2018a, 2018b, 2018c; Raisi et al., 2010).
In the present study, we focused on culturable bacteria and fungi because of their high interactions with other air pollutants and sensitivity to meteorological parameters, such as air temperature, etc. So, we attempted to investigate the concentration of atmospheric culturable bacteria and fungi in relation to ambient PM2.5, location, dates and meteorological factors in different sites of Abadan city over 6 months (autumn and winter). To the best of our knowledge, so far, there is no study on outdoor bioaerosols concentration and the effect of meteorological factors and ambient PM2.5 on their concentration conducted in this area of Iran. This study can provide references for a better understanding of outdoor air quality in industrialized and dusty areas which is useful in emission control planning and health risk assessment studies.
2 Material and methods
2.1 Study area and sampling sites
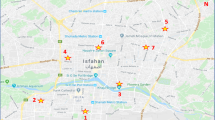
The study was carried out in Abadan, a city of Khuzestan province, southwest of Iran located at 30,339,167° north and 48,304,167° east, with a population of approximately 231,500 people. Abadan has a desert climate with mild, dry winter (16–20 °C) and warm, wet summer (around 45 °C) and experiences a few sand and dust storms per year. The prevailing wind direction is most often from the north (25.4%) and northwest (40.2%) with average wind speeds of 10.1 mph, which is maximum in June and minimum in October. The whole area is flat but some elevated features are created artificially in some area. Two rivers named Arvandrud and Bahmanshir estuaries of Karun river flow through the city and down to 50 km join to the Persian Gulf. The vegetation area is mostly located around the coastal plain of these rivers. Abadan is an industrial city having a large oil refinery site and petrochemical complex located at the heart of the city. Ambient air samples were collected from 10 representative sites selected according to different characteristics that are shown in Table 1, and such a way to cover all areas of the city (Fig. 1). Sampling was conducted for 20 non-consecutive days, each day from 10 cities, from October 2017 to March 2018 (6 months).
2.2 Air sampling methods for bioaerosols
The samples were collected with a single-stage viable Andersen cascade impactor (MAS 100 Merck) linked to a vacuum pump (SKC MCS Flite; 0–20 l/min) with an average flow rate of 12 l/min, calibrated by a rotameter, for 2–3 min sampling time. The ambient air sampling set was located on a stand 1.5 m above ground level as a representative of human respiratory height and at a distance of at least 2 m away from nearby physical barriers (Fang et al., 2005; Wu et al., 2000). The sampler with tube linings was disinfected with ethanol 70% and air-dried before each sample collection. In total 400 air samples (200 samples for bacteria and 200 samples for fungi) were collected among the 6 months. For stability, all samples were collected during the day at times between 9:00 to 15:00. The culture plates were transported to the site and then to the laboratory by a cold box.
2.3 Quantification of culturable bacteria and fungi
The air samples were directed into agar Petri dishes located in the impactor, one containing Tryptone Soya Agar (TSA) media for the culturing of bacteria and other containing Sabouraud Dextrose Agar (SDA) media for fungal culture. Cycloheximide (500 mg/L) was used to inhibit fungal growth in the bacteria culture media while chloramphenicol (100 mg/L) was used to suppress bacteria growth in the fungal culture media. After wrapping samples with sterilized aluminium foil, the specimens were brought back to the laboratory in a cold box and incubated at 35 ± 0.5 °C for 28–48 h for the bacteria samples, and at 25 ± 0.5 °C for 72 h for the fungal samples (Fang et al., 2005; Haas et al., 2014). The total numbers of bacteria and fungi in the ambient air were determined as colony-forming units per cubic meter of air (CFU/m3) using the positive-hole correction method based on the following formula:
where T is the number of bacteria or fungi colonies, t (min) is the duration of sampling, F (l/min) is the pump flowrate and 1000 is the conversion factor of litre to the cubic meter.
