Abstract
The presence of bioaerosols in the airborne fine particulate matters (PM2.5) has raised public concern due to their pathogenic effects on human. The objective of this study was to investigate the levels of airborne bacteria and fungi and their association with PM2.5 and meteorological parameters in Isfahan. Air samples were collected from seven different locations of Isfahan city using SKC pumps connected to glass impingers containing a buffer solution and analyzed by the cell culture method. The meteorological variables were obtained from the provincial meteorological office, and the data of atmospheric PM2.5 were taken from the Department of Environment. The highest and the lowest bacterial levels were found at station 7 (114.4 (40–254.2) CFU/m3) and station 3 (39.6 (12–67.5) CFU/m3), respectively. While, the highest and the lowest fungal levels were observed at station 5 (61.1 (22.5–117)) and station 4 (26.9 (0–50)) CFU/m3, respectively. The most predominant bacteria were Gram-positive bacilli, and that for fungi were Dematiaceous and Yeast. The microbial levels varied between various months and the mean concentration of 76 CFU/m3 for bacteria and 46 CFU/m3 for fungi were obtained. The average of airborne PM2.5 concentrations was 25 µg/m3, with mean maximum occurring in the cold season. A positive association between PM2.5 and bioaerosols levels were observed, while the correlation between PM2.5 and meteorological parameters was not significant (P < 0.05). Air temperature significantly impacted both the airborne bacterial and fungal concentration (P < 0.05). Also, there was a strong correlation between the bacteria level and wind speed (P < 0.05).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Urbanization and industrialization have resulted in increasing use of fossil fuel in the industries and transportation leading to air pollution. Various industries with high pollutant emission rate have accentuated the problem of air pollution in Isfahan, one of the largest and most industrialized cities in Iran. There are two biggest thermal power plants, one is ‘Shahid Montezey power plant’ located in the north of Isfahan, producing 1600 MWh, and another is ‘Isfahan power plant’ placed in 75 km southwest of Isfahan, generating 954 MWh. Furthermore, there are two large iron and steel companies called ‘Isfahan steel company’ in the west and ‘Mobarakeh steel company’ in the southwest of Isfahan. Unfortunately, no proper air pollution control systems have been installed to the off-gas streams of the aforementioned plants. Besides, a cement plant in the west and an oil refinery in the north of Isfahan emit more air pollutants to the atmosphere (Rabiei et al. 2017). Another major source of pollutant emission is light, and heavy-duty vehicles using gasoline and diesel as fuel and traveling in the city. Majority of vehicles have no smoke-filter or catalyzer box installed in their exhaust. So, it is estimated that about one million vehicles and 1.5 million motorcycles are traveling in the city each day and producing near 80% of air pollution (Ghaffarpasand et al. 2020). Also, there are two large wastewater treatment plants located in the southeast and northeast of the city and a landfill and solid waste composting sites placed in the east. These plants along with sewerage collection network account for major sources of bioaerosols emission.
Atmospheric particulate matter, especially fine particle (PM2.5), has been regarded as one of the most important pollutants in reducing air quality (Niu et al. 2016). PM2.5 is a combination of various components including black carbon, ammonium, nitrates, organic matter, sulfates, plant debris and microbial matters. The microbial fraction of particulate matter which is called bioaerosols is 25% of the all aerosols in the air and can be extremely harmful to the atmospheric environment and human health (Du et al. 2018). Due to the lack of nutrients, the atmosphere is not considered as an appropriate inhabitant for microorganism to grow, however, microorganisms exist in the form of aerosol and suspended matters in air. Exposure to bioaerosols has been shown to have adverse effects on human health due to their inflammatory nature (Roy et al. 2020). Bioaerosols are made of a verity of biological-origin airborne particles consisting of microorganisms (bacteria, fungi and viruses) and the organic particles of microorganisms (endotoxin, metabolites, toxin, cell fragment). Due to low weight and small size of bioaerosols (ranging from 0.001 to 100 um), they can move from one environment to another (Mandal and Brandl 2011). Fungi and bacteria have been known as microbial damaging factors in indoor and outdoor air. The natural sources including fresh and marine water, soil, plants, animals and leaf surfaces, and anthropogenic sources such as farming, livestock breeding, wastewater treatment and composting facilities can release bioaerosols (Li et al. 2019; Xu et al. 2019). Human activities (e.g., coughing, washing and talking, walking, sneezing and sweeping floors) are also capable of generating bioaerosols. Owing to spread of some pathogenic bacteria and fungi from these sources, exposure to bioaerosols have contended to a wide range of adverse health effects and respiratory disorders, including infectious diseases, hypersensitivity pneumonitis, respiratory tract diseases, acute toxic effects, allergies, upper airway inflammation and cancer (Jahne et al. 2015). Evidence showed that these microorganisms are one of the most important factors for the biogeochemical cycle. Part of the fungi spores and bacteria that are airborne, can enter indoor areas through ventilation systems. About 10% of people show allergic respond to fungi in indoor and outdoor environments (Pasanen et al. 1996).
