Abstract
Exposure to bioaerosols can have adverse effects on human health and contribute to allergies, asthma, infectious diseases and pulmonary conditions. Bioaerosols are airborne particles of microbial, animal and plant origin. Animal- and plant-derived particles, which may disperse over long distances, are being increasingly addressed by environmental studies in addition to microorganisms. In this study, air samples were collected in various planned areas of three cities in China (Beijing, Hangzhou and Wuhan) using a new liquid-based sampler. Animal, plant and bacterial components were quantified according to the expression levels of housekeeping genes via real-time PCR and analysis of cycle threshold (Ct) values. The level of animal-derived particles in the air of Hangzhou was higher than those in the other two cities. More bacterial particles than animal or plant particles were detected in the air in Beijing compared with other cities. We also found a negative correlation between the bacterial content of bioaerosols and relative humidity and a positive correlation between bacterial content and temperature. By contrast, bioaerosol animal content was positively correlated with relative humidity and negatively correlated with temperature. In summary, high levels of bacterial particles were detected in atmospheric samples in China, although the lower concentrations of animal- and plant-derived particles should not be ignored. These data may provide important evidence for structural analyses of animal- and plant-derived bioaerosols as well as for risk assessment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Bioaerosols, also known as atmospheric biological particles, can originate from a variety of natural and anthropogenic sources (e.g., husbandry, industrial and agricultural activities) and a variety of locations (e.g., land, ocean, freshwater, soil, forest, desert and urban areas) (Brodie et al. 2007; Gandolfi et al. 2013; Yoo et al. 2017). Bioaerosols have significant effects on atmospheric chemistry, physics and cloud formation and influence the global climate (Pöschl 2005; Morris et al. 2014). Moreover, exposure to bioaerosols may be detrimental to human health and contribute to infectious diseases, acute toxic effects, allergies, cardiovascular disease and cancers (Humbal et al. 2018; Bowers et al. 2011; Walser et al. 2015). Generally, biological aerosol particles can include microorganisms and many types of intact or fragmented biological cells, dispersal units, or tissues (Després et al. 2012; Yoo et al. 2017). Microorganism-derived materials include live or dead intact microbes (e.g., fungi, bacteria, viruses and protozoa) as well as fragments including endotoxins, mycotoxins, spores, proteins, nucleic acids and metabolic products (Veillettea et al. 2018). Human activities, such as agriculture, animal feed production, traffic, wastewater treatment and composting, are the major causes of airborne microorganisms in urban areas (Skotak et al. 2016). Bioaerosols account for approximately 30% of the aerosol load in urban air (Després et al. 2012; Huffman et al. 2013; Fröhlich-Nowoisky et al. 2016). In recent years, the temporal and spatial distributions of bioaerosols (bacteria, fungi and viruses) in modern cities around the world have been investigated using culture-based, molecular or optical methods. Numerous studies have demonstrated that bioaerosol levels vary across seasons and regions (Bowers et al. 2013; Gabey et al. 2013; Haas et al. 2013; Helin et al. 2017; Huffman et al. 2012; Hurtado et al. 2014; Innocente et al. 2017; Lee et al. 2016; Wu et al. 2012; Maki et al. 2015; Xie et al. 2018a, b; Cao et al. 2014; Gao et al. 2016; Lu et al. 2018a, b; Xie et al. 2018a, b a).
Most published studies of bioaerosols have focused on detecting airborne microorganisms (especially bacteria) under different weather conditions or in high-risk work environments such as farms, livestock or poultry feeding areas and slaughterhouses (Chien et al. 2011; Hu et al. 2015; Lutgring et al. 1997; Millner 2009; Straumfors et al. 2016; Veillettea et al. 2018) for the purpose of risk assessment. However, bioaerosols of animal and plant origin have often been overlooked. Animal- and plant-derived particles contain uncultivable, dead, or fragmented plant and animal cells derived from multiple sources including leaf litter, algae, brochosomes, pollen, grains, aerosolized feed, feces droplets, dandruff, insect parts, feathers, flaked bird skin debris and broken feather barbules (Cox and Clark 1973; Després et al. 2012; Chien et al. 2011; Millner 2009; Wittmaack et al. 2005). Such particles may be emitted from trees, grasses, rocks, animal skin or hair, poultry and livestock, food processing, agricultural production and composting facilities. Bioaerosol constituents may have adverse health impacts despite their generally low concentrations at a distance from the source. Exposure to such particles may produce a variety of clinical responses including allergy, asthma, pneumonia, chronic bronchitis, organic dust toxic syndrome and other chest illnesses (Iversen 1999; Millner 2009; Straumfors et al. 2016). In addition, animal- and plant-derived bioaerosols vary in size from 0.001 µm to 100 µm in diameter (Pöschl 2005) but are normally at the larger end of this range; for example, pollen from anemophilous plants has aerodynamic diameters of 17–58 µm (Després et al. 2012; Yoo et al. 2017). These particles have high settling velocities, and their residence time in the atmosphere influences the distance they can travel (Després et al. 2012). Some particles may be lifted to high altitudes and have long residence times (Diehl et al. 2001; Després et al. 2012). They may also be resuspended from the ground and travel short or long distances along with dust in the air due to wind-driven or traffic-related effects (Esmaeil et al. 2014; Hallar et al. 2011; Jochner et al.2015; Fröhlich-Nowoisky et al. 2016; Ortiz-Martínez et al. 2015). Dust aerosols can be transported over thousands of kilometers from their source (Goudie 2014; Yoo et al. 2017). Animal- and plant-derived particles in complexes with dust can become carriers for airborne microorganisms and provide nutrition for their growth (Maron et al. 2005; Park et al. 2018; Yamaguchi et al. 2012). In addition, proteins including airborne allergens may be contained in coarse biological particles such as pollen grains, which can form from plant debris (Pöschl 2005). Since the specific components that are responsible for health threats from bioaerosol exposure remain unclear (Yoo et al. 2017), biological aerosol particles originating from animal and plant sources should be monitored for occupational safety and public health purposes.