2.4 Measurement of PM2.5 and meteorological parameters
In addition to bacteria and fungi sampling of ambient air in each selected site, we measured airborne PM2.5 concentrations in the same point by a portable DustTrak Aerosol Monitor (Model TSI-8520, Italy). Relative humidity and temperature also were measured at the same time by TSI-8520 digital during the sampling period (Fang et al., 2008). The other meteorological parameters such as wind velocity and wind direction data were obtained from the Isfahan Weather Forecast Organization and included in the study.
2.5 Statistical analysis
One-way analysis of variance (ANOVA) was carried out to compare means in different sites. To find out the normality of the data, the one-sample Kolmogorov–Smirnov test was used. Nonparametric Spearman’s rank correlation analysis was employed to evaluate the relationship between the concentration of airborne bacteria and fungi and meteorological parameters, as well as PM2.5. A p-value of < 0.05 was considered as a significant difference for a confidence level of 95%. IBM SPSS ver. 24.0 was used for all the data analysis.
3 Results
3.1 Spatial variation of airborne bacteria and fungi concentrations in the city
The mean concentration of bacteria and fungi at different sampling sites during the study period are presented as CFU/m3 in Table 2. There were extreme differences in the concentration of the bioaerosols in the air of all sites, while the highest level of airborne bacteria and fungi were detected at sites 1, amounting to 878.81 ± 676.81 and 706.05 ± 479.94 CFU/m3, respectively. The lowest concentration of bacteria and fungi were measured at site 8, accounting for 312.12 ± 208.12 and 272.72 ± 162.14 CFU/m3, respectively. Figure 2 illustrates the concentration of airborne bacteria and fungi in representative sites during the sampling period. The microbial content of ambient air displayed a significant difference between different sites of Abadan (p < 0.05).
3.2 Temporal variation of airborne bacteria and fungi concentrations during the study period
The variation of airborne bacteria and fungi during the sampling period are shown in Table 3. The mean concentration of airborne bacteria and fungi for the entire study period were 569.57 ± 312.64 and 482.73 ± 242.86 CFU/m3, respectively. The corresponding higher mean value for bacteria was in December with 1287.87 CFU/m3 and that for fungi, was in January with 909.09 CFU/m3, whereas the lowest concentration of bacteria and fungi was in November with 127.27 and 136.36 CFU/m3, respectively. The mean concentration of airborne bacteria was higher than fungi. In addition, there was a significant correlation between the concentration of airborne bacteria and fungi (p < 0.01). Also, as can be seen in Figs. 3 and 4 the frequency and the dispersion of total bacteria and fungi during the sampling period did not show a regular trend. However, a higher concentration of both bacteria and fungi was detected in December and January.
3.3 Association of airborne bacteria and fungi levels with PM2.5 and meteorological factors
Table 4 illustrates the averages of PM2.5 concentrations in the sampling sites during the study period. The mean concentration of PM2.5 in the ambient air over the study period was 93.24 ± 14.05 µg/m3 with the maximum level of 504.95 µg/m3 in January and the minimum level of 10.94 µg/m3 in November. As demonstrated in Table 5, there was a strong correlation between the concentration of bacteria and fungi, and PM2.5 (p < 0.05). Among all the measured factors, PM2.5 was the most correlated factor with the concentration of airborne bacteria and fungi. Figures 5 and 6 also present the association between airborne bacteria and fungi with PM2.5 concentrations, respectively.
The meteorological variables in the dates of sampling are shown in Table 4. The air temperature was in a range of 38.6–16.0 °C (average: 23.28 °C), with the highest in October and the lowest in December. The average relative humidity was 45%, the highest was 72% occurring in November and the lowest was 22% cast in February. Furthermore, the average wind velocity was 3.7 m/s within a range of 0–15 m/s with a maximum average of 5.3 ± 0.64 m/s. The Spearman correlation coefficients between the concentration of airborne bacteria and fungi versus temperature, relative humidity, and wind velocity are shown in Table 5. There was a significant negative correlation between temperature and bacteria, and temperature and fungi concentrations (p < 0.05). Whereas, significant positive correlation between relative humidity and fungi concentration was found (p < 0.05), while there was no correlation between relative humidity and bacteria concentration (p > 0.05). In addition, the wind speed showed no statistically significant correlation with both bacteria and fungi concentration (p > 0.05).