Bioaerosols have attracted much attention in recent years. By now, numerous studies have been carried out to quantify feature of airborne bacteria and fungi in indoor and outdoor environments of different places such as residential and occupational area (Mandal and Brandl 2011; Moon et al. 2014), public buildings (Lee et al. 2012; Stanley et al. 2008), solid waste disposal and wastewater treatment plant (Fathi et al. 2017; Nikaeen et al. 2009), educational environments and urban traffic area(Fang et al. 2008; Hussin et al. 2011), health care places (Hoseinzadeh et al. 2013; Park et al. 2013), and agriculture or food processing industries (Rajasekar and Balasubramanian 2011; Tsapko et al. 2011). These studies have attempted to explain that regional and seasonal changes greatly affect the distributions of sizes and concentrations of microorganisms, depending on biotic and abiotic factors, including the species of microorganisms, existing environmental conditions, vegetation, air pollution and human being activities (Li et al. 2017; Segvić Klarić and Pepeljnjak 2006). For example, the concentration of total airborne cultivable fungi ranged from 2.4 × 10 to 1.4×\({10}^{4}\) CFU/m3 in Beijing, China (Fang et al. 2008), While, the total airborne fungi concentration was in the ranges of 6455 CFU/m3 in Tehran, Iran (Shams-Ghahfarokhi et al. 2014). Research on bioaerosols has identified a tightly-coupled relationship between meteorological parameters (e.g., temperature, relative humidity and wind speed etc.) and their concentration in the air (Gao et al. 2016). However, according to the literature, there is no study investigating the amounts of bacterial and fungal aerosols in the ambient air of Isfahan and their association with the concentration of airborne PM2.5. The present study aimed to determine the concentration of bioaerosols including bacteria and fungi in the ambient air of Isfahan and their spatial and temporal changes from February to July 2018. The effect of meteorological parameters on the concentration of bioaerosols was also investigated.
2 Method and materials
2.1 Sampling locations
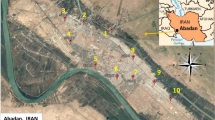
The study was carried out in Isfahan (39° 21′ and 33°9′ N latitude and 59° 39′ and 53°12′ E longitude, 1570 m above sea level) with 106,179 km2 area and a population of about 1.54 million which is located in the center of Iran and surrounded by Zagros mountains and desert. It is a semi-arid area based on its climate region with fairly cold and dry winters (Peel et al. 2007). The average annual temperature and rainfall typically fluctuate from 10–16 °C and 110–160 mm, respectively. The most rainfalls occur within 7 months of a year from November to May, with the highest in March and December. In this study, as there are seven active air pollution monitoring stations in the city, the air samples were collected from seven sites adjacent to these stations which include sites 1 (Azadi Sq.), 2 (Vahid St.), 3 (Khajoo St.), 4 (Alyadaran St.), 5 (Valiasr St.), 6 (Ostandari St.) and 7 (Ahmad Abad Sq). For selecting the sampling points, the following criteria were concerned: close to the air pollution monitoring stations, the urban traffic area, enough free space, distance from the chimneys and air extraction systems. Metrological variables such as UV index, ambient temperature, wind speed and direction and relative humidity (RH) were continuously obtained from the province Metrological Office. The airborne PM2.5 data of the seven monitoring stations were taken from Isfahan Department of Environment. The position of sampling sites and study area are shown in Fig. 1.