It is estimated that only 1% of microorganisms in bioaerosols can be cultured (Després et al. 2012), and detection of animal- and plant-derived particles is currently not possible using culture methods. Currently, PCR-based assays play an important role in the analysis of environmental samples. Housekeeping genes, which are expressed at relatively constant levels, provide ideal targets for discriminating taxonomic groups (Hebert et al. 2003; Radonić et al. 2004). Thus, airborne biological particles derived from animal, plant and bacterial sources can be quantified by analyzing the levels of these housekeeping genes. In this study, the spatial distributions of animal-, plant- and bacteria-derived atmospheric biological particles were investigated in three cities in China using real-time PCR. The correlations between bioaerosol levels and meteorological factors were also evaluated.
2 Materials and methods
2.1 Design and evaluation of housekeeping gene primers and probes
The universality and specificity of housekeeping genes for animals, plants and bacteria were evaluated. Primers and TaqMan probes used for the detection and amplification of housekeeping genes were synthesized by Invitrogen (Thermo Fisher Scientific, Waltham, MA, USA; Table 1). Pork, beef, fish and chicken purchased from the Yonghui Supermarket in Beijing were selected as representative sources of animal DNA to test the designed primers and probes. For plant samples, DNA from two types of vegetables (Brassica napus and Lactuca sativa), leaves of outdoor plants (Ilex paraguariensis, Prunus persica, Eucommia ulmoides and Ginkgo biloba) and leaves of pot-cultured plants (Alocasia macrorrhiza and Scindapsus aureus) were used to assess the specificity and sensitivity of the primers and probes (Gu et al. 2014; Song et al. 2018). Staphylococcus aureus, Escherichia coli, Salmonella typhimurium and Streptococcus faecium were chosen as representative bacterial species to evaluate the primers and probes (Wan 2015).
DNA was extracted from meat and bacterial cultures using the Blood and Tissue Kit (QIAGEN GmbH, Hilden, Germany) according to the manufacturer’s protocol. Plant DNA samples were extracted using the Plant Genomic DNA Kit (TIANGEN Inc., Beijing, China). Real-time PCR assays were performed using an ABI 7500-Fast real-time PCR system (Applied Biosystems, Thermo Fisher Scientific, Waltham, MA, USA). The instrument’s on-board software was used for data analysis. Assays were carried out in a 25-µL reaction volume with an ABI one-step RT-PCR kit (Applied Biosystems, Thermo Fisher Scientific, Waltham, MA, USA). Each PCR mixture contained 1 µL of 25 × enzyme mix, 12.5 µL of 2 × PCR buffer, 1 µL each of the forward and reverse primers, 1 µL of probe, 7 µL of DNA template and 2.5 µL of DNase/RNase-free water. The PCR cycling conditions were as follows: 95° C for 9 min, followed by 40 cycles of 95° C for 15 s and 60° C for 1 min. Cycle threshold (Ct) values were recorded after each experiment. Genomic DNA from nontarget organisms was used as a negative control, and deionized water was used as a blank control for each target gene.
2.2 Field sampling
2.2.1 Sampling sites
Geographically, China consists of seven major regions: northeast, north, east, central, south, southeast and southwest. In this study, sampling of ambient bioaerosols was carried out in three prosperous cities with high population densities (> 10 million individuals): Beijing, Hangzhou and Wuhan. These three cities represent the northern, eastern and central regions, respectively, and have continental, subtropical monsoon and temperate monsoon climates, respectively (Fig. 1). Beijing (39.4° –41.6° N, 115.7° –117.4° E), the capital of China, is located in the northern part of the North China Plain and is surrounded by the Taihang Mountains to the west and the Yanshan Mountains to the north and northeast. The terrain is high in the northwest of the city (1000–1500 m above sea level) and low in the southeast (20–60 m above sea level). The city has a total population of 21 million individuals and an area of 16,410 km2. Beijing has four distinct seasons with a long summer and winter and a shorter spring and autumn. The average annual temperature is 12.6 °C and 620.6 mm of precipitation fell in 2017. Hangzhou (29°11′–30°33′N, 118°21′–120°30′E), the capital of Zhejiang Province, is located along the southeastern coast of China and is in the southern wing of the Yangtze River Delta. The world’s longest canal (the Grand Canal) winds through the city, in addition to the Qiangtang River. The city has a total population of 10.1 million individuals and an area of 16,853 km2. Hangzhou has a subtropical monsoon climate with plentiful rainfall and sufficient sunshine. Its average annual temperature is 17.8 °C, the average relative humidity is 70%, and the average annual rainfall is 1454 mm. Wuhan (29°58′–31°22′N, 113°41′–115°05′E, 19.2–873.7 m above sea level), the only subprovincial city in central China, is the capital of Hubei Province. The city has a total population of 10.9 million individuals and an area of 8,494 km2. The world’s third longest river (the Yangtze) flows through the city. Wuhan has a humid subtropical monsoon climate with abundant rainfall, abundant sunshine and four distinct seasons. The average annual temperature is 15.8–17.5 °C, and the average precipitation is 1150–1450 mm.
Seven sampling areas were selected in the cities of Beijing, Wuhan and Hangzhou based on the Ambient Air Quality Standards (National Standard GB3095–2012). Each site represented a different planned area of the city (Table 2). Environmental functional zones were divided into three levels: level 1, natural protection and scenic areas; level 2, residential-commercial mixed areas, residential areas, cultural industry parks, industrial parks and rural areas; and level 3, specific industrial areas with enhanced restrictions. In each city, the seven sampling areas included two level 1 zones (rich in vegetation and/or forests) and five level 2 zones (including areas with high human population density and rural areas where dairy farms are located). Therefore, within the three cities that represent different climactic regions, we selected seven sites for sampling while covering different environmental functional zones.
2.2.2 Collection of environmental samples
Three teams were assigned for air sampling in each city from July 30 to August 20, 2018, using the BioCapture-6 sampler (Fig. 2; Bio-enrichment Technology Co., Ltd., Hangzhou, China). Each team worked at an individual sampling site for three continuous days. All sampling time arrangements are listed in Supplementary Table 1, and the temperatures and relative humidities are listed in Supplementary Table 3. Air (1000 L) was sampled continuously using a BioCapture-6 sampler at a rate of 80 L/min. Each sample took 13 min to obtain. The sampler was designed by the Bio-enrichment Technology Co., Ltd. It is a lithium battery-powered bioaerosol sampler and weighs 4.8 kg. The sampler was operated at flow rate of 80 L/min, and airborne particles were effectively collected into liquids under inertia and centrifugal force. The sampler has a dismountable sampling head as well as a sampling bottle. Before sampling, the plastic head and sampling bottles were disinfected using 75% ethanol, cleaned with deionized water and then baked at 80 °C in an oven overnight.