4 Discussion
Bacteria and fungi concentrations were alterable by sites. In site 1 the concentrations were approximately triple times higher than those in site 8. Moreover, higher bacteria and fungi concentrations were detected in sites 10 and 4, respectively. Fang et al. found a positive correlation between ambient bioaerosols and human activities, population density and region type (Fang et al., 2005). A possible reason for the high bioaerosol levels of these sites may be their release from domestic and industrial wastewaters produced in the city and discharged to the rivers near these sites. Whereas, lower microbial concentration was detected in the air near the green lands where has relatively clean air. In addition, the plants can decrease the concentration of bioaerosols by their volatile products (Fang et al., 2008). High airborne bacterial and fungal concentrations were also observed in sites 5 and 6, the downtown, populated and industrial zones. In a study in Mexico, airborne microbial levels varied from an average of 230 ± 130 CFU/m3 in the coastal reference site to an average of 40,100 ± 21.689 CFU/m3 in the Tijuana River valley. The highest concentration has been reported near the populated river area with relatively intense human and animal activities (Hurtado et al., 2014). In contrast, we found a significant concentration of bacteria and fungi around the industrial regions. The concentrations we reported here for the sites located near the suburban and low polluted areas followed the study results in Guangzhou, China (Chen et al., 2012). However, Rodríguez et al. evaluated the temporal variability of PM2.5 and its associated microorganisms in the central Iberian Peninsula and found no temporal differences in microbial counts (Rodríguez et al., 2020).
Several studies have been conducted to quantify the level of airborne bacteria and fungi in different areas, however, we could not find any recommendation or standard for their level in the ambient air. Our study intended to supply initial data in this field and to compare them with similar studies conducted worldwide. The mean values of airborne bacteria and fungi detected in this study were nearly similar to the reports of Li et al. (bacteria: 565 ± 464 and fungi: 399 ± 371 CFU/m3) (Li et al., 2017), Du et al. (bacterial: 20–383 and fungal: 60–930 CFU/m3) (Du et al. 2018a; 2018b; 2018c), Wang et al. (bacteria: 440 ± 77 CFU/m3) (Wang et al., 2012) and Bragoszewska and Pastuszka (bacterial: 50–300 CFU/m3) (Brągoszewska & Pastuszka, 2018). However, we detected different ranges compared to the reports of Gao et al. (mean viable bacteria: 1110 ± 976 and fungi: 948 ± 978 CFU/m3) (Gao et al., 2016) and O’Gorman et al. (mean viable fungi: 915 CFU/m3) (O’Gorman & Fuller, 2008). There were no obvious reasons for these differences, but they may correspond with different experimental and sampling approaches, different types of culture media (bacterial and fungal growth substrate), geographic location, and mainly various meteorological and environmental conditions in these regions (Shaffer & Lighthart, 1997).
Given the geographic and meteorological conditions, Khuzestan, especially Abadan city experiences a long period of hot climate (March-October). The average temperature in this period of study was 23.27 ± 1.43 °C. Previous studies have shown that temperature over 24 °C decreases growth and survival of gram-positive and gram-negative bacteria, such as Pseudomonas, Salmonella and Escherichia (Handley & Webster, 1993). Thus, dry weather and strong solar radiation on high-temperature days can increase the die-off rate of airborne bacteria and fungi, and significantly affect their culturability (Li et al., 2017). Xu AL et al. reported that unfavourable conditions for microorganisms in winter decrease their concentration in the atmosphere (Xu et al., 2017). Furthermore, a study in Silesia, Poland reported that the seasonally average concentration of culturable bacteria was maximum in the spring and minimum in the winter (Brągoszewska & Pastuszka, 2018).