2.2 Sampling methods and cell cultivation
Air sampling was carried out using SKC pumps with a flow rate of 10 L/min, calibrated before application, connected to glass impingers containing 50 ml phosphate buffer solution. Samples were taken from the seven selected sites at a height of 1.5 m above the ground level, in the midday, twice each month, during six months from February to July 2018. During the study period, altogether 84 samples were collected. The sampling duration was adjusted such a way to pull approximately 1800 L of air into the impinger. All samples were transferred to the laboratory at 4 °C cold box and in isolated conditions. Then, shortly after arrival, all needed processes were applied to analyze the bioaerosols by cell culture method. For analysis of total bacteria and fungi, Tryptic soy agar (TSA) and Sabouraud dextrose agar (SDA) were used as the culture media, respectively. To suppress the growth of bacteria and inhibit fungal growth, Chloramphenicol and Nystatin were added to the SDA and TSA media, respectively. Upon arrival in the laboratory, 15 ml of each impinger content was poured into15 ml plastic falcon and centrifuged for 20 min at 6000 rpm. Then, the solution plated onto duplicated TSA growth media and SDA media. To determine the total number of bacteria and fungi, bacteria containing plates were incubated at 35–37 °C, and fungi cultured plates were kept at room temperature (25–30 °C). Bacterial colony formation was investigated after 48 h, the fungal colony was examined after 3–5 days, and the total number of colonies were reported. Grown colonies on TSA medium were directly macroscopically analyzed based on Gram-stained and morphological method, and calculated by colony counter forming units per cubic meter (CFU/m3) of air. Grown colonies on SDA medium were identified based on colony's type and other methods of morphological spores using a microscope under magnifications of 10.40.100 and measured as colony-forming units per cubic meter (CFU/m3) of air (Fathi et al. 2017; Mirhoseini et al. 2015, 2016).
2.3 Statistical analyses
Data management and analysis were performed using R software, version 3.5.1. The data were normalized using the Shapiro–Wilk test. Given the fact that the distribution of data was not normally obtained, the non-parametric method was applied. The Spearman correlation analysis was performed to address the relationship between airborne bacteria, fungi, PM2.5 and metrological parameters. The relationship between the average of bacteria and fungi concentrations within the different months was tested using Kruskal–Wallis test. A P-value smaller than 0.05 was considered as a statically significant difference. The ArcGIS software, version10.1 was also applied to identify the temporal and spatial variation of the mean airborne microbial concentration in the study area.
3 Results
3.1 Airborne bacterial and fungal concentration in various sites
Figure 2 illustrates and compares the average concentration of airborne bacteria and fungi and their variation ranges at different sampling sites during the study period. The highest mean bacterial concentration was obtained at sampling site 7 (114 CFU/m3) which is very crowded and high traffic area, and that at site 5 (92 CFU/m3) and site 6 (88 CFU/m3) ranked the rest. The lowest level of airborne bacteria was observed at site 3 (47 CFU/m3). In case of fungi, the highest mean fungal level was seen at sampling site 5 (61 CFU/m3) which is located in the northeast of Isfahan metropolis. Among the different sampling sites, similar to bacteria, mean fungal concentration in site 7 was the highest (57 CFU/m3), and site 6 ranked the second (55 CFU/m3). The lowest values of airborne fungi count were achieved in sampling site 4 (27 CFU/m3). However, there was no significant difference between the levels of bioaerosols over the various sampling sites (P > 0.05).
3.2 Variation of airborne bacterial and fungal levels during the study period
The bacterial mean concentrations varied widely among different months. Figure 3 compares the level of bacterial bioaerosols at different sampling sites from February to July 2018. The highest amount of airborne viable bacteria was observed in February (mean 116 CFU/m3, from 52 to 254 CFU/m3), and that in March ranked the second (mean 101 CFU/m3, from 35 to 190 CFU/m3); while the minimum amount of that was measured in June (mean 35 CFU/m3, from 13 to 61 CFU/m3). Overall, the concentration of airborne bacteria in cold months was higher than that in hot months. As shown in Fig. 4, the maximum concentration of fungal microorganisms also occurred in June (mean 79 CFU/m3, from 41 to 117 CFU/m3), followed by July (mean 48 CFU/m3, from 26 to 73 CFU/m3), and the lowest value occurred in March (mean 28 CFU/m3, from 0 to 59 CFU/m3). Statistics analysis showed that there was a positive correlation between bacteria and fungi values in February (P = 0.034, Rho = 0.82), while in the other months of study, no link was detected between fungal and bacterial aerosols. According to the results of Kruskal–Wallis test, no significant difference was found for bacteria (P > 0.05), while the difference for mean fungi concentration throughout the study period was statistically significant (P < 0.05). Mapping the concentration distribution of bacteria and fungi in Fig. 5 and Fig. 6 show that the higher concentration area of airborne bacteria and fungi bioaerosols is related to site 7.
