Abstract
In virtue of a curved insertion path inside tissues, needle steering techniques have revealed the potential with the assistance of medical robots and images. The superiority of this technique has been preliminarily verified with several maneuvers: target realignment, obstacle circumvention, and multi-target access. However, the momentum of needle steering approaches in the past decade leads to an open question—“How to choose an applicable needle steering approach for a specific clinical application?” This survey discusses this question in terms of design choices and clinical considerations, respectively. In view of design choices, this survey proposes a hierarchical taxonomy of current needle steering approaches. Needle steering approaches of different manipulations and designs are classified to systematically review the design choices and their influences on clinical treatments. In view of clinical consideration, this survey discusses the steerability and acceptability of the current needle steering approaches. On this basis, the pros and cons of the current needle steering approaches are weighed and their suitable applications are summarized. At last, this survey concluded with an outlook of the needle steering techniques, including the potential clinical applications and future developments in mechanical design.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The term “minimally invasive surgery (MIS)” was first coined in 1984 by John Wickham [9]. Over time, MIS procedures are more approved by patients in comparison to open surgery with upsides of fewer complications, shorter hospital stays, and superior long-term overall survival [10]. Needles, characterized by elongated bodies and invasive tips, are considered the least invasive medical tools in MIS, where needle insertion is performed to reach the target region to conduct subsequent local diagnosis or therapies [11, 12].
In virtue of advanced medical image techniques and specialized surgical robots, surgeons are able to comprehend the spatial structure of the anatomy and work in tandem with robots to perform new procedures that were considered too dangerous in previous times [21,22,23]. Despite the purported advantages of image-guided robotic-assisted needle interventions, needle insertion still suffers major challenges in some clinical applications including tumor surgery and neurosurgery. First, there is a notable challenge in accurately targeting a tumor. Needles will deviate from the intended trajectory due to unavoidable errors such as imaging quality, tissue heterogeneity, and respiration affection [24]. This could lead to serious problems ranging from false negatives in diagnosis [25] to local tumor recurrence in therapy [26]. Second, it is difficult to find a safe path to avoid anatomical obstacles (e.g., bones) and sensitive parts (e.g., vessels) to reach the target in brain surgery [27]. In some cases, a safe path does not even exist [28].
Needle steering techniques enable a needle to reach targets with a curved insertion path, showing great potential to solve the aforementioned challenges and expand the current medical practices in the past two decades. Various needle steering approaches have been developed to achieve the curved insertion path. A technological roadmap in Fig. 1 shows the major achievements of needle steering approaches, from which it is known that various types of active needles become a significant issue in recent years. Active needles, which can be directly manipulated intracorporeally, possess larger steering potential and controllability than passive needles (traditional needles which can only be interfered with extracorporeally) and have drawn a lot of attention from researchers in recent years. The progress in a variety of design choices and their supporting actuation systems have induced and facilitated the development of some potential steering approaches. The earliest practice of manipulation to steer the needle can be traced back to the time when physicians performed needle insertion. They discovered that bevel-tip needles (the most common asymmetric-tip needle) can naturally bend when inserted into tissue, resulting in a deflection error on the tip. Instead of decreasing the deflection error (e.g., using needles with larger bending stiffness), physicians exploited such error to counterbalance other errors through some extracorporeal manipulation (e.g., rotation of the needle base) [29]. With the development of surgical robots and medical image techniques, some robotic-assisted image-guided extracorporeal manipulation (e.g., duty-cycled rotation [30]) were developed to manipulate the traditional needle, enabling more sophisticated steering motions. For passive needles, it is unable to control the part of needles inside the tissue. Thus, the manipulation can only be applied extracorporeally; for active needles, the manipulations are based on the mechanical and actuation design of the needles. The active parts of the needles can be manipulated to help with needle steering. For instance, concentric tube needles (CTNs) consist of several concentric tubes that can steer along a time-independent curve outside the tissue [31]. This steering approach is considered to have the potential for new surgical procedures via cavity such as transbronchial needle aspiration [32]. In short, over the last two decades, a variety of needle steering approaches have been developed, and active needles with different design choices have shown great potential in clinical procedures.
Motivations
Our survey is motivated and structured by several factors based on the surveys related to needle steering techniques in the last ten years. In comparison with them, this survey enables a more definite taxonomy of design choices, a more systematic discussion of design considerations, and a more complete summary of potential applications, as briefed in Table 1. Specifically, the motivations are concluded as follows:
-
First, an organized taxonomy of needle steering approaches is required. It has been almost a decade since the highest-cited survey was published [13], in which needle steering approaches are classified as the needle is either active or passive. This classification was reasonable at that time and provided guidance for subsequent designs; however, since a variety of design choices then emerged, a modified classification based on the previous literature is in urgent need. Recently, a classification has been proposed [15], which focuses on the principles that implement three-dimensional (3D) steering motion based on several combinable motion choices. However, the mechanical designs as well as their clinical considerations are not discussed at length.
-
Second, the applicability of needle steering approaches for specific medical treatment needs to be considered. On the one hand, although the needle steering approaches proposed in recent years have advantages in steerability, some manipulations, and design choices may increase clinical risks or even conflict with medical demand. On the other hand, some needle steering approaches have potential benefits for clinical treatment such as improving imaging quality. Thus, an insightful discussion of clinical considerations is deeply needed for a specific procedure.
-
Last, needle steering techniques have shown superiority in some promising clinical treatments. To the best of our knowledge, the most instructive summary is concluded in 2006 [33]. Over the decade, more potential applications such as in transbronchial lung biopsy [34] are being explored. Although some of them are also mentioned in recent surveys [17, 35], the reviewed applications are partial and not related to the needle steering approaches, which is not conducive to demand-oriented design for engineers.
Contributions
This survey gives a comprehensive summary of needle steering approaches to answer an open question—“How to choose an applicable needle steering approach for a specific clinical application?” Since the concerned topics of modeling, path planning, and control of needle steering have been well reviewed recently [14, 16, 18, 19], we discuss the topic related to design choices in this survey. The contributions are listed as follows:
-
This survey first proposes a hierarchical taxonomy of needle steering approaches based on two unambiguous and rational classification criteria. To this end, the taxonomy can provide insight into the characteristics and considerations of current steering approaches. In addition, the hierarchical structure also provides room for newly developed steering approaches.