The influence of relative humidity on bacteria and fungi concentrations is complicated. In this study, we could not find a significant correlation between bacteria concentration and relative humidity, while fungi showed a positive correlation. Desired temperature and relative humidity for the growth of fungi has been reported to be 19.4 ± 5.1 °C and 70.7 ± 12.5% (Li et al., 2017) Li et al. also reported a high concentration of airborne fungi in the autumn which is following our study; whereas, the death ratios of bacteria was increased with increasing relative humidity (Li et al., 2017). Another study in Sao Paulo reported a negative correlation between the ambient level of colourless ascosporic fungi and humidity and suggested that low humidity associated with heavy traffic could suspend the road dust and be the reason for high aerosolization of the crustal elements, therefore increasing the release of bioaerosols from vegetation and soil (Degobbi et al., 2011).
In the present study, the concentration of ambient PM2.5 ranged from 10.94 to 504.95 µg/m3, and with increasing PM2.5 level the concentration of ambient bacteria and fungi were increased. The results showed that the bacteria and fungi are a significant portion of the PM2.5 (p < 0.05). As presented in Table 4, the maximum concentration of bacteria and fungi was obtained in the days that PM2.5 concentrations were higher, amounting to 252.3 and 504.95 µg/m3. Studying temporal variability of PM2.5 and its associated microorganisms in the central Iberian Peninsula positive correlations between PM2.5 and relative humidity, and PM2.5 and cultivable microorganisms have been reported (Rodríguez et al., 2020). A consistent trend in the proportion of ambient bacteria and fungi with haze degree during the study were also observed. Water bodies, soil, plants, and anthropogenic activities are recognized as the primary sources of airborne bioaerosols (Xie et al., 2021). Under the unforeseen condition, due to the intense deficit of vegetation and neighbouring to the desert area in Khuzestan, Iraq and Saudi Arabia, episodes of dust storms occur periodically in Abadan (Goudarzi et al., 2014). This behaviour mainly caused bacteria and fungi to attach to the surface of suspended particulate matter. Joen et al. reported that each gram of desert soil may include a large number (about 109) of bacterial cells (Jeon et al., 2011). Liu et al. reported that the abundance of pathogenic bacteria was highest in heavily polluted air (Liu et al., 2018). Following our findings, Goudarzi et al. maintained that the greatest bacterial concentrations were observed during dust events compared with normal days (Goudarzi et al., 2014). Also, Li et al. observed the highest value of airborne viable microbes on hazy days and the lowest on rainy days (Li et al., 2017). In contrast, Gao et al. concluded that on hazy days the concentrations of airborne bacteria were low because the compositions of heavily polluted and hazy air might cause damage to the total bacteria (Gao et al., 2015). These findings provide essential information in exposure control programs and health risk assessment, however, further studies are needed to understand the source apportionment, atmospheric diffusion and transport pathways of bioaerosols.
5 Conclusion
In this study, we investigated the relationships between the concentration of culturable airborne bacteria and fungi and meteorological parameters and atmospheric PM2.5. The concentrations of airborne bacteria and fungi, as well as the ambient PM2.5, were relatively high in the air of the studied area compared to the worldwide similar studies, especially in the cold months. The results indicated that the community of airborne bacteria and fungi were more affected by temperature variation than the other meteorological parameters. Also, it seems to be a significant relationship between the concentration of fungi and relative humidity, while there is no meaningful correlation between bacterial concentration and relative humidity. Moreover, the bacterial and fungal concentrations were significantly correlated to the level of PM2.5 concentrations. These findings provide appropriate information for exposure mitigation programs and health risk assessment.