3.3 Characteristics of bacterial and fungi bioaerosols
The results, as presented in Fig. 7, indicated that the most predominant bacteria were Gram-positive bacteria which accounted for 99% of the total count. Only trace amounts of Gram-negative bacteria (1%) were detected in all the cultured media. The most common bacterial species of all the collected air samples were bacilli. According to Fig. 8, during the 6 months of the study, the types of fungi that were predominated include Dematiaceous fungi (mostly Cladosporium sp. and Alternaria sp.), Yeasts, Aspergillus and Penicillium sp, respectively. The lowest percentage of fungal species in the ambient air was related to Mycelia Sterilia (5%) and Rhizopus (1%), and Dematiaceous with 34% was the highest species in the atmosphere during the sampling period.
3.4 Association of airborne bioaerosols with ambient PM 2.5
Table 1 shows the summary of atmospheric PM2.5 concentrations in the seven air pollution monitoring stations from February to July 2018. The PM2.5 concentration ranged between the highest value of 49 µg/m3 obtained in February, and the lowest value of 15 µg/m3 detected in April. The monthly averages of PM2.5 were the highest in February (mean 40.4 µg/m3) and the lowest in April (16.6 µg/m3). Zoning of the mean concentration of PM2.5, in Fig. 5 shows that the highest amount of PM2.5 was detected in site 5. The distribution map of PM2.5 concentrations in the city according to the data obtained from the monitoring stations are shown in Fig. 6 separately for the cold and warm seasons. In the cold season, the highest value of PM2.5 (site 5, 7 and 6), bacteria (site 5) and fungi (site 6) were recorded. The present study showed a significant correlation between the concentration of fungal aerosols and PM2. (P < 0.05) in the sampling period.
3.5 Association of bacterial and fungal bioaerosols with metrological factors
The effects of common metrological parameters including wind speed, relative humidity, temperature, visibility and UV index on the concentration of airborne bioaerosols were also investigated in this study. According to Table 1, both the outdoor ambient temperature and UV index were continuously increased from February to July. Meantime, the relative humidity was decreased from 58.2 to 12.7%, whereas the changes in other parameters were not meaningful.
To evaluate the potential association between the concentration of bioaerosols, PM2.5 and the meteorological parameters Spearman correlation analysis was conducted, and the results were presented in Table 2. There was a moderate negative correlation between bacterial aerosols concentration and temperature (P < 0.05, rs = − 0.41) and an strong negative correlation between bacterial aerosols and wind speed (P < 0.05, rs = − 0.79), as well as a positive meaningful correlation between airborne fungi and temperature (P < 0.05, rs = 0.44).
A positive correlation was detected between PM2.5 and airborne fungi levels during the study period, however, the differences were not statistically significant for the concentration of bacteria. The Spearman correlation test showed no meaningful relationship between the amount of PM2.5 and the environment parameters (P > 0.05), while significant difference was found between the average concentration of bacteria and fungi (P < 0.05).
4 Discussion
There is no approved international standard for an acceptable level of bioaerosols in the ambient air. However, the most obvious finding of this study is that the mean concentration of total bacteria and fungi in the ambient air of Isfahan is lower than that reported for most cities of the world (Gao et al. 2015; Haas et al. 2013; Lee et al. 2006). In a study in Xian, China, (Li et al. 2017) the concentrations of viable bacteria varied from 97 to 1909 with the average values of 565 ± 464 CFU/m3 which are higher than the results of the present study (mean,76 CFU/m3, from 12 to 254 CFU/m3). A possible explanation of this difference may be dissimilar environmental and metrological factors in various regions and different sampling methods. some previous studies have suggested that the process of aerosolization and impingement collection can physically hurt the cell walls of bacteria (Lundholm 1982; Terzieva et al. 1996). From the climatic point of view, Isfahan is located in an arid or semi-arid region and strong solar radiation, and dry weather can provide an undesirable condition for the survival of airborne bioaerosols.
In this study, the highest and lowest amount of viable bacterial bioaerosols were seen on the cold and warm months, respectively. Similarly, Li et al. reported the lowest airborne bacteria concentration in spring and summer (79–83 CFU/m3). It seems that in summer the intensity of solar radiation and high temperature affect the bacterial life. Temperatures above 24 °C may influence the survival rate of airborne bacteria (Tang 2009). Nevertheless, the results of the present study contradict with the results of some previous research (Fang et al. 2008; Lee et al. 2016). According to Lee et al. (Lee et al. 2016), the lowest bacterial level should be in winter owing to inactivate bacterial sources and inappropriate weather condition in the cold season. However, in Isfahan, the temperature does not decline as much to inactivate the microorganism (Table 1). Furthermore, the association of bioaerosols with PM2.5 and increased concentration of PM2.5 in winter due to the occurrence of thermal inversion, foggy and hazy days result in a higher level of bioaerosols in this season. There was a significant difference between the seasonal variation of airborne fungi and bacteria. The maximum concentration of fungi was detected in spring (June), while its minimum amount was in late winter (March). Adhikari et al. reported maximum levels of fungal spore in the May and June (Adhikari et al. 2006). The low value of airborne fungi in this research was in agreement with the results of some previous studies (Fang et al. 2008; Gao et al. 2016). As described by Fang et al. (Fang et al. 2008), nutrient levels, environmental factors and propagation and growth of plants mostly affect fungal spore. Due to cold and dry weather as well as declining the plant cover during winter, fungal spore concentration in the air also diminishes.