-
This survey summarizes the clinical considerations of current needle steering approaches, providing comprehensive considerations for peers when they solve related clinical problems. These considerations are summarized in connection with engineering and clinical issues. Deep thinking on these issues is a prerequisite for a medical-graded steerable needle system for a specific application.
-
This survey presents the potential clinical applications and outlooks of the mechanical design of the needle steering approaches. The potential applications of brain, lungs, liver, kidneys, and prostate are discussed from four perspectives, i.e., leading diseases, promising treatments, steering strategies, and beneficial results. Furthermore, we conclude the design development into three aspects: miniaturization of needle structure, integration of steering approaches, and intellectualization toward needle-like robot.
Organization
The structure of this survey is organized as hereunder mentioned. In “Overview and Hierarchical Classification” section, an overview of the needle steering techniques is presented, where the concept of the needle is identified, including the concept, structures, and mechanisms of the needle steering. Based on the overview, a hierarchical taxonomy of current needle steering approaches is proposed. Accordingly, the needle steering approaches can be first divided into two groups: passive steering approaches in “Passive Steering Approaches” section and active steering approaches in “Active Steering Approaches” section. For each subcategory, the mechanism, working principles, design choices, and clinical applicability are detailedly reviewed. In “Discussions” section, we discuss the clinical considerations of needle steering and weigh up the pros and cons of the current steering approaches for specific clinical applications. At last, in “Outlooks” section, we summarize the five most potential clinical applications as expectations for the future and put forward the future developments of steerable needles in terms of mechanical design.
Overview and Hierarchical Classification
This section demonstrates an overview for a better and deeper understanding of the needle steering techniques. We first differentiate the needles from other easily confused interventional tools. Then, some key mechanical components and functional sections of needle steering, which are continued to use in this survey, are clarified. After that, the concepts of passive and active needle steering mechanisms are carried out and elaborated with an example. At last, a hierarchical taxonomy is proposed to classify the current needle steering approaches.
Differentiating Needles from Catheters and Wires
Previous research findings in the engineering field into needle steering confuse the needle with some other interventional tools due to their similarity in structure (mostly catheters and wires); however, these interventional tools are markedly different in clinical usage. To reduce confusion, we draw a distinction between the needles and the catheters and wires in the context of interventional radiology (a medical specialty that performs various image-guided MIS procedures).
In interventional radiology, needles are medical tools with invasive tips, helping create access inside the body through insertion motion in the tissue. Wires are usually introduced into vessels after the access is created by needles. Catheters are always advanced over a wire to avoid scraping the vessel lumen or are introduced in the internal cavity [36]. Although the catheters and wires can be used with invasive tips [37, 38], the two are notably different from the needles in the tissue environment [39].
Mechanical Components and Functional Sections
Figure 2a shows a schematic diagram of the insertion of a trocar needle into tissue with a bevel tip, in which most of the needle-steering-related components are included. The template and sheath are two assistant components commonly used in some needle interventions. For instance, the grid templates are used in prostate brachytherapy (BT) to precisely determine the distance between seeds [40]. The sheaths are used in some percutaneous needle insertion procedures to lend support and provide controlled access [41].
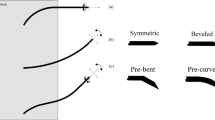
Overview of needle steering inside the tissue. a The insertion of a bevel-tip trocar needle into tissue, in which six mechanical components and three functional sections are labeled. b Two types of needle tips: symmetric tip and asymmetric tip. The latter can be reclassified into three types according to tip asymmetry: bevel, pre-bend, and pre-curve. The asymmetry of the needle tip produces a resultant lateral load, which causes a flexible needle naturally bending. c An example of obstacle avoidance during insertion illustrates the differences between passive (Needle 1) and active (Needle 2) steering mechanisms. The letter “S” in the figure represents the word “Step”
The components of the needle are divided into three sections according to their functions: (1) needle base (manipulation section), through which the cannula and stylet are manipulated, (2) needle body (curve section), which is flexible and bends during needle steering, and (3) needle tip (invasion section), which overcomes cutting force to cut the tissue. Of note, both stylet and cannula can be invasive. However, in the context of needle steering, the cannula is often used to prevent stylet from contacting tissue or to actively bend [42, 43]. Thus, the cannula in Fig. 2a is illustrated in a non-invasive pattern.
Needle Steering Mechanisms
Although there are many steering approaches based on various types of needles, we suppose the steering mechanisms can be categorized as one of two: (1) passive steering mechanism and (2) active steering mechanism. The essential difference between these two mechanisms is whether the force that bends the needle body is generated passively during insertion or actively by interventions.
The following parts introduce the two steering mechanisms in detail and end with an example to better illustrate their major differences.
Passive Steering Mechanism
The passive steering mechanism depends on tip asymmetry during needle insertion. Needles with asymmetric tips bend unavoidably during insertion due to the resultant lateral load, as presented in Fig. 2b. The bevel-tip needle (the most common type of asymmetric-tip needle) is used as an example to illustrate this mechanism from mechanical and phenomenological aspects.
Mechanically, after the bevel-tip needle punctures the tissue, the needle insertion force can be mechanically divided into three parts: friction, cutting force, and tissue deformation force [44, 45]. Among them, only the cutting force has a component perpendicular to the insertion direction. Due to the flexibility of the needle body, the needle bends and compresses the tissue, leading to the tissue deformation force [46]. In the above process, the asymmetric cutting force caused by the asymmetry of the tip during insertion is passively generated, which is the direct cause of the needle steering. This kind of mechanism is called the passive steering mechanism.
Phenomenologically, a bevel-tip needle steering inside the tissue can be considered a constant-curvature continuum robot abiding by follow-the-leader deployment [47]. The follow-the-leader manner refers to the unchanged state of the curved shape of the needle body despite the advance of the needle tip [28]. This is because the needle body is relatively more flexible than the tissue. The steering path of the bevel-tip needle is approximately a curve with a constant radius of curvature (ROC), which is velocity independent while determined by both the mechanical parameters of the needle and tissue [33, 48].