References
Brągoszewska, E., & Pastuszka, J. S. (2018). Influence of meteorological factors on the level and characteristics of culturable bacteria in the air in Gliwice, Upper Silesia (Poland). Aerobiologia, 34(2), 241–255. https://doi.org/10.1007/s10453-018-9510-1
Brodie, E. L., DeSantis, T. Z., Moberg Parker, J. P., Zubietta, I. X., Piceno, Y. M., & Andersen, G. L. (2007). Urban aerosols harbor diverse and dynamic bacterial populations. Proceedings of the National Academy of Sciences of the United States of America, 104(1), 299–304. https://doi.org/10.1073/pnas.0608255104
Cao, C., Jiang, W., Wang, B., Fang, J., Lang, J., Tian, G., et al. (2014). Inhalable microorganisms in Beijing’s PM2.5 and PM10 pollutants during a severe smog event. Environmental Science and Technology, 48(3), 1499–1507. https://doi.org/10.1021/es4048472
Chen, X., Ran, P., Ho, K., Lu, W., Li, B., Gu, Z., et al. (2012). Concentrations and size distributions of airborne microorganisms in guangzhou during summer. Aerosol and Air Quality Research, 12(6), 1336–1344. https://doi.org/10.4209/aaqr.2012.03.0066
Degobbi, C., Lopes, F. D. T. Q. S., Carvalho-Oliveira, R., Muñoz, J. E., & Saldiva, P. H. N. (2011). Correlation of fungi and endotoxin with PM2.5 and meteorological parameters in atmosphere of Sao Paulo, Brazil. Atmospheric Environment, 45(13), 2277–2283. https://doi.org/10.1016/j.atmosenv.2010.12.005
Du, P., Du, R., Lu, Z., Ren, W., & Fu, P. (2018a). Variation of bacterial and fungal community structures in PM2.5 collected during the 2014 APEC summit periods. Aerosol and Air Quality Research, 18(2), 444–455. https://doi.org/10.4209/aaqr.2017.07.0238
Du, P., Du, R., Ren, W., Lu, Z., & Fu, P. (2018b). Seasonal variation characteristic of inhalable microbial communities in PM2.5 in Beijing city, China. Science of the Total Environment, 610–611, 308–315. https://doi.org/10.1016/j.scitotenv.2017.07.097
Du, P., Du, R., Ren, W., Lu, Z., Zhang, Y., & Fu, P. (2018c). Variations of bacteria and fungi in PM2.5 in Beijing, China. Atmospheric Environment, 172, 55–64. https://doi.org/10.1016/j.atmosenv.2017.10.048
Fang, Z., Ouyang, Z., Hu, L., Wang, X., Zheng, H., & Lin, X. (2005). Culturable airborne fungi in outdoor environments in Beijing, China. Science of the Total Environment, 350(1–3), 47–58. https://doi.org/10.1016/j.scitotenv.2005.01.032
Fang, Z., Ouyang, Z., Zheng, H., & Wang, X. (2008). Concentration and Size distribution of culturable airborne microorganisms in outdoor environments in Beijing, China. Aerosol Science and Technology, 42(5), 325–334. https://doi.org/10.1080/02786820802068657
Franzetti, A., Gandolfi, I., Gaspari, E., Ambrosini, R., & Bestetti, G. (2011). Seasonal variability of bacteria in fine and coarse urban air particulate matter. Applied Microbiology and Biotechnology, 90(2), 745–753. https://doi.org/10.1007/s00253-010-3048-7
Gao, M., Qiu, T., Jia, R., Han, M., Song, Y., & Wang, X. (2015). Concentration and size distribution of viable bioaerosols during non-haze and haze days in Beijing. Environmental Science and Pollution Research, 22(6), 4359–4368.