In the present study, Gram-positive bacteria, accounting for more than 99% prevailed in all the collected samples, which was in agreement with other findings (Wei 1999; Zhu et al. 2003; Roy et al. 2020). The possible explanation is that types of Gram-negative bacteria do not grow in an aerosolized form as well as Gram-positive bacteria (Pascual et al. 2003), and the reason is associated with fairly hard and protective cell wall around Gram-positive bacteria. However, in a study in Tempe, Arizona, Zhu et al. reported that Gram-negative bacteria were remarkably higher inside the office environment than outside. This is maybe because Gram-positive bacteria have more resistance and survival ability than Gram-negative bacteria outside under unfavorable conditions such as intense sun radiation (Zhu et al. 2003). In this study, the fungal genera including dematiaceous (mainly Cladosporium sp.), Yeast, Aspergillus, Penicillium and Mycelia Sterilia were identified with dominancy of the dematiaceous genus with 34%. The melanin pigments in the cell wall of dematiaceous can be contributed to promoting its survival in the environment (Jones and Harrison 2004; Li et al. 2012). As the pathogenic fungi (Penicillium and Aspergillus) can be harmful to the human being in high value due to the production of mycotoxins, their monitoring in the air is important. Similarly, Basilico et al. and Khan et al. found that Penicillium and Aspergillus are presented in outdoor and indoor air at much lower concentrations than Cladosporium and Alternaria (Basilico et al. 2007; Khan and Wilson 2003). In research on airborne fungi concentration in Basra, Iraq, Cladosporium was identified as predominant fungal species with 31.3% followed by Penicillium notatum (11.9%), Alternaria alternate (10.0%) and Aspergillus Niger (5.8%) (Muhsin and Adlan 2012). Roy et al. have found Aspergillus, Cladosporium, and Penicillium sp. As predominant fungal species in both urban and rural area (Roy et al. 2020). In a study conducted by Barati et al., the most predominant species of fungi was Dematiaceous, and the second one was Yeast in the outdoor environment of Qeshm Island, Iran (Barati et al. 2009). Also, in conformity with this, Chadeganipour et al. reported that Yeast accounts for 18.7% of total fungi in the air of Isfahan (Chadeganipour et al. 2010).
Both the internal biological agent and external environmental parameters including relative humidity, temperature and wind speed can affect bioaerosols concentrations (Larsen 1981). The current study found that temperature has a significant influence on the airborne bioaerosols during the study period. It positively correlated with fungi and inversely with bacteria level (P < 0.05). These results are in agreement with findings that showed a positive significant relationship between airborne spore counts and temperature (Herrero et al. 2006). Also, statistical analysis showed a positive correlation of bacteria concentrations against wind speed, and intensely negative correlation versus UV (P < 0.05). Di Giorgio et al. measured airborne bacteria at rural, coastal and urban places and observed that the concentration increases with temperature and wind speed; but contrary to expectations, they did not find a significant difference between the bioaerosols concentrations and relative humidity (Di Giorgio et al. 1996). However, these results are consistent with those of reported a positive correlation between concentrations of viable microorganisms versus relative humidity (Li et al. 2015). Further long-term monitoring of bioaerosols is needed in the study area to aid exposure and health risk assessment and therefore eliminate the sources of emission.