The minimum ROC of the steering path becomes a key parameter that embodies the steering ability and has been examined in many needle steering approaches. Steering with smaller ROC by optimizing the geometric parameters of the bevel tip can achieve more delicate manipulation, which has been well concluded in the existing literature [13]. As shown in Fig. 2b, needles with pre-bent and pre-curved tips (collectively called pre-shaped tips in this survey) perform smaller ROC than bevel-tip needles [49]. When it comes to the long pre-curve arc lengths, the ROC of the steering path was found close to the ROC of the pre-curve [50]. In addition to the efforts to reduce the ROC of the steering path, real-time change of the ROC is also needed to enlarge the manipulability of steering motion.
Active Steering Mechanism
The active steering mechanism is the result of needles actively bending. In general, steerable needles based on the active steering mechanism can be considered needle-like hyper-redundant or continuum robots working inside the tissue and overcoming the tissue deformation force to reach the target [1]. To this end, most of the approaches with the active steering mechanism are independent of tissue and insertion, which enables more steering strategies than approaches with the passive steering mechanism. That means they can steer outside the tissue and steer without insertion motion. In addition, because the steering motions do not involve the cutting force, the active steering mechanism enables smaller ROC than passive steering mechanisms [51, 52].
Structurally, most of the needles with the active steering mechanism have more complicated mechanical designs with active degrees of freedom (DOFs), which results in diversified steering strategies. For example, these needles can continue to bend during insertion for realignment [53], or, can approach the target through a large bend motion after insertion [54]. In addition, surgery, such as transoral surgery and enteric surgery, can be operated by going through curved cavities to reach the target [55].
However, active steering mechanisms usually have problems with complex mechanical and actuation designs. Under the condition of the narrow environment limited by tissue, the design work becomes more challenging. Furthermore, steerable needles based on the active steering mechanism usually fail to achieve the follow-the-leader manner, which is only realizable with special designs [56].
Obstacle Avoidance Example
As shown in Fig. 2c, an example of obstacle avoidance is presented to better illustrate the differences between the two steering mechanisms. This operation is helpful in some medical practices such as reaching the prostate with interference [53]. Needle 1 (bevel-tip needle) and needle 2 (symmetric-tip needle) are manipulated based on passive and active steering mechanisms, respectively. Needle 1 can rotate axially and needle 2 can actively bend. The following four steps render how the two needles use the two steering mechanisms to achieve obstacle avoidance.
-
Step 1: Two needles are inserted into the tissue. Needle 1 bends naturally and avoids the obstacle. Needle 2 remains straight.
-
Step 2: Needle 1 is axially rotated \(180^\circ\) to change the bevel-tip orientation. Needle 2 actively bends to avoid the obstacle.
-
Step 3: Needle 1 is naturally steered along a curved path and reaches the target. Needle 2 actively bends in the opposite direction to reach the target.
-
Step 4: Needle 2 reaches the target along a straight tip path.
This example clearly illustrates some of the characteristics and differences of the two steering mechanisms. First, the working principles are totally different. Second, the tip trajectory of needle 1 is a curve with constant ROC, while the trajectory of needle 2 is made up of some lines with abrupt curves. Third, it can be obviously judged from the trajectories of the needle body that only needle 1 is steered in line with the follow-the-leader manner.
Hierarchical Classification of Needle Steering Approaches
This section presents a scientific classification of state-of-the-art needle steering approaches with a hierarchical structure. We set two classification criteria, by which the steering approaches are classified into four categories. Then, based on the characteristics of manipulations and mechanical designs, we classified the four categories into ten subcategories. The complete diagram of the hierarchical classification is presented in Fig. 3. The two classification criteria are proposed as follows.
-
(1)
The needle steering is based on the passive mechanism or active mechanism.
-
(2)
The needle steering is carried out by passive needles or active needles.
Criterion (1) distinguishes between passive and active steering mechanisms. The essential difference between these two mechanisms is whether the force that bends the needle body is generated passively during insertion or actively by interventions, which have been summarized in “Needle Steering Mechanisms” section. Criterion (2) distinguishes between passive and active needles. Active needles have driving DOFs and can be directly manipulated intracorporeally while the steering of the passive needle can only be interfered with by extracorporeal manipulation. These two kinds of needles appear successively with the development of the needle steering technique, which is briefly summarized in “Introduction” section.
According to Criterion (1), the needle steering approaches can be classified into (1) passive steering approaches and (2) active steering approaches. For passive steering approaches, needle steering is inevitable. Manipulations and needle designs only play a supplementary role in steering. According to Criterion (2), passive steering approaches can be further divided into (1) axial rotation, namely, rotating the needle base, and (2) active-tip needle, namely, actively changing the shape of the needle tip. For active steering approaches, needle steering is realized by actively compressing the tissue. According to Criterion (2), active steering approaches can be further divided into (1) lateral manipulation, an extracorporeal manipulation to laterally compress the tissue, and (2) active-body needle, namely, actively bending the needle body to achieve steering.
Each of the four categories is further subdivided into multiple subcategories based on the characteristics of manipulations and mechanical designs, as shown in Fig. 3. This classification based on these two criteria also provides room for newly developed steering approaches.
Passive Steering Approaches
Passive steering approaches exploit the tip asymmetry of the flexible needles that naturally bend due to the resultant lateral load. Due to the follow-the-leader manner, the insertion trajectory is considered a constant-ROC curve. By performing some specific manipulations extracorporeally and designing some active mechanisms with different structures, a more complicated steering curve (a 3D curve with ROC variable) can be achieved.
In this section, we classified the passive steering approaches into (1) axial rotation, which is the only accessible extracorporeal manipulation during insertion due to the limitation of the needle entry point, and (2) active-tip needle, of which the shape of the tip can be actively changed due to specifically designed structures during insertion.
Axial Rotation
Axial rotation of the needle base is a common and effective manipulation choice in needle steering, which aims at changing the orientation of the needle tips. According to the patterns and functions of rotation, we classified the axial rotation into two subcategories: (1) intermittent rotation, namely the rotation is intermittent during insertion to change the steering direction, and (2) duty-cycled rotation, namely the rotation motion is periodic with duty cycles. In this way, the ROC of the steering curve can be modified during insertion.