Gao, M., Yan, X., Qiu, T., Han, M., & Wang, X. (2016). Variation of correlations between factors and culturable airborne bacteria and fungi. Atmospheric Environment, 128, 10–19. https://doi.org/10.1016/j.atmosenv.2015.12.008
Genitsaris, S., Stefanidou, N., Katsiapi, M., Kormas, K. A., Sommer, U., & Moustaka-Gouni, M. (2017). Variability of airborne bacteria in an urban Mediterranean area (Thessaloniki, Greece). Atmospheric Environment, 157, 101–110. https://doi.org/10.1016/j.atmosenv.2017.03.018
Goudarzi, G., Shirmardi, M., Khodarahmi, F., Hashemi-Shahraki, A., Alavi, N., Ankali, K. A., et al. (2014). Particulate matter and bacteria characteristics of the Middle East Dust (MED) storms over Ahvaz, Iran. Aerobiologia, 30(4), 345–356. https://doi.org/10.1007/s10453-014-9333-7
Haas, D., Habib, J., Luxner, J., Galler, H., Zarfel, G., Schlacher, R., et al. (2014). Comparison of background levels of culturable fungal spore concentrations in indoor and outdoor air in southeastern Austria. Atmospheric Environment, 98, 640–647. https://doi.org/10.1016/j.atmosenv.2014.09.039
Handley, B. A., & Webster, A. J. F. (1993). Some factors affecting airborne survival of Pseudomonas fluorescens indoors. Journal of Applied Bacteriology, 75(1), 35–42. https://doi.org/10.1111/j.1365-2672.1993.tb03404.x
Hurtado, L., Rodríguez, G., López, J., Castillo, J. E., Molina, L., Zavala, M., & Quintana, P. J. E. (2014). Characterization of atmospheric bioaerosols at 9 sites in Tijuana, Mexico. Atmospheric Environment, 96, 430–436. https://doi.org/10.1016/j.atmosenv.2014.07.018
Innocente, E., Squizzato, S., Visin, F., Facca, C., Rampazzo, G., Bertolini, V., et al. (2017). Influence of seasonality, air mass origin and particulate matter chemical composition on airborne bacterial community structure in the Po Valley, Italy. Science of the Total Environment, 593–594, 677–687. https://doi.org/10.1016/j.scitotenv.2017.03.199
Jeon, E. M., Kim, H. J., Jung, K., Kim, J. H., Kim, M. Y., Kim, Y. P., & Ka, J. O. (2011). Impact of Asian dust events on airborne bacterial community assessed by molecular analyses. Atmospheric Environment, 45(25), 4313–4321. https://doi.org/10.1016/j.atmosenv.2010.11.054
Kellogg, C. A., & Griffin, D. W. (2006). Aerobiology and the global transport of desert dust. Trends in Ecology and Evolution. https://doi.org/10.1016/j.tree.2006.07.004
Li, Y., Lu, R., Li, W., Xie, Z., & Song, Y. (2017). Concentrations and size distributions of viable bioaerosols under various weather conditions in a typical semi-arid city of Northwest China. Journal of Aerosol Science, 106, 83–92. https://doi.org/10.1016/j.jaerosci.2017.01.007
Liu, H., Zhang, X., Zhang, H., Yao, X., Zhou, M., Wang, J., et al. (2018). Effect of air pollution on the total bacteria and pathogenic bacteria in different sizes of particulate matter. Environmental Pollution, 233, 483–493. https://doi.org/10.1016/j.envpol.2017.10.070
Maki, T., Kakikawa, M., Kobayashi, F., Yamada, M., Matsuki, A., Hasegawa, H., & Iwasaka, Y. (2013). Assessment of composition and origin of airborne bacteria in the free troposphere over Japan. Atmospheric Environment, 74, 73–82. https://doi.org/10.1016/j.atmosenv.2013.03.029
Maron, P. A., Lejon, D. P. H., Carvalho, E., Bizet, K., Lemanceau, P., Ranjard, L., & Mougel, C. (2005). Assessing genetic structure and diversity of airborne bacterial communities by DNA fingerprinting and 16S rDNA clone library. Atmospheric Environment, 39(20), 3687–3695. https://doi.org/10.1016/j.atmosenv.2005.03.002
Niu, J., Rasmussen, P. E., Wheeler, A., Williams, R., & Chénier, M. (2010). Evaluation of airborne particulate matter and metals data in personal, indoor and outdoor environments using ED-XRF and ICP-MS and co-located duplicate samples. Atmospheric Environment, 44(2), 235–245. https://doi.org/10.1016/j.atmosenv.2009.10.009
O’Gorman, C. M., & Fuller, H. T. (2008). Prevalence of culturable airborne spores of selected allergenic and pathogenic fungi in outdoor air. Atmospheric Environment, 42(18), 4355–4368. https://doi.org/10.1016/j.atmosenv.2008.01.009
Raisi, L., Lazaridis, M., & Katsivela, E. (2010). Relationship between airborne microbial and particulate matter concentrations in the ambient air at a Mediterranean site. In Global nest journal (Vol. 12, pp. 84–91). doi:https://doi.org/10.30955/gnj.000694
Rodríguez, A., Seseña, S., Sánchez, E., Rodríguez, M., Palop, M. L., del Rodríguez Martín-Doimeadios, R. C., & Rodríguez Fariñas, N. (2020). Temporal variability measurements of PM2.5 and its associated metals and microorganisms on a suburban atmosphere in the central Iberian Peninsula. Environmental Research, 191, 110220. https://doi.org/10.1016/j.envres.2020.110220
Shaffer, B. T., & Lighthart, B. (1997). Survey of culturable airborne bacteria at four diverse locations in Oregon: Urban, rural, forest, and coastal. Microbial Ecology, 34(3), 167–177. https://doi.org/10.1007/s002489900046
Shahsavani, A., Naddafi, K., Haghighifard, N. J., Mesdaghinia, A., Yunesian, M., Nabizadeh, R., et al. (2012). The evaluation of PM10, PM2. 5, and PM1 concentrations during the Middle Eastern Dust (MED) events in Ahvaz, Iran, from april through september 2010. Journal of Arid Environments, 77, 72–83.