A bottle was filled with 45 mL of phosphate-buffered saline (PBS) as the sampling liquid, and then 150 µL of magnetic bead solution (Bio-enrichment Technology Co., Ltd., Hangzhou, China) was added to the sampling liquid. These magnetic beads were specially developed for bioaerosol collection. The collected organisms are adsorbed onto the surface of the beads during sampling, where they aggregate and survive better due to reduced levels of reaerosolization. The air sampler was set at a height of 1.2–1.5 m above the ground. Temperature and relative humidity were also recorded during sampling. After sampling, the sampling head was disassembled and immersed in 75% ethanol for disinfection. The sampling liquid was placed on the magnetic rack of the sampler for 3 min at room temperature. Subsequently, the supernatant was carefully discarded, and the magnetic beads were resuspended in 200 µL of PBS. The mixture was then transferred to a microcentrifuge and transported to the laboratory for the next experiment.
Nucleic acids were extracted using a DNeasy Power Water Kit (QIAGEN GmbH, Hilden, Germany). The presence of genetic material derived from animal, plant and bacterial components in environmental samples was analyzed by real-time PCR as described in Sect. 2.1. Ct values were recorded after each experiment. DNA extracted from pork and lettuce was used as the positive control for animal-derived and plant-derived amplifications, respectively. DNase/RNase-free water was used as the blank control for all amplifications.
2.2.3 Data analysis
Data analysis was performed using SAS 8.2 software (SAS Institute, Inc.). An independent-sample t test or Wilcoxon two-sample test was used to compare Ct values between datasets. A one-way analysis of variance (ANOVA) or Kruskal–Wallis test was used to assess differences in Ct values across three or more datasets. Differences were considered statistically significant at P < 0.05. Linear regression was performed using Prism version 6.02 (GraphPad Software, Inc.).
3 Results
3.1 Evaluation of housekeeping genes
Each pair of primers and probes listed in Table 1 was evaluated separately. Mitochondrial 16S rRNA-encoding genes were detected as the housekeeping genes for animals. Following assessment of primers and probes, the average Ct values for pork, beef, fish and chicken were 14.8, 11.3, 26.66 and 26.02, respectively. The chloroplast tRNALeu gene was used as the housekeeping gene for detecting plant-derived aerosols (General Administration of Quality Supervision, Inspection and Quarantine of the People's Republic of China 2003). The probe sequences for the chloroplast tRNA genes of different plants were designed in our laboratory. Following assessment of the primers and probes, the Ct values for Brassica napus, Lactuca sativa, Ilex paraguariensis, P. persica, E. ulmoides, Ginkgo biloba, Alocasia macrorrhiza and Scindapsus aureus were 12.88, 11.64, 24.55, 13.85, 19.0, 22.13, 15.45 and 12.93, respectively. Pure cultures of Staphylococcus aureus, E. coli, Salmonella typhimurium, and S. faecium were used for nucleic acid extraction and amplification to evaluate the primers and probes. The Ct values for the corresponding genomic DNA samples were 15.81, 12.62, 12.07 and 9.05, respectively. These results showed that housekeeping genes from different organisms could be successfully amplified by real-time PCR and that these amplification methods could be used for the detection of animal-, plant- and bacteria-derived particles in air.
3.2 Variations in bioaerosol particle distributions from the atmospheres of three cities
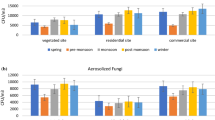
Animal-, plant- and bacteria-derived particles were collected and detected (Table 3). Their relative amounts were quantified based on the Ct values of the housekeeping genes. The Ct values (< 40) of housekeeping genes detected in the collected samples are listed in Supplementary Table 2 and Supplementary Fig. 1. In Beijing, bioaerosols in urban functional area I did not differ significantly from those in functional area II regardless of particle type (animal: t = − 1.31, P > 0.05, plant: t = 0.50, P > 0.05, bacteria: z = − 0.71, P > 0.05; Fig. 3a). The contents of animal-, plant- and bacteria-derived particles for each city as a whole were also evaluated and were found to differ significantly (F = 279.74, P < 0.0001; Fig. 3d). The average Ct values for animal-, plant- and bacteria-derived aerosol particles were 36.62, 35.65 and 22.32, respectively. In Hangzhou, the levels of animal- and bacteria-derived particles in urban functional areas I and II were similar (animal: z = 0.64, P > 0.05, bacteria: t = 0.17, P > 0.05; Fig. 3b), whereas the levels of plant-derived particles in these two functional areas were significantly different (t = − 3.33, P < 0.05). Thus, more plant-derived particles were detected in functional area I than in functional area II (Ct value: 30.55 versus 35.11). The levels of animal-, plant- and bacteria-derived particles in the city as a whole were also different (χ2 = 27.01, P < 0.0001; Fig. 3d). The Ct values for animal-, plant- and bacteria-derived aerosol particles were 33.60, 33.71 and 25.74, respectively. Student–Newman–Keuls grouping analysis showed that there were no statistically significant differences in the quantity of animal- and plant-derived particles. In Wuhan, none of the three types of bioaerosol particles in urban functional area I differed significantly in quantity from those in functional area II (animal: t = − 0.83, P > 0.05, plant: z = 1.17, P > 0.05, bacteria: t = − 0.31, P > 0.05; Fig. 3c). The Ct values for animal-, plant- and bacteria-derived particles in the city were 38.16, 35.65 and 24.59, respectively. Thus, the levels of animal-, plant- and bacteria-derived particles were significantly different (χ2 = 25.99, P < 0.0001; Fig. 3d), and more bacterial particles than plant-derived and animal-derived particles were detected.