Notably, the two rotation choices can be implemented in combination to realize a more complicated curve while the minimum ROC of the curve is limited [59]. The minimum ROC depends on both the mechanical properties of tissue and needles. Although the manipulation of axial rotation owns the convenience that transforms the steering path without the requirement of needle design during insertion, the insertion accuracy and tissue damage problems are newly introduced by this manipulation.
Intermittent Rotation
Needle steering with intermittent rotation is achieved by dividing the needle insertion process into several stages. A rotation motion on the needle base is applied for each stage to change the tip orientation. The direction of needle steering is discretely modified due to these rotation motions [60]. Rotating the needle base \(180^{\circ }\) is an effective way to realize a relatively complex curve in the plane [61], as shown in Fig. 4. This method is applicable to many current surgical requirements such as avoiding obstacles in the straight-line insertion path and in-plane target realignment. In addition, intermittent rotation can achieve a 3D steering curve with constant ROC (changing the torsion angle) [62]. Steering in 3D space not only can compensate for out-of-the-plane errors [63] but also can implement a 3D insertion path in complex environments [64].
When twisting the needle base, the radical friction between the tissue and the needle body causes a discrepancy between the base and tip twist angles (e.g., a lag of more than 45\(^\circ\) for a 10 cm insertion depth [65]) due to the torque. This can cause a large out-of-plane error when performing a unidirectional, large-angled rotation. To reduce the error, the torsional dynamics are modeled and the controller is designed for error compensation [66, 67]. However, the risk of rupturing the needle maintains when relatively fine needles are used such as in brain surgery.
Remarkably, pre-shaped-tip needles are commonly used in needle steering to achieve smaller ROC of the steering curve [68]. In comparison with the rotation of bevel-tip needles, the rotation of needles with pre-shaped tips involves some new problems. On the one hand, the pre-shaped tip is subjected to larger lateral force while rotating, leading to aggravation of tip angle lagging. On the other hand, rotation of kinked pre-shaped tips causes larger tissue damage—the rigid nature of the kinked tip leads to a steering path with a diameter larger than the needle body. To handle the problems, a flexure-based needle tip is proposed to simultaneously maintain the maximum curvature while minimizing the tissue damage [57].
Duty-Cycled Rotation
Duty-cycled rotation is another extracorporeal manipulation that can real-time modify the ROC of the steering path when inserted into tissue; however, this manipulation cannot decrease the minimum ROC of the steered needle [30, 69, 70]. It is based on the demonstrated hypothesis that a straight insertion path with a bevel-tip needle can be achieved by spinning the needle with applicable rotation speed and insertion speed during insertion. In fact, the trajectory of the insertion path is helical but appears straight [71]. Then, by changing the duty cycle of the spinning, different ROC can be achieved. Figure 4 presents the insertion of a bevel-tip needle using the duty-cycled approach. When \(\text {duty cycle}=0\%\), the ROC of the steering path is equivalent to that of directly inserting the bevel-tip needle.
Tissue wind-up is a severe problem caused by friction between the needle shaft and tissue when the needle rotates. This phenomenon is especially serious in duty-cycled rotation due to the increment of rotation cycles. Needle insertion experiments in phantom [72] and ex vivo beef tissue [73] both show that unidirectional rotation rises concerns about this problem. According to [73], the wind-up phenomenon is more serious in ex vivo beef tissue (trials have to be discontinued due to tissue wind-up). In addition, continuous unidirectional rotation can also cause cable wind-up issues of instrumentation. Thus, some bidirectional rotation methods are proposed by periodically reversing the direction of axial rotation [72]. Modified duty-cycled strategies are proposed to elegantly solve the cable wind-up issues of instrumentation with no hardware changing requirement [74]. By introducing a cannula outside the needle, the tissue wind-up could also be eliminated.
Illustration of rotatable joint-tip needles with their joint structure and actuation choices emphasized. a Cable-driven joint-tip needle. Berg et al. [3]. b Shape memory alloy wire-actuated joint-tip needle. Konh et al. [75]. c Magnetic-actuated joint-tip needle. Pratt et al. [2]. d Joint-tip needle with a close-loop cable. Adebar et al. [76]. e Joint-tip needle with a flexure joint. Berg et al. [77]. f Joint-tip needle of which the joint is made of cables. Scali et al. [78]
Active-Tip Needle
With specially designed mechanical structures and supporting actuation systems, active-tip needles can actively change their tip during insertion. The objective of changing the tip shape is to change the tissue lateral reaction force, so that based on the passive steering mechanism the ROC of the insertion path can be adjusted. Active-tip needles achieve higher steering ability by means of mechanical and actuation design. Rotation-free 3D steering motion is in demand by active needles to eliminate the aforementioned rotation-related problems such as base-tip lag and tissue damage. Correspondingly, larger design and control complexities are introduced in active-tip needles.
We classified current active-tip needles into three subcategories based on different mechanical structures: (1) rotatable joint-tip needle, (2) programmable bevel-tip needle, and (3) retractable joint-tip needle. Among them, the actuation of rotatable joint-tip needles is challenging because the active joint is required to be actuated inside the tissue.
Rotatable Joint-Tip Needle
Joint-tip needles possess an active joint near the tip, providing a rotational motion between the needle tip and the body. Due to the narrow working environment along the flexible needle, the actuation choices of the joint-tip needles are limited. Thus, joint-tip needles can be mainly actuated in one of three ways: cable-driven actuation, shape memory alloy (SMA) wire-based actuation, and magnetic actuation.
Among the three actuation methods, cable-driven actuation is the most universal way to drive the active joint. Composed of a cable system, the motors can drive the joint remotely outside the tissue [79]. As shown in Fig. 5a, the length between the two parts of the joint is changed by pulling the cables. This method is also capable of rapid manually hand–eye operation in combination with medical images [77, 80]. SMA wires are self-actuated and also used to actuate the joint [75, 81, 82], as shown in Fig. 5b. The actuation process is similar to the cable-driven actuation. The differences lie in the two ends of the SMA wires are fixed and the length of the SMA wires is self-changed. This method saves limited operating space due to the unnecessary external actuation systems. However, the SMA actuation includes some major problems in electric and heat insulation issues as well as the time efficiency [83]. Unlike the aforementioned two actuation choices, magnetic actuation is an untethered way that using the magnetic field for actuation, which is presented in Fig. 5c. This actuation enables a simpler structure (a ball joint and a magnetic tip) [2, 84,85,86,87]. However, the control method is relatively complex and this method is not suitable for the magnetic resonance imaging (MRI) environment.