Soleimani, Z., Goudarzi, G., Naddafi, K., Sadeghinejad, B., Latifi, S. M., Parhizgari, N., et al. (2013). Determination of culturable indoor airborne fungi during normal and dust event days in Ahvaz, Iran. Aerobiologia, 29(2), 279–290. https://doi.org/10.1007/s10453-012-9279-6
Wang, W., Ma, Y., Ma, X., Wu, F., Ma, X., An, L., & Feng, H. (2012). Diversity and seasonal dynamics of airborne bacteria in the Mogao Grottoes, Dunhuang, China. Aerobiologia, 28(1), 27–38. https://doi.org/10.1007/s10453-011-9208-0
Wu, P. C., Su, H. J. J., & Ho, H. M. (2000). A comparison of sampling media for environmental viable fungi collected in a hospital environment. Environmental Research, 82(3), 253–257. https://doi.org/10.1006/enrs.1999.4017
Xie, W., Li, Y., Bai, W., Hou, J., Ma, T., Zeng, X., et al. (2021). The source and transport of bioaerosols in the air: A review. Frontiers of Environmental Science & Engineering, 15(3), 1–19.
Xu, A. L., Song, Z. W., Lang, X. L., Chen, X., & Xia, Y. (2017). Seasonal variability in bacterial and fungal diversity and community composition of the near-surface atmosphere in coastal megacity. Aerobiologia, 33(4), 555–575. https://doi.org/10.1007/s10453-017-9489-z
Zhen, Q., Deng, Y., Wang, Y., Wang, X., Zhang, H., Sun, X., & Ouyang, Z. (2017). Meteorological factors had more impact on airborne bacterial communities than air pollutants. Science of the Total Environment, 601–602, 703–712. https://doi.org/10.1016/j.scitotenv.2017.05.049
Acknowledgements
This research was supported by the research chancellery of Isfahan University of Medical Sciences (Grant no: 396576). The collaboration of Jondishapur University of Medical Sciences is greatly acknowledged.
Funding
Isfahan University of Medical Sciences, Grant No: 396576.
Author information
Authors and Affiliations
Contributions
The individual contributions of authors to the manuscript are specified as follows: YH: Supervision, Methodology, Data curation; GG: Conceptualization, Methodology, Supervision, HT: Writing—Review and Editing; FK: Investigation, Methodology, Writing—Original Draft; MH: Analytical assistance. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
Not applicable.
Consent for publication
Not applicable.
Data availability
All the necessary data generated or analysed during this study are included in this published article. However, the datasets for statistical analysis are available from the corresponding author on reasonable request.
Rights and permissions
About this article
Cite this article
Keishams, F., Goudarzi, G., Hajizadeh, Y. et al. Influence of meteorological parameters and PM2.5 on the level of culturable airborne bacteria and fungi in Abadan, Iran. Aerobiologia 38, 233–245 (2022). https://doi.org/10.1007/s10453-022-09744-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10453-022-09744-9