The differences in the distribution of bioaerosols from Beijing, Hangzhou and Wuhan were also analyzed. There were no statistically significant differences in the levels of animal-derived particles detected in urban functional area I of the three cities (χ2 = 4.90, P > 0.05; Fig. 4a). In urban functional area II, the concentration of animal-derived particles in Hangzhou (Ct value: 33.34) was higher than that in the two other cities (χ2 = 14.80, P < 0.05; Fig. 4a). There were no significant differences in the concentrations of animal-derived particles between Beijing (Ct: 36.81) and Wuhan (Ct: 38.32). The highest levels of plant-derived particles were detected in urban functional area I of Hangzhou (F = 7.96, P < 0.05; Fig. 4b). The Ct values for plant-derived particles in Hangzhou, Beijing and Wuhan were 30.55, 36.04 and 37.00, respectively. The quantities of plant-derived particles in urban functional area II of the three cities were not significantly different (χ2 = 1.08, P > 0.05; Fig. 4b). Airborne bacteria levels in urban functional area I of Beijing (Ct: 22.27) were higher than those in Hangzhou (Ct: 25.81) and Wuhan (Ct: 24.49), with no differences observed between the levels of the latter two cities (F = 7.41, P < 0.05; Fig. 4c). In urban functional area II, the levels of airborne bacteria differed among the three cities (F = 41.42, P < 0.05; Fig. 4c), with the levels in Beijing being the highest, followed by those in Wuhan and Hangzhou (22.34 < 24.63 < 25.72).
Bioaerosol particles from different sources in the three cities were analyzed without considering the functional areas. Compared with Beijing and Wuhan, Hangzhou contained more animal-derived particles (χ2 = 19.94, P < 0.0001; Fig. 4d). The corresponding Ct values for animal-derived particles in Hangzhou, Beijing and Wuhan were 33.60, 36.62 and 38.16, respectively. No differences were observed in the levels of airborne plant-derived particles in the three cities (F = 2.30, P > 0.05; Fig. 4d). However, the levels of airborne bacteria in the three cities were significantly different (F = 43.77, P < 0.0001; Fig. 4d). The levels of airborne bacteria in Beijing were highest, followed by those in Wuhan and Hangzhou (22.32 < 24.59 < 25.74).
3.3 Linear correlations between bioaerosol levels and meteorological factors
Data from all three cities were pooled for analysis. Overall, the levels of bacteria-derived particles in the air were much higher than those of animal- and plant-derived particles (χ2 = 85.03, P < 0.0001; Fig. 5a). The average Ct values for bacteria-, plant- and animal-derived particles were 24.13, 34.95 and 36, respectively. Regardless of their origin, the bioaerosol levels in functional area I were not significantly different from those in functional area II (animal: z = 0.01, P > 0.05; plant: z = 0.15, P > 0.05; bacteria: t = 0.55, P > 0.05; Fig. 5b). No differences in bioaerosol levels of any origin were detected among the seven planned areas of any city (animal: χ2 = 2.27, P > 0.05; plant: F = 0.86, P > 0.05; bacteria: χ2 = 3.68, P > 0.05; Fig. 6).
The influences of temperature, relative humidity and altitude on bioaerosol distributions were evaluated using linear correlation analyses for animal-, plant- and bacteria-derived bioaerosols (Fig. 7). The Ct values for animal-derived bioaerosols were positively correlated with relative humidity (r = 0.4425, R2 = 0.1958, P = 0.0038, Fig. 7d) but negatively correlated with temperature (r = -0.3800, R2 = 0.1444, P = 0.014, Fig. 7g). Thus, as relative humidity rose, the levels of airborne animal-derived particles increased. By contrast, the levels of these particles decreased as the relative temperature increased. None of these three environmental parameters were correlated with the levels of plant-derived particles (P = 0.1220, P = 0.1132, and P = 0.4928 for temperature, relative humidity and altitude, respectively; Fig. 7b, e and h). The Ct values for bacteria-derived particles were negatively correlated with relative humidity and altitude (altitude: r = − 0.5495, R2 = 0.3019, P = 0.0001, Fig. 7c; relative humidity: r = -0.3165, R2 = 0.1002, P = 0.0363, Fig. 7f) but positively correlated with temperature (r = 0.35377, R2 = 0.1251, P = 0.0185, Fig. 7i). Thus, bacterial levels in the air decreased with increasing relative humidity and altitude but increased with increasing temperature.
Linear correlations between biological particle levels and environmental factors. a Animal-derived particles versus altitude; b plant-derived particles versus altitude; c bacteria versus altitude, R2 = 0.3019, r = − 0.5495; d animal-derived particles versus relative humidity, R2 = 0.1958, r = 0.4425; e plant-derived particles versus relative humidity; f bacteria versus relative humidity, R2 = 0.1002, r = -0.3165; g animal-derived particles versus temperature, R2 = 0.1444, r = − 0.3800; h plant-derived particles versus temperature; i bacteria versus temperature, R2 = 0.1251, r = 0.3577
4 Discussion
4.1 Geographical and ecological factors
In this study, atmospheric bioaerosols of animal, plant and bacterial origin from Beijing, Hangzhou and Wuhan were analyzed using real-time PCR. Beijing, Hangzhou and Wuhan, which are located in northern, eastern and central China, respectively, are densely populated and have undergone rapid urbanization and industrialization over the last two decades. Each city was divided into two functional areas. Compared with urban functional area II, urban functional area I has abundant vegetation and is sparsely populated. However, increased levels of plant-derived particulates were only detected in urban functional area I of Hangzhou, and these levels were higher than those in area I of the other two cities. Based on geographical effects, Hangzhou has the highest plant coverage, at 69.8%, followed by Wuhan (65.1%) and Beijing (43.5%) (Ma et al. 2019; Huang and Xie 2009). Findings of previous studies support these results. For example, the plentiful vegetation and forest coverage in Hangzhou resulted in higher levels of plant-derived particles during summer (Song and Zheng 2012; Tang et al. 2019; Wang et al. 2009; Shen et al. 2011). In addition, despite their very low concentrations, animal-derived particles were still detected in urban areas. More than one-third of the Ct values measured in our study were below 36, with a minimum of 29. The levels of animal-derived particles in the air of Hangzhou were higher those in the other two cities. A previous study showed that the distributions and habitats of bird populations were positively correlated with plant coverage in scenic areas and national parks in Hangzhou (Zhou 2018). The large bird population may explain the higher levels of animal-derived particles in the city of Hangzhou (Lu et al. 2010).
Considering the influence of altitude, microorganisms have been detected in the air at elevations as high as 20–70 km (Després et al. 2012; Womack et al. 2010). In the present study, altitude as a geographical factor ranging from − 20 to 1100 m was also evaluated, although more than 85% of measurements were below 100 m. More systematic data are needed to thoroughly assess the relationship between bioaerosol level and altitude.