Except for actuation choices, the design of the active joint is also essential in joint-tip needles. As shown in Fig. 5a and b, the most common design is a rotating pair actuated by a variable-length tendon (in the form of cables or SMA wires) to rotate the joint. In general, one open-loop variable-length tendon can only generate 0.5-DOF on the needle tip (providing unidirectional rotation). Thus, four variable-length tendons with four actuators are required for a ball joint to realize 2-DOF rotation motion [3]. A close-loop method is proposed in Fig. 5d, where the cable is secured to the distal hinge section with adhesive [76], realizing 1-DOF planar rotation with one actuator. As shown in Fig. 5e, another way to decrease the actuator number is introducing the flexible joints, which utilize the elasticity of the joint to recover the rotation angle when releasing the cables [41]. Figure 5f presents a joint made of three relatively thick cables. In that way, the needle could be designed smaller [78].
Recently, a waterjet needle is proposed that has its invasive tip a “waterjet” [88, 89]. Although the cutting force is not the bending source of the needle. We still classified this needle into a rotatable joint-tip needle for the waterjet tip is rotatable and the steering motion is a concomitant motion of the insertion that is produced by the tissue reaction force. Of note, the ROC of the waterjet needle is not limited to the tissue stiffness but is decided by the water velocity.
Programmable Bevel-Tip Needle
The programmable bevel-tip needle (PBN) is a biomimetic concept design proposed in 2010 [90]. With a dozen years of development, improvements have been made in many aspects. The original prototype, a two-segments mechanism with 12 mm in outer diameter, can only verify the steerability in principle [91]. The up-to-date mechanism is a clinically sized (2.5 mm in outer diameter), medical-graded product, comprising four slender segments connect by the interlocking mechanism [92]. By adjusting the relative axial distance between the four segments, the bevel shape of the tip can be ”programmed,” enabling different ROC and torsion angles of the steering curve.
Certain studies ignore the inner connectivity during mechanical design and fail to use the needle in some applications such as drug delivery and aspiration. In PBN, each segment has a single lumen, providing a channel from tip to base. These channels not only meet the medical needs but also provide ways for tethered sensors, such as electromagnetic sensors for pose estimation [4, 93] and fiber Bragg grating sensors for shape reconstruction [94]. Not like the joint-tip needles that would cause tissue damage when rotating the needle tip, the “program” process of PBN does not compress the tissue. Thus, the steering process is safer. Due to the four-segment construction and the smaller interlocking mechanism, it is challenging to apply the PBN to high-gauge needle applications.
Retractable Trocar-Tip Needle
Retractable motion in trocar can also be used to change the tip shapes. As far as the best knowledge of authors, there are two types of constructions. One construction consists of a relative stiffness cannula and a stylet with pre-bent tips pre-hidden in the cannula [95, 96]. Due to the large stiffness of the cannula, the needle appears to be approximately straight. By increasing the stick-out length of the stylet tip, more pre-curved-tip shapes can be achieved. Another construction consists of a flexible stylet with an exposed asymmetric tip and a relative stiffness cannula [97]. A flexure joint is formed near the tip by sticking out the stylet, and the joint would be forced to bend to form a pre-bend tip during insertion. More pre-bend-tip shapes can be achieved by increasing the stick-out length.
A relatively small diameter (e.g., 0.46 mm in [97]) can be achieved due to the simple structure and extracorporeal actuation pattern, which satisfies most of the clinical applications of high-gauge needles. However, on account of the requirements of the stiffness discrepancy between the stylet and cannula, the steering ability is limited.
Active Steering Approaches
Active steering approaches achieve the desired needle configuration by actively bending motion based on mechanical and actuation design. The tissue is compressed during the bending so that most of the active steering approaches do not qualify for the follow-the-leader manner. Since the steering motion is not generated by cutting the tissue but inside the needle, the steering motion can be achieved outside the tissue, and its ROC is not limited by the mechanical property of the tissue.
In this section, we classified the active steering approaches into (1) lateral manipulation, namely the needle is laterally manipulated to achieve steering motion, and (2) active-body needle, namely the needles could actively change their body shape to help with steering motion.
Lateral Manipulation
Lateral manipulation is the earliest robotic-assisted steering approach, in which the needle acts as the end effector. In manipulating the six DOFs of the needle, four of them contribute to compressing the tissue (excluding axial rotation and translation insertion). However, in some cases, the four contributory DOFs are constrained due to assisted medical tools (manipulation with a template) or damage considerations (manipulation within the entry point). Hence, we classified the lateral manipulation into (1) unconstrained lateral manipulation and (2) constrained lateral manipulation.
Of note, tissue manipulation is another way that is similar to lateral manipulation [98, 99]. Nevertheless, this method requires squeezing the tissue in the outer contour to bend the needle, which limits the applications (mostly in the breast). With its more limited application and poorer function, we excluded this method from our needle steering approaches.
Unconstrained Lateral Manipulation
Unconstrained lateral manipulation can apply arbitrary motion on the needle base. Pioneer works were carried out on formulating the relationship between the base and tip velocities. Both the numerical model that formulates a needle manipulation Jacobian to describe the base-tip motion relation [100, 101] and the analytical model based on a linear beam supported by virtual springs are developed [5]. In comparison with the numerical model, the analytical one allows combination with the medical imaging device and realizes real-time closed-loop control for needle insertion procedures [102, 103]. However, the steering ability decreases as the insertion depth increases due to the increase of tissue deformation force.
To this end, needle steering methods that fuse lateral manipulation at the base and bevel-tip needle-based axial rotation are proposed. The research point lies in the control law design to operate the two steering methods collaboratively as well as reduce the tissue deformation [104, 105].