5 Conclusion
In this study, the concentration of culturable airborne bacteria and fungi in different sites of Isfahan and their association with PM2.5 level and environmental factors were investigated. The most obvious finding of this study is that the average concentrations of viable bacteria and fungi in Isfahan are lower than those reported in most cities in the world. Airborne bioaerosols values were generally higher for bacteria compared to fungi in all sites of the city. Monthly averages of PM2.5 were varied from 16.6 to 40.4 µg/m3 among the study period, and it showed a positive association with the community of airborne microorganisms. Gram-positive bacteria prevailed in all the sites accounting for more than 99% of the total counted bacteria. The most predominant fungal genera were dematiaceous (mainly Cladosporium sp.) accounting for 34% of the total counted fungi. We found that meteorological parameters including wind speed and UV index affect the concentration of bacteria, while the only factor affecting both the airborne bacteria and fungi was temperature. The present results can be contributed to provide valuable information for prospective exposure assessment, can be used in source identification and emission reduction programs and the development of official standard concerning bioaerosols in the atmospheric air.
References
Adhikari, A., Reponen, T., Grinshpun, S. A., Martuzevicius, D., & LeMasters, G. (2006). Correlation of ambient inhalable bioaerosols with particulate matter and ozone: A two-year study. Environmental Pollution, 140(1), 16–28.
Barati, B., Ghahri, M., & Sorouri, R. (2009). Isolation and characterization of bacteria and fungi in ambient air of Qeshm Island. Hormozgan Medical Journal, 13(2), 101–108.
Basilico, M. D. L. L. Z., Chiericatti, C., Aringoli, E. E., Althaus, R. L., & Basilico, J. C. (2007). Influence of environmental factors on airborne fungi in houses of Santa Fe city, Argentina. Science of the Total Environment, 376(1–3), 143–150.
Chadeganipour, M., Shadzi, S., Nilipour, S., & Ahmadi, G. (2010). Airborne fungi in Isfahan and evaluation of allergenic responses of their extracts in animal model. Jundishapur Journal of Microbiology, 3(4), 155.
Di Giorgio, C., Krempff, A., Guiraud, H., Binder, P., Tiret, C., & Dumenil, G. (1996). Atmospheric pollution by airborne microorganisms in the city of Marseilles. Atmospheric Environment, 30(1), 155–160.
Du, P., Du, R., Ren, W., Lu, Z., Zhang, Y., & Fu, P. (2018). Variations of bacteria and fungi in PM2.5 in Beijing, China. Atmospheric Environment, 172, 55–64.
Fang, Z., Ouyang, Z., Zheng, H., & Wang, X. (2008). Concentration and size distribution of culturable airborne microorganisms in outdoor environments in Beijing, China. Aerosol Science and Technology, 42(5), 325–334.
Fathi, S., Hajizadeh, Y., Nikaeen, M., & Gorbani, M. (2017). Assessment of microbial aerosol emissions in an urban wastewater treatment plant operated with activated sludge process. Aerobiologia, 33(4), 507–515.
Gao, M., Qiu, T., Jia, R., Han, M., Song, Y., & Wang, X. (2015). Concentration and size distribution of viable bioaerosols during non-haze and haze days in Beijing. Environmental Science and Pollution Research, 22(6), 4359–4368.
Gao, M., Yan, X., Qiu, T., Han, M., & Wang, X. (2016). Variation of correlations between factors and culturable airborne bacteria and fungi. Atmospheric Environment, 128, 10–19.
Ghaffarpasand, O., Talaie, M. R., Ahmadikia, H., Khozani, A. T., & Shalamzari, M. D. (2020). A high-resolution spatial and temporal on-road vehicle emission inventory in an Iranian metropolitan area, Isfahan, based on detailed hourly traffic data. Atmospheric Pollution Research, 11(9), 1598–1609.
Haas, D., Galler, H., Luxner, J., Zarfel, G., Buzina, W., Friedl, H., et al. (2013). The concentrations of culturable microorganisms in relation to particulate matter in urban air. Atmospheric Environment, 65, 215–222.
Herrero, A. D., Ruiz, S. S., Bustillo, M. G., & Morales, P. C. (2006). Study of airborne fungal spores in Madrid, Spain. Aerobiologia, 22(2), 133.
Hoseinzadeh, E., Samarghandi, M. R., Ghorbani Shahna, F., & Chavoshi, E. (2013). Isoconcentration mapping of particulate matter in H amedan intercity bus stations. Water and Environment Journal, 27(3), 418–424.
Hussin, N. H. M., Sann, L. M., Shamsudin, M. N., & Hashim, Z. (2011). Characterization of bacteria and fungi bioaerosol in the indoor air of selected primary schools in Malaysia. Indoor and Built Environment, 20(6), 607–617.
Jahne, M. A., Rogers, S. W., Holsen, T. M., Grimberg, S. J., & Ramler, I. P. (2015). Emission and dispersion of bioaerosols from dairy manure application sites: Human health risk assessment. Environmental science and technology, 49(16), 9842–9849.