4.2 Anthropogenic factors
Bioaerosols are important components of atmospheric aerosols, and 80% of total airborne microorganisms are bacteria (Cao et al. 2014; Fröhlich-Nowoisky et al. 2016; Jaenicke 2005). In our study, we found significantly higher levels of bacteria-derived particles than of plant- or animal-derived particles in the air for all cities. Based on previous studies on the analysis of aerosol pollutant levels among these three cities in summer from 2014–2016, PM2.5 levels were higher in Beijing than in Hangzhou and Wuhan (Jia et al. 2018; Li 2017; Lu et al. 2018a, b). PM2.5 levels reflect the pollutant levels of particles with aerodynamic diameters less than 2.5 μm, including bacteria. This may be the reason for the higher levels of bacterial than of animal and plant particles in Beijing compared with other cities. This result is consistent with those of a previous analysis of PM2.5 pollutant levels among these three cities. This finding may be attributed to the fact that Beijing is a super metropolis with a total population of 21 million individuals. A high population density with large gatherings in public places, as well as a windy dry climate, promotes suspension of bacteria in the air (Ma et al. 2018; Wu et al. 2012).
4.3 Meteorological factors
Bioaerosols play important roles in atmospheric chemistry and the nucleation process and may influence the global climate (Morris et al. 2014). Meteorological factors, including temperature, relative humidity, wind direction, wind speed, solar radiation, precipitation and chemical factors, have significant impacts on the generation, composition, concentration and transmission of bioaerosols (Bertolini et al. 2013; Ferguson et al. 2019; Xie et al. 2018a, b a; Zhen et al. 2017).
For bacterial aerosols, temperature and relative humidity are two key factors that influence the viability and growth of microorganisms in the atmosphere (Harrison et al. 2005; Lu et al. 2018a, b; Mouli et al. 2005; Smets et al. 2016). Brągoszewska and Pastuszka (2018) reported that the propagation and survival of airborne bacteria increase in fall and winter and decrease in spring and summer (Brągoszewska and Pastuszka 2018). A study in western Oregon, USA, found that the abundance of airborne bacteria was positively correlated with temperature but negatively correlated with relative humidity (Tong and Lighthard 2000). Šantl-Temkiv et al. (2018) showed that bacterial densities were positively correlated with air temperature and negatively correlated with relative humidity in western Greenland during mid-summer. In our study, data from all three cities were pooled for linear correlation analysis with environmental factors. The temperatures ranged from 26° to 38° C. Positive correlations were observed between bacteria-derived particle levels and temperature, and negative correlations were observed between bacterial content and relative humidity. These results are consistent with those of previous studies (Uetake et al 2019; Šantl-Temkiv et al. 2018; Tong and Lighthard 2000; Brągoszewska and Pastuszka 2018). Microorganisms grow faster at higher temperatures (Xie et al. 2018a, b). Thus, our study is consistent with other published reports and supports the idea that high relative humidity keeps the ground surface wet. The resulting surface tension may keep particles attached to the surface and reduce the levels of bacteria-derived particles in the air (Jones and Harrison 2004; Šantl-Temkiv et al., 2018; Bragoszewska and Pastuszka 2018; Gao et al. 2016; Ferguson et al. 2019; Dybwad et al. 2014; Zhai et al. 2018). In our study, no correlation was detected between the level of plant-derived particles and temperature or humidity. Because of data collection limitations, we could not identify the correlations between pollen levels and meteorological factors. This finding was inconsistent with the results of Lo and Levetin (2007), who reported positive correlations between pollen levels and temperature. In future studies, specific primers and probes for detecting specific families or species of plants, including pollen, will be used in selected regions from each city to assess the correlations between meteorological factors and bioaerosol levels derived from specific plant species. Moreover, the levels of airborne animal-derived particles were negatively correlated with temperature and positively correlated with relative humidity. Generally, biological particles of animal origin have large size distributions and settle quickly due to gravitational forces. Once settled, particles may be re-suspended by wind or aeolian dust events and dispersed over long distances by air flow (Lutgring et al. 1997; Millner 2009; Park et al. 2018; Serrano-Silva and Calderón-Ezquerro 2018). However, recent research for the influence mechanism of meteorological conditions including temperature and relative humidity on the concentration and species composition of animal-derived bioaerosol is still insufficient. In future research, wind speed and precipitation must be considered as meteorological factors, and their effects on our monitoring results should be studied.
4.4 Uncertainties and limitations
As this was a preliminary study, it should be noted that Ct values instead of DNA copy numbers were used to quantify relative bioaerosol levels. Although copy number is a better indicator, we believe that our results still provide useful estimates. The experimental design needs to be refined in follow-up studies. More significant sampling sites will be selected with better climatic characteristics, including those in temperate zones, subtropical zones, tropical zones, plateaus and mountainous areas. In addition, long term monitoring is necessary to obtain more accurate temporal and spatial information on bioaerosol distributions. Samples must be taken at different locations simultaneously during all four seasons. The resulting data can be used for multiple analyses; for example, bacterial or viral detection can be carried out through culture methods or next-generation DNA sequencing approaches, but this is contingent on funding. Such an approach would enable more detailed and conclusive analyses of species diversity in relation to the impact of bioaerosols on human health. Further studies may focus on particle size distributions, compositions, microscopic structures, origins, dispersal capabilities and correlations with anthropogenic air, and these will be crucial to further developing processes to study transmission routes, animal sources of viruses and pathogenesis of global respiratory diseases.
References
Bertolini, V., Gandolfi, I., Ambrosini, R., Bestetti, G., Innocente, E., Rampazzo, G., et al. (2013). Temporal variability and effect of environmental variables on airborne bacterial communities in an urban area of Northern Italy. Applied Microbiology and Biotechnology, 97(14), 6561–6570.
Bowers, R. M., Clements, N., Emerson, J. B., Wiedinmyer, C., Hannigan, M. P., & Fierer, N. (2013). Seasonal variability in bacterial and fungal diversity of the near-surface atmosphere. Environmental Science and Technology, 47(21), 12097–12106.
Bowers, R. M., McLetchie, S., Knight, R., & Fierer, N. (2011). Spatial variability in airborne bacterial communities across land-use types and their relationship to the bacterial communities of potential source environments. The ISME journal, 5(4), 601–612.