Constrained Lateral Manipulation
Some clinical procedures conduct needle insertion with a template, which introduces a cylindrical pair constraint near the needle base. Thus, lateral manipulation can be only implemented on the needle body between the tissue and the template. In [58], A mechanics-based deflection model is established, which is compatible with multi-layer tissue. In addition, a model-based control algorithm is presented to minimize needle deflection at the final insertion depth [40].
Except for the usage of templates, limiting the motion of the needle entry point is also considered a beneficial constraint to reduce the damage caused by tearing the entry point. By introducing this remote-center-of-motion constraint in the form of control law or mechanical design, the needle can only be manipulated within the entry point, which reduces the tissue damage [106, 107].
Active-Body Needle
Active-body needles are considered needle-like hyper-redundant or continuum robots, which compete with the tissue to achieve the desired configurations. Thus, active-body needles have multifarious design choices with different working principles. Based on this, we classified the active-body needles into three categories: (1) bendable joint-body needle, (2) concentric tube needle, and (3) compliant mechanism needle. Among them, only the concentric tube needle is the only one that has both abilities of out-of-the-tissue steering and follow-the-leader deployment, which can steer through the intracorporeal cavity to reach the target.
Bendable Joint-Body Needle
Joint-body needles are similar to joint-tip needles in structure, i.e., owning the active joints and their supporting actuation. The difference lies in the joints of joint-body needles are on purpose to bend the needle body. In order to avoid excessive local bend angle, the joints are generally multiple and distributed in the needle body.
Although SMA wire-actuated method requires relatively more complex considerations in comparison with cables, it is commonly used in joint-body needles due to its high power density to compete with tissue deformation force. The considerations include: (1) the SMA wires are generally heated by Joule heat generated electrically so the thermal and electrical insulation issues need to be considered, (2) the process of heating and naturally cooling down the SMA wires is too slow, and (3) the nonlinearity and hysteresis of the SMA materials embarrass the modeling accuracy of the orientation angle of the needle tip.
Many studies of joint-body needles have been proposed [108,109,110,111], and two pieces of work are representative. The first study proposed a discretely actuated joint-body needle using the bending mode of SMA wires [1, 83, 112]. The needle consists of two joints and can be actuated by one power supply on a pulse width modulation-based control scheme. The needle owns a hollow core for the delivery of medical tools. The second study developed a needle with a set of slits on its body machined by laser (distributed flexure joints). The SMA wire is clamped at the ends of the joint and uses tension mode rather than bending mode for high force generation [113]. The optical heating method is used instead of Joule heating to actuate the SMA wires, which avoids artifacts created by current in MRI environment [114,115,116]. However, this needle has a solid core and the steering motion is only unidirectional.
Notably, most of the bendable joint-body needles have their needle body soft, while the needle bodies become rigid when actuated to change the insertion angle. In comparison with abruptly applying a large joint angle, it is preferable to gradually increase the joint angle during the insertion procedure to reduce tissue damage.
Concentric Tube Needle
Concentric tube needles (CTNs) are a concept established in the context of concentric tube robots, which are continuum robots that comprise a series of pre-curved elastic tubes that are translated and rotated with respect to each other to control the shape of the robot and tip pose [117]. The main advantage of CTNs that distinguishes them from other active-body needles is that the CTNs can realize the follow-the-leader deployment on the premise of meeting some design requirements [28]. This expands the application of the original needles—needles can steer through open or liquid-filled cavities to enter the tissue [6, 55].
The follow-the-leader manner has been the major design guideline for clinical-demanded CTNs [118,119,120]. In [28], the follow-the-leader solution of special cases including circular tubes and helical tubes is analyzed, and a metric for evaluating the similarity of an approximate follow-the-leader deployment is also proposed. Recently, In [119], more comprehensive follow-the-leader possibilities are analyzed. It is found that deformed helices with exponentially varying curvature magnitude can be a candidate for follow-the-leader design. Moreover, the potentially exploitable kinematic possibilities are also discussed. It has been proved that CTNs with variable stiffness tubes in association with additional motions can increase the possibilities of motion and geometry in the parts of the needles [119]. Several studies have combined CTNs with the aforementioned bendable joint-body needles, in which the joint-body needles act as the inner tube (stylet) of the CTNs (cannula) [121]. In [122], the joint-body needle and the bevel tip are integrated into needles to raise the operability.
Although the CTNs can accomplish a theoretical follow-the-leader manner, the stiffness of the proximal tube decreases with the hierarchical deployment of the tubes. Thus, affected by tissue reaction forces, the proximal tubes will suffer a relatively large offset [28]. In addition, the deployment of CTNs involves snapping problems, which is an unexpected release of the elastic potential energy. The energy is accumulated due to tube bending and twisting, which may damage tissue and needles. To handle this, a redundant CTN is proposed to achieve snap-free motion [123], and a nonlinear model predictive controller is designed to steer the CTN away from dangerous configuration [124].
Compliant Mechanism Needle
Compliant mechanism needle exploits structural mechanics to actuate the needle body with base manipulation. The compliant mechanism is a kind of mechanism that has no actual joints and achieves motions by elastic deformation of the flexible part. Current studies machined the needle (or part of them) into two axially parallel parts. By changing the relative angle or position of the two parts, a bending motion can be realized. This method realizes both the intuitive operability mechanism and the simple internal structure. A loop-shaped compliant mechanism is proposed with a modeling method based on constant-curvature assumption [43, 54]. Models based on the pseudo-rigid body method with parallel structures are also proposed [7, 128].
Compliant mechanism needles have simple actuation patterns with large steering ability. However, the design choices are limited due to the slender body of the needle, i.e., a closed-loop mechanical transmission chain is required in a slender structure to bend the needle body. Furthermore, the fabrication of the complex structure also limits the miniaturization of compliant mechanism needles.
Discussions
Currently, some of the current research focuses on the design and optimization of the needle steering approaches while ignoring or taking incomprehensive consideration of their target application. In fact, needle steering should be application oriented to solve concrete clinical problems. With a large and growing number of needle steering choices, clinical considerations should play the guiding role in selecting appropriate steering approaches as well as detailed design choices.