Jones, A. M., & Harrison, R. M. (2004). The effects of meteorological factors on atmospheric bioaerosol concentrations—a review. Science of the Total Environment, 326(1), 151–180.
Khan, N. N., & Wilson, B. L. (2003). An environmental assessment of mold concentrations and potential mycotoxin exposures in the greater Southeast Texas area. Journal of Environmental Science and Health, Part A, 38(12), 2759–2772.
Larsen, L. S. (1981). A three-year-survey of microfungi in the air of Copenhagen 1977–79. Allergy, 36(1), 15–22.
Lee, B. U., Hong, I. G., Lee, D. H., Chong, E.-S., Jung, J. H., Lee, J. H., et al. (2012). Bacterial bioaerosol concentrations in public restroom environments. Aerosol and Air Quality Research, 12(2), 251–255.
Lee, B. U., Lee, G., & Heo, K. J. (2016). Concentration of culturable bioaerosols during winter. Journal of Aerosol Science, 94, 1–8.
Lee, T., Grinshpun, S. A., Martuzevicius, D., Adhikari, A., Crawford, C. M., & Reponen, T. (2006). Culturability and concentration of indoor and outdoor airborne fungi in six single-family homes. Atmospheric Environment, 40(16), 2902–2910.
Li, H., Zhou, X.-Y., Yang, X.-R., Zhu, Y.-G., Hong, Y.-W., & Su, J.-Q. (2019). Spatial and seasonal variation of the airborne microbiome in a rapidly developing city of China. Science of The Total Environment, 665, 61–68. https://doi.org/10.1016/j.scitotenv.2019.01.367
Li, Y., Fu, H., Wang, W., Liu, J., Meng, Q., & Wang, W. (2015). Characteristics of bacterial and fungal aerosols during the autumn haze days in Xi’an, China. Atmospheric Environment, 122, 439–447.
Li, Y., Lu, R., Li, W., Xie, Z., & Song, Y. (2017). Concentrations and size distributions of viable bioaerosols under various weather conditions in a typical semi-arid city of Northwest China. Journal of Aerosol Science, 106, 83–92.
Li, Y., Qiu, X., Li, M., Ma, Z., Niu, T., & Feng, Y. (2012). Concentration and size distribution of airborne actinomycetes in a municipal wastewater treatment plant. Polish Journal of Environmental Studies, 21(5), 1305–1311.
Lundholm, I. M. (1982). Comparison of methods for quantitative determinations of airborne bacteria and evaluation of total viable counts. Applied and Environment Microbiology, 44(1), 179–183.
Mandal, J., & Brandl, H. (2011). Bioaerosols in indoor environment—a review with special reference to residential and occupational locations. The Open Environmental and Biological Monitoring Journal, 4(1), 83–96.
Mirhoseini, S. H., Nikaeen, M., Khanahmad, H., Hatamzadeh, M., & Hassanzadeh, A. (2015). Monitoring of airborne bacteria and aerosols in different wards of hospitals—particle counting usefulness in investigation of airborne bacteria. Annals of Agricultural and Environmental Medicine, 22(4), 670–673.
Mirhoseini, S. H., Nikaeen, M., Satoh, K., & Makimur, K. (2016). Assessment of airborne particles in indoor environments: Applicability of particle counting for prediction of bioaerosol concentrations. Aerosol and Air Quality Research, 16(8), 1903–1910.
Moon, K. W., Huh, E. H., & Jeong, H. C. (2014). Seasonal evaluation of bioaerosols from indoor air of residential apartments within the metropolitan area in South Korea. Environmental Monitoring and Assessment, 186(4), 2111–2120.
Muhsin, T. M., & Adlan, M. M. (2012). Seasonal distribution pattern of outdoor airborne fungi in Basrah city, southern Iraq. Journal of Basrah Researches (Sciences), 38(1A), 90–98.
Nikaeen, M., Hatamzadeh, M., Hasanzadeh, A., Sahami, E., & Joodan, I. (2009). Bioaerosol emissions arising during application of municipal solid-waste compost. Aerobiologia, 25(1), 1–6.
Niu, X., Cao, J., Shen, Z., Ho, S. S. H., Tie, X., Zhao, S., et al. (2016). PM2. 5 from the Guanzhong plain: Chemical composition and implications for emission reductions. Atmospheric Environment, 147, 458–469.
Park, D.-U., Yeom, J.-K., Lee, W. J., & Lee, K.-M. (2013). Assessment of the levels of airborne bacteria, gram-negative bacteria, and fungi in hospital lobbies. International journal of environmental research and public health, 10(2), 541–555.