Bragoszewska, E., & Pastuszka, J. S. (2018). Influence of meteorological factors on the level and characteristics of culturable bacteria in the air in Gliwice, Upper Silesia (Poland). Aerobiologia, 34(2), 241–255.
Brodie, E. L., DeSantis, T. Z., Parker, J. P. M., Zubietta, I. X., Piceno, Y. M., & Andersen, G. L. (2007). Urban aerosols harbor diverse and dynamic bacterial populations. Proceedings of the National Academy of Sciences of the United States of America, 104(1), 299–304.
Cao, C., Jiang, W., Wang, B., Fang, J., Lang, J., Tian, G., et al. (2014). Inhalable microorganisms in Beijing’s PM2.5 and pollutants during a severe smog event. Environmental Science and Technology, 48(3), 1499–1507.
Chien, Y. C., Chen, C. J., Lin, T. H., Chen, S. H., & Chien, Y. C. (2011). Characteristics of microbial aerosols released from chicken and swine feces. Journal of the Air and Waste Management Association, 61(8), 882–889.
Cox, R. N., & Clark, R. P. (1973). Natural-convection flow about the human body. Revue Generale de Thermique, 12(133), 11–19.
Després, V. R., Huffman, J. A., Burrows, S. M., Hoose, C., AleksandrS, S., Buryak, G., et al. (2012). Primary biological aerosol particles in the atmosphere: A review. Tellus B: Chemical and Physical Meteorology, 64(1), 145–153.
Diehl, K., Quick, C., Matthias-Maser, S., Mitra, S. K., & Jaenicke, R. (2001). The ice nucleating ability of pollen: Part I: laboratory studies in deposition and condensation freezing modes. Atmospheric Research, 58(2), 75–87.
Dybwad, M., Skogan, G., & Blatny, J. M. (2014). Temporal variability of the bioaerosol background at a subway station: Concentration level, size distribution, and diversity of airborne bacteria. Applied and environmental microbiology, 80(1), 257–270.
Esmaeil, N., Gharagozloo, M., Rezaei, A., & Grunig, G. (2014). Dust events, pulmonary diseases and immune system. American Journal of Clinical and Experimental Immunology, 3(1), 20–29.
Ferguson, R., Garcia-Alcega, S., Coulon, F., Dumbrell, A. J., Whitby, C., & Colbeck, I. (2019). Bioaerosol biomonitoring: Sampling optimization for molecular microbial ecology. Molecular ecology resources, 19(3), 672–690.
Fröhlich-Nowoisky, J., Kampf, C. J., Weber, B., Huffman, J. A., Pöhlker, C., Andreae, M. O., et al. (2016). Bioaerosols in the Earth system: Climate, health, and ecosystem interactions. Atomospheric Research, 182, 346–376.
Gabey, A. M., Vaitilingom, M., Freney, E., & Boulon, J. (2013). Observations of fluorescent and biological aerosol at a high-altitude site in central France. Atmospheric Chemistry and Physics, 13(15), 7415–7428.
Gandolfi, I., Bertolini, V., Ambrosini, R., Bestetti, G., & Franzetti, A. (2013). Unravelling the bacterial diversity in the atmosphere. Applied Microbiology and Biotechnology, 97(11), 4727–4736.
Gao, M., Yan, X., Qiu, T. L., Han, M. L., & Wang, X. M. (2016). Variation of correlations between factors and culturable airborne bacteria and fungi. Atmospheric Environment, 128, 10–19.
General Administration of Quality Supervision, Inspection and Quarantine of the People's Republic of China. (2003). Protocol of the real-time PCR for detecting genetically modified plants and their derived products. SN/T 1204—2003.
Goudie, A. S. (2014). Desert dust and human health disorders. Environment International, 63, 101–113.
Gu, X., Yao, L., Wang, L., et al. (2014). PCR detection method of plant ingredient in frozen surimi. Chinese Fishery Quality and Standards, 4(2), 44–49.
Haas, D., Galler, H., Luxner, J., & Zarfel, G. (2013). The concentrations of culturable microorganisms in relation to particulate matter in urban air. Atmospheric Environment, 65(2), 215–222.
Hallar, A. G., Chirokova, G., McCubbin, I., Painter, T. H., Wiedinmyer, C., & Dodson, C. (2011). Atmospheric bioaerosols transported via dust storms in the western United States. Geophysical Research Letters, 38, 2–7.
Harrison, R. M., Jones, A. M., Biggins, P. D., Pomeroy, N., Cox, C. S., & Kidd, S. P. (2005). Climate factors influencing bacterial count in background air samples. International Journal of Biometeorology, 49(3), 167–178.
Hebert, P. D. N., Cywinska, A., Ball, S. L., & deWaard, J. R. (2003). Biological identifications through DNA barcodes. Proceedings: Biological Sciences, 270(1512), 313–321.
Helin, A., Sietiö, O. M., Heinonsalo, J., Bäck, J., Riekkola, M. L., & Parshintsev, J. (2017). Characterization of free amino acids, bacteria and fungi in size-segregated atmospheric aerosols in boreal forest: Seasonal patterns, abundances and size distributions. Atmospheric Chemistry and Physics, 17, 13089–13101.
Huang, J., & Xie, Z. (2009). Analysis for monitoring vegetation coverage change of Wuhan based on remote sensing data of MODIS. Meteorological and Environmental Science, 32(2), 16–20.
Huffman, J. A., Prenni, A. J., DeMott, P. J., Pöhlker, C., Mason, R. H., Robinson, N. H., et al. (2013). High concentrations of biological aerosol particles and ice nuclei during and after rain. Atmospheric Chemistry and Physics, 13, 6151–6164.
Huffman, J. A., Sinha, B., Garland, R. M., & Sneepollmann, A. (2012). Size distributions and temporal variations of biological aerosol particles in the Amazon rainforest characterized by microscopy and real-time UV-APS fluorescence techniques during AMAZE-08. Atmospheric Chemistry and Physics, 12(12), 11997–12019.
Humbal, C., Gautama, S., & Trivedib, U. (2018). A review on recent progress in observations, and health effects of bioaerosols. Environment International, 118, 189–193.
Hurtado, L., Rodríguez, G., Lopez, J., Castillo, J. E., Molina, L., Zavala, M., et al. (2014). Characterization of atmospheric bioaerosols at 9 sites in Tijuana Mexico. Atmospheric Environment, 96(7), 430–436.