In the aforementioned paragraphs, we have proposed a taxonomy to classify the current needle steering approaches (deriving ten subcategories), which gives insight into the current needle steering approaches. For each approach (subcategory), corresponding design choices and clinical applicability are reviewed. In this section, we first discuss the clinical considerations of the classified steering approaches. Some influential literature with key information is concluded and presented in Table 2. Then, we weigh up the pros and cons of these steering approaches in terms of clinical applications. The discussed results are presented in Table 3. The objective is to answer the core question—“How to choose an applicable needle steering approach for a specific clinical application?” in terms of mechanical design.
Clinical Considerations
We concluded and discussed the clinical considerations from two aspects: steerability (i.e., the steering ability) and clinical acceptability. From the aspect of steerability, is the primary guarantee for the success of specific surgery and acceptability ensures the safety of the operation. Meanwhile, for steering approaches that are capable of specific applications, clinical acceptability should be considered to lower surgical risks and achieve better outcomes of treatment.
Steerability
In general, stronger steerability means more complex manipulation or mechanical design. To avoid redundant complexity, getting the right match for the application and steerability is significant. At present, the concept of steerability is not clearly defined. In different studies, different connotations are adopted such as needle manipulation Jacobian [101], steering index and steering condition number [129], and maximum curvature [8]. However, none of these measures is capable of giving an intuitive trade-off of the current steering approaches. Hence, based on previous research, we consider the steerability of the classified approaches qualitatively and quantitatively in this survey.
Qualitatively, based on existing clinical demands, we concluded the steerability with four types of steering strategies: target realignment, obstacle circumvention, multiple target access, and steering via cavity. Target realignment is the simplest steering strategy since only a mild adjustment to the steering motion is required. This strategy is essential in all highly accurate insertion conditions to achieve the specified trajectory in combination with feedback control. Obstacle circumvention is also a commonly used method to circumvent obstacles in the way of needle insertion such as vessels and bones. In general, a planar constant-ROC steering motion is enough to deal with the potential interference. However, a safe planar circumvention path may not exist under the complex manipulation environment (e.g., involving large or multiple obstacles), which requires a 3D variable-ROC curve. Multiple target access is similar to complex obstacle circumvention while a more accurate steering path is required to allow the needle path through the desired points. Steering via cavity is required in some transbronchial and transintestinal procedures, which requires some steering approaches that can steer outside the tissue as well as abide by the follow-the-leader manner.
Quantitatively, several considerable indexes are used to describe the steerability of needle steering approaches. The indexes include the DOF, minimum ROC, and maximum deflection. Among them, the DOF is a universal index, while the minimum ROC is usually adopted by steering approaches that abide by the follow-the-leader manner (passive steering approaches and concentric tube needles). The maximum deflection is mostly used when the follow-the-leader manner is inapplicable (other needle steering approaches). In general, 1-DOF needle steering indicates the relationship between needle tip position and insertion depth is determined immutably, which means the steering path requires to be preoperatively determined. 2-DOF needle steering has two modules: one module is the insertion path extended into 3D space while the relationship between depth and deflection magnitude remains unchanged; the other module can customize the deflection magnitude when steering in the plane. 3-DOF needle steering integrates the functions of the above two modules. Minimum ROC and maximum deflection are two parameters with similarity in function, either of which can sufficiently estimate the realizable maximum bending capability of a steerable needle. Unlike the minimum ROC which is not related to the insertion depth, the maximum deflection is usually dependent on depth. The maximum deflection is measured immediately after the steerable needle bends or after inserting the needle some distance. In some specific cases such as joint-body needles, the maximum joint angle is an alternative to maximum deflection. Table 2 lists the related information of recent remarkable research.
Acceptability
Tissue damage is a considerable factor in needle steering techniques, which increases blood loss (especially in the liver and kidney), risk of serious complications (especially in the lumbar and breast), and intraoperative pain. Most of the needle steering approaches would cause varying degrees of tissue damage, such as tissue wind-up of axial rotation, major tissue compression of needles with the active steering mechanism, and minor tissue compression of joint-tip and trocar-tip needles. Of note, for PBN, the steering motion does not cause additional tissue damage. Except for friction damage and compressing damage, some improper actuation designs integrated into the needle can also cause tissue damage, such as excessive temperature by heating the SMA wire and tendons exposed outside the needle.
Medical imaging modalities, such as computed tomography (CT), ultrasound (US), and MRI play an important role in needle steering techniques. The assistance can be concluded in two aspects: (1) preoperative path planning, which provided key anatomical information to plan the needle insertion path, and (2) intraoperative path realignment and replanning, which can real-time adjust the steering trajectory and avoid undesired obstacles, whereas different imaging modalities may have good or bad effects on different steering approaches, and vice versa. CT can accurately detect small differences in the density of various tissues (e.g., the structure and calcifications) in a lateral anatomical plane while having low contrast to soft tissue in some cases. Thus, this imaging modality is often introduced for preoperative path planning of needle steering. However, due to the hazardous ionizing radiation, this modality cannot be used in feedback control to real-time guide the insertion path. US is an imaging method with almost no hazards and low cost and is a commonly used method to improve insertion accuracy. Due to the feature of small size and real-time imaging, this imaging modality is the first priority to provide real-time dynamic images for intraoperative path realignment and replanning. However, in the US images, needles are not always visible. Decreasing the angle between US beam and needle and needle diameter would make visualization difficult. Furthermore, the real-time imaging mode of US may decrease the visibility of needles [130]. A study shows that needles with compliant joints may increase the visibility of needles [131]. MRI has unique advantages over other medical imaging modalities, including high spatial resolution, real-time imaging, and no ionizing radiation hazard to patients. Although MRI does not provide real-time imaging, the produced high-quality images can ensure the correctness of the preoperatively planned path. However, the material and actuation of the needle steering system must conform to MRI compatibility, which includes electromagnetic interference, material incompatibility, etc. [21, 132, 133].
Needle diameter is a considerable parameter in needle-based interventions. An appropriate diameter is mainly determined by surgical requirements, tissue types, and patient characteristics. However, most of the steering approaches with active needles achieve large steerability at the expense of increasing the needle diameter. Composed of active joints, multiple parallel tubes, actuation parts, etc., a high-gauge (small-diameter) active needle is hard to design and fabricate. Among them, the retractable trocar-tip needles can achieve a relatively large ROC with a small diameter due to the relatively simple structure.