Pasanen, A.-L., Lappalainen, S., & Pasanen, P. (1996). Volatile organic metabolites associated with some toxic fungi and their mycotoxins. Analyst, 121(12), 1949–1953.
Pascual, L., Pérez-Luz, S., Yanez, M. A., Santamaría, A., Gibert, K., Salgot, M., et al. (2003). Bioaerosol emission from wastewater treatment plants. Aerobiologia, 19(3–4), 261–270.
Peel, M. C., Finlayson, B. L., & McMahon, T. A. (2007). Updated world map of the Köppen–Geiger climate classification. Hydrology and Earth System Sciences Discussions, 4(2), 439–473.
Rabiei, K., Hosseini, S. M., Sadeghi, E., Jafari-Koshki, T., Rahimi, M., Shishehforoush, M., et al. (2017). Air pollution and cardiovascular and respiratory disease: Rationale and methodology of CAPACITY study. ARYA Atherosclerosis, 13(6), 264.
Rajasekar, A., & Balasubramanian, R. (2011). Assessment of airborne bacteria and fungi in food courts. Building and Environment, 46(10), 2081–2087.
Roy, R., Jan, R., Joshi, U., Bhor, R., Pai, K., & Satsangi, P. G. (2020). Characterization, pro-inflammatory response and cytotoxic profile of bioaerosols from urban and rural residential settings in Pune, India. Environmental Pollution, 264, 114698. https://doi.org/10.1016/j.envpol.2020.114698
Segvić Klarić, M., & Pepeljnjak, S. (2006). A year-round aeromycological study in Zagreb area, Croatia. Annals of Agricultural and Environmental Medicine, 13(1), 55–64.
Shams-Ghahfarokhi, M., Aghaei-Gharehbolagh, S., Aslani, N., & Razzaghi-Abyaneh, M. (2014). Investigation on distribution of airborne fungi in outdoor environment in Tehran, Iran. Journal of Environmental Health Science and Engineering, 12(1), 54.
Stanley, N. J., Kuehn, T. H., Kim, S. W., Raynor, P. C., Anantharaman, S., Ramakrishnan, M., et al. (2008). Background culturable bacteria aerosol in two large public buildings using HVAC filters as long term, passive, high-volume air samplers. Journal of Environmental Monitoring, 10(4), 474–481.
Tang, J. W. (2009). The effect of environmental parameters on the survival of airborne infectious agents. Journal of the Royal Society Interface, 6(Suppl_6), S737–S746.
Terzieva, S., Donnelly, J., Ulevicius, V., Grinshpun, S. A., Willeke, K., Stelma, G. N., et al. (1996). Comparison of methods for detection and enumeration of airborne microorganisms collected by liquid impingement. Applied and Environment Microbiology, 62(7), 2264–2272.
Tsapko, V. G., Chudnovets, A. J., Sterenbogen, M. J., Papach, V. V., Dutkiewicz, J., Skórska, C., et al. (2011). Exposure to bioaerosols in the selected agricultural facilities of the Ukraine and Poland—a review. Annals of Agricultural and Environmental Medicine, 18(1), 19–27.
Wei, S. L. S. W. S. (1999). Study on Airborne Bacteria Pollution in Shanghai. Shanghai Environmental Sciences, 6, 258–260.
Xu, J., Jia, C., He, J., Xu, H., Tang, Y.-T., Ji, D., et al. (2019). Biomass burning and fungal spores as sources of fine aerosols in Yangtze River Delta, China—using multiple organic tracers to understand variability, correlations and origins. Environmental Pollution, 251, 155–165. https://doi.org/10.1016/j.envpol.2019.04.090
Zhu, H., Phelan, P. E., Duan, T., Raupp, G. B., Fernando, H. J., & Che, F. (2003). Experimental study of indoor and outdoor airborne bacterial concentrations in Tempe, Arizona, USA. Aerobiologia, 19(3–4), 201–211.
Acknowledgements
This research was carried out with financial support of the vice chancellery for research of Isfahan University of Medical Sciences (Grant No: 396637) as M.Sc. dissertation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hosseini, N., Hajizadeh, Y., Nikaeen, M. et al. Spatiotemporal variation of ambient bioaerosols in a large and industrialized metropolis of Iran and their association with PM2.5 and meteorological factors. Aerobiologia 37, 105–117 (2021). https://doi.org/10.1007/s10453-020-09672-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10453-020-09672-6