Hu, D., Wang-Li, L., Simmons, O. D., III., Classen, J. J., & Osborne, J. A. (2015). Spatiotemporal variations of bioaerosols in the vicinity of an animal feeding operation facility in the US. Journal of Environmental Protection, 6, 614–627.
Innocente, E., Squizzato, S., Visin, F., Facca, C., Rampazzo, G., Bertolini, V., et al. (2017). Influence of seasonality, air mass origin and particulate matter chemical composition on airborne bacterial community structure in the Po Valley, Italy. Science of The Total Environment, 593–594, 677–687.
Iversen, M., (1999). Humans effects of dust exposure in animal confinement buildings. Proceedings of the Dust control in animal production facilities International Symposium, Jutland, Denmark, 131–139.
Jaenicke, R. (2005). Abundance of cellular material and proteins in the atmosphere. Science, 308(5718), 73.
Jia, X., Yan, P., Dong, P., Zhang, X., Li, Y., & Guo, W. (2018). Variations of PM mass concentration at Chaoyang Stie in Beijing during. Meteorological Monthly, 44(11), 1489–1500.
Jochner, S., Lüpke, M., Laube, J., Weichenmeier, I., Pusch, G., Traidl-Hoffmann, C., et al. (2015). Seasonal variation of birch and grass pollen loads and allergen release at two sites in the German Alps. Atmospheric Environment, 122, 83–93.
Jones, A. M., & Harrison, R. M. (2004). The effects of meteorological factors on atmospheric bioaerosol concentrations - a review. Science Total Environmental, 326, 151–180.
Lee, B. U., Lee, G., & Heo, K. J. (2016). Concentration of culturable bioaerosols during winter. Journal of Aerosol Science, 94, 1–8.
Li, Z. (2017). Temporal and spatial distribution of PM2.5 and PM10 and correlation of particulate matters and meteorological factors in Wuhan. Journal of Green Science and Technology, 10, 66–71.
Lo, E., & Levetin, E. (2007). Influence of meteorological conditions on early spring pollen in the Tulsa atmosphere from 1987–2006. Journal of Allergy and Clinical Immunology, 119(1), S101.
Lutgring, K. R., Linton, R. H., Zimmerman, N. J., Peugh, M., & Heber, A. J. (1997). Distribution and quantification of bioaerosols in poultry-slaughtering plants. Journal of Food Protection, 60(7), 804–810.
Lu, Z., Wu, C., Yue, W., Feng, K., & Huang, M. (2010). The assessment on residential ecological environment in the central city of Hangzhou. Acta Ecologica Sinica., 30(11), 2856–2863.
Lu, R., Li, Y. P., Li, W. X., Xie, Z. S., Fan, C. L., Liu, P. X., et al. (2018). Bacterial community structure in atmospheric particulate matters of different sizes during the haze days in Xi’an, China. Science of The Total Environment, 637–638, 244–252.
Lu, S., Wu, Y., & Wang, Y. (2018). Analysis on the characteristics of spatial and temporal distribution and cause in Hangzhou PM2.5. Journal of Mudanjiang University, 27(4), 43–46.
Maki, T., Hara, K., Kobayashi, F., Kurosaki, Y., Kakikawa, M., Matsuki, A., et al. (2015). Vertical distribution of airborne bacterial communities in an asian-dust downwind area, noto peninsula. Atmospheric Environment, 119, 282–293.
Maron, P. A., Lejon, D. P. H., Carvalho, E., Bizet, K., Lemanceau, P., Ranjard, L., et al. (2005). Assessing genetic structure and diversity of airborne bacterial communities by DNA fingerprinting and 16S rDNA clone library. Atmospheric Environment, 39(20), 3687–3695.
Ma, J., Li, C., Kwan, M., & Chai, Y. (2018). A multilevel analysis of perceived noise pollution, geographic contexts and mental health in Beijing. Int J Environ Res Public Health, 15(7), 1479.
Ma, M., Xu, F., & Dang, A. (2019). Study on the spatial temporal change of vegetation coverage between the belts of Beijing’s main urban area based on dynamic remote sensing data. Journal of Environmental Engeering Technology, 9(4), 404–413.
Ma, Z., Zhang, H., & Zhang, J. (2009). The analysis on vegetation coverage of Hangzhou based on MODIS system. Journal of Anhui Agri Sci, 34(18), 4618–4619.
Millner, P. D. (2009). Bioaerosols associated with animal production operations. Bioresource Technology, 100(22), 5379–5385.
Morris, C. E., Conen, F., Alex, H. J., Phillips, V., Pöschl, U., & Sands, D. C. (2014). Bioprecipitation: A feedback cycle linking earth history, ecosystem dynamics and land use through biological ice nucleators in the atmosphere. Global Change Biology, 20(2), 341–351.
Mouli, P. C., Mohan, S. V., & Reddy, S. J. (2005). Assessment of microbial (bacteria) concentrations of ambient air at semi-arid urban region: Influence of meteorological factors. Applied Ecology and Environmental Research, 3(2), 139–149.
Ortiz-Martínez, M. G., Rodríguez-Cotto, R. I., Ortiz-Rivera, M. A., Pluguez-Turull, C. W., & Jiménez-Vélez, B. D. (2015). Linking endotoxins, African dust PM10 and asthma in an urban and rural environment of Puerto Rico. Mediators of Inflammation. https://doi.org/10.1155/2015/784212.
Park, J., Li, P. F., Ichijo, T., Nasu, M., & Yamaguchi, N. (2018). Effects of Asian dust events on atmospheric bacterial communities at different distances downwind of the source region. Journal of Environmental Sciences, 72, 133–139.
Pöschl, U. (2005). Atmospheric aerosols: Composition, transformation, climate and health effects. Angewandte Chemie International Edition, 37(7), 7520–7540.
Radonić, A., Thulke, S., Mackay, I. M., Landt, O., Siegert, W., & Nitsche, A. (2004). Guideline to reference gene selection for quantitative real-time PCR. Biochemical and Biophysical Research Communications, 313(4), 856–862.
Šantl-Temkiv, T., Gosewinkel, U., Starnawski, P., Lever, M., & Finster, K. (2018). Aeolian dispersal of bacteria in Southwest Greenland: Their sources, abundance, diversity and physiological states. FEMS Microbiology Ecology, 94, 1–10.
Serrano-Silva, N., & Calderón-Ezquerro, M. C. (2018). Metagenomic survey of bacterial diversity in the atmosphere of Mexico City using different sampling methods. Environmental Pollution, 235, 20–29.
Shen, Q., Qin, J., & Cao, L. (2011). Quantitative Classification and Ordination of Shrub-grass Vegetation on Hangzhou’s Xixi Wetland. Journal of Zhejiang International Studies University., 4, 92–100.
Skotak, K., Degorska, A., Ulanczyk, R., & Pecka, T. (2016). Carbonaceous aerosol. An indicator of the human activity impact on environment and health. Przemysl Chemiczny, 95(3), 548–553.
Smets, W., Moretti, S., Denys, S., & Lebeer, S. (2016). Airborne bacteria in the atmosphere: presence, purpose, and potential. Atmospheric Environment, 139, 214–221.
Song, B., Sun, Y., & Xu, D. (2018). Distribution of artemisia pollen and its effect on airway responsiveness in patients with allergic rhinitis. Chinese Medical Digest, 33(5), 385–387.
Song, G., Zheng, X. (2012). The Analysis on types and environmental functions of the wetland in Zhejiang province. 2012 2nd International Conference on Applied Social Science, 361–366.
Straumfors, A., Heldal, K. K., Eduard, W., Wouters, I. M., Ellingsen, D. G., & Skogstad, M. (2016). Cross-shift study of exposure–response relationships between bioaerosol exposure and respiratory effects in the Norwegian grain and animal feed production industry. Occupational and Environmental Medicine, 73(10), 685–693.
Tang, Z., Zeng, X., & Zhao, H. (2019). Research on tree array landscape in urban open space of Hangzhou and Its Influence on environment factors in summer. Journal of Chinese Forestry., 17(2), 1–5.
Tong, Y., & Lighthart, B. (2000). The annual bacterial particle concentration and size distribution in the ambient atmosphere in a rural area of the Willamette Valley Oregon. Aerosol Science Technology, 32, 393–403.
Uetake, J., Tobo, Y., Uji, Y., Hill, T. C. J., DeMott, P., Kreidenweis, S., & Misumi, R. (2019). Seasonal changes of airborne bacterial communities over tokyo and influence of local meteorology. Front Microbiol., 10, 1572.
Veillettea, M., Bonifaita, L., Mbarechea, H., Marchandb, G., & Duchainea, C. (2018). Preferential aerosolization of Actinobacteria during handling of composting organic matter. Journal of Aerosol Science, 116, 83–91.
Walser, S. M., Gerstnera, D. G., Brennera, B., Büngerb, J., Eikmannc, T., Janssena, B., et al. (2015). Evaluation of exposure–response relationships for health effects of microbial bioaerosols–A systematic review. International Journal of Hygiene and Environmental Health, 218(7), 577–589.
Wan, S. H. (2015). The study of universal real-time PCR detection methods for polluted microorganisms in food. South China University of Technology. 1–6.
Wang, Q., Ge, J., Xu, X., & Wu, Z. (2009). Research on vegetation coverage in Wuhan Donghu. Science and Technology Innovation Herald, 8, 223–224.
Wittmaack, K., Wehnes, H., Heinzmann, U., & Agerer, R. (2005). An overview on bioaerosols viewed by scanning electron microscopy. Science of the Total Environment, 346(1–3), 244–255.
Womack, A. M., Bohannan, B. J. M., & Green, J. L. (2010). Biodiversity and biogeography of the atmosphere. Philosophical Transactions: Biological Sciences, 365(1558), 3645–3653.
Wu, Y., Chan, C., & Chew, G. L. (2012). Meteorological factors and ambient bacterial levels in a subtropical urban environment. International Journal of Biometeorology, 56, 1001–1009.
Xie, Z. S., Fan, C. L., Lu, R., Liu, P. X., Wang, B. B., Du, S. L., et al. (2018). Characteristics of ambient bioaerosols during haze episodes in China: A review. Environmental Pollution, 243, 1930–1942.
Xie, Z., Li, Y., Lu, R., Li, W., Fan, C., Liu, P., et al. (2018). b). Characteristics of total airborne microbes at various air quality levels. Journal of Aerosol Science, 116, 57–65.
Yamaguchi, N., Ichijo, T., Sakotani, A., Baba, T., & Nasu, M. (2012). Global dispersion of bacterial cells on Asian dust. Scientific Reports, 2, 525.
Yoo, K., Lee, T. K., Choi, E. J., Yang, J., Shukla, S. K., Hwang, S., et al. (2017). Molecular approaches for the detection and monitoring of microbial communities in bioaerosols: A review. Journal of Environmental Sciences, 51, 234–247.
Zhai, Y., Li, X., Wang, T., Wang, B., Li, C., & Zeng, G. (2018). A review on airborne microorganisms in particulate matters: Composition, characteristics and influence factors. Environment International, 113, 74–90.
Zhen, Q., Deng, Y., Wang, Y., Wang, X., Zhang, H., Sun, X., et al. (2017). Meteorological factors had more impact on airborne bacterial communities than air pollutants. Science of the Total Environment, 601–602, 703–712.
Zhou, W. (2018). Study on plant landscape of birds habitat in urban green space at City parks in Hangzhou. Zhejiang Agriculture and Forestry University Master Degree Thesis. 54–57.
Acknowledgements
This study was supported by a Grant (2016YFF0103103) from the National Key R&D Program of China, the Chinese Academy of Inspection and Quarantine (CAIQ 2018JK015) and the National Key R&D Program of China (2018ZX10101003-002-004). Special thanks are due to all personnel involved in completing the experiments.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Supplementary information
Below is the link to the electronic supplementary material.
Supplementary file 1
Graph of housekeeping gene amplification in collected samples. (a) Animal-derived amplification, (b) plant-derived amplification, and (c) bacteria. (TIF 4246kb)
Rights and permissions
About this article
Cite this article
Ma, X., Zhang, Q., Liu, K. et al. Spatial distribution of atmospheric bioaerosols in Beijing, Hangzhou and Wuhan, China. Aerobiologia 37, 155–170 (2021). https://doi.org/10.1007/s10453-020-09680-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10453-020-09680-6