Choosing Appropriate Needle Steering Approaches
Having summarized the clinical considerations, we put forward the suitable applications of the ten subcategories of needle steering approaches by evaluating their steerability, characteristics, limitations, cons, and pros. Table 3 summarizes the above topics, where two subcategories of rotation manipulations are combined into the axial rotation approach and two subcategories of lateral manipulations are combined into the lateral manipulation approach for their similarity in the summarized topics.
The axial rotation and lateral manipulation are the only two methods that can use traditional (passive) needles to conduct steering motions. In comparison with active needles, passive needles are low cost and can be adopted in some surgery requiring high-gauge needles. Moreover, they can be easily integrated into other active needles to enlarge their performances. Lateral manipulations have large steerability in shallow tissue by compressing tissue, while the compression is hard to pass from the needle base to the deep tissue. The insertion depth also influences the performance of axial rotation approaches due to the increased friction during rotation, which can reduce rotation accuracy as well as introduce the risk of needle fracture in high-gauge conditions.
Active needles based on the passive steering mechanism possess the follow-the-leader motion inherently because they only actively change the tip shapes to further change the magnitude of lateral cutting force. Correspondingly, the minimum ROC is limited by the mechanical properties of the insertion media. Rotatable joint-tip needles can directly manipulate the tip orientation to realize a 3D steering curve. The ROC of the curve can be modified smaller by increasing the tip angle and the tip length. However, the small ROC is dependent on the large diameter of the needle tip, which generates a large lateral reaction force. Thus, this approach is inapplicable to applications requiring high-gauge needles. The PBNs have multiple channels from tip to base, which can easily integrate fiber sensors and conduct treatments such as drug delivery and aspiration. Because the shape-changing process do not change the maximum diameter of the PBN, no additional tissue damage would produce during modifying the ROC during steering motion. Retractable trocar-tip needle has a relatively simple structure among all the active needles. On this basis, this needle can simultaneously achieve a relatively small ROC than passive needles during steering and a relatively simple structure for high-gauge needle purposes, whereas only 1-DOF steering can be conducted by this needle providing the axial rotation manipulation is not introduced.
Active needles based on the active steering mechanism possess large steerability because their needle bodies can actively bend. To this end, needles can perform insertion-free steering motion and can steer outside the tissue. Correspondingly, most of these needles do not abide by the follow-the-leader deployment (except for some of the CTNs) and cause relatively large tissue damage than passive steering approaches. Rotatable joint-body needles, which are similar to rotatable joint-tip needles in structure and actuation design, are also not suitable for high-gauge applications. And their mechanical structures are more complex. Since the active bending motion is insertion independent, this type of needle can real-time change the tip position after reaching the target. This characteristic is helpful for surgery such as BT to keep the distance from the needle tip to the target in time without insertion adjustment. CTNs are the only type of needles that can achieve follow-the-leader deployment as well as steer outside the tissue simultaneously. Thus, this needle is the most applicable choice for surgery with the requirement of reaching targets through internal ducts and cavities of the human body, such as transbronchial needle aspiration. The applications of compliant mechanism needles are similar to the rotatable joint-body needle due to their similar ways of bending. The discrepancy lies in the bending of compliant mechanism needles is driven by the mechanical structure of the needle body cut by laser. Thus, compliant mechanism needles possess a relatively compact structure. Nevertheless, the slenderness of the needle body limits the design of compliant mechanisms. Compliant mechanism needles of 2 DOFs are not only difficult to design, and the structure of the needle body is relatively fragile.
Outlooks
The recent progress in needle steering techniques reveals potential advantages in the medical field, while much work needs to be done to clinically implement these techniques. In this section, as presented in Fig. 6, we summarize five potential applications of needle steering techniques related to five vital organs (brain, lungs, liver, kidneys, and prostate). For each of the organs, we demonstrate the importance of needle steering to it from four perspectives: the current leading disease, promising treatment to the disease, steering strategies applied in the treatment, and the beneficial result beneath the strategies. At last, we highlight the future developments of needle steering techniques in terms of mechanical design.
Five potential initial application areas of the needle steering from different organs of the body, including brain, lungs, liver, kidney, and prostate. For each organ, the current leading disease, promising treatment to the disease, steering strategies applied in the treatment, and the beneficial results the strategies bring are concluded
Potential Application
Brain
Keyhole neurosurgery (mostly in the brain) aims at achieving maximal surgical efficiency with minimum trauma and has been preferentially chosen with the clear advantage of reducing the risk of complications [134]. In this surgery, needles are commonly used by surgeons to reach the target for further operation through a small dura opening [135].
The use of needles covers many promising keyhole neurosurgery. First, brain biopsy (usually fine needle biopsy) is effective in the case of uncertainty to obtain samples of intracranial tissue for diagnostic purposes [136]. Second, deep brain stimulation (DBS, placement of a needle-like neurostimulator in the brain) is highly effective in controlling movement disorders in patients, such as Parkinson’s disease [137]. Third, convection-enhanced delivery (CED, using bulk flow rather than conventional diffusion) is adopted in brain drug delivery to bypass the blood–brain barrier and raise treatment efficiency [138].
Needle steering techniques can reduce the risk of intracranial hemorrhage (ICH), which is the most frequent and devastating complication in the brain [27]. Two key factors cause the ICH: on the one hand, due to the resolution limitations of current image devices, the needle injures some small-diameter vessels that are undetectable preoperatively [139]; on the other hand, due to the insertion accuracy, the needles may accidentally puncture the vessel near the insertion path. To handle the ICH complication, needle steering techniques can perform intraoperative circumvention of vessels as well as raise insertion accuracy to reduce the risk [140, 141]. Indeed, sometimes a safety straight-line insertion path that avoids all the vessels and keeps enough distance threshold is even unfindable [28]. However, needle steering techniques can provide a safe 3D curve to guide the needle reaching the target [142, 143].
Lungs
Lung cancer is leading cancer that causes the greatest number of deaths [144]. Transbronchial needle aspiration (TBNA) is a promising investigation approach for diagnosing and staging this cancer. In comparison with the conventional approach consisting of several investigative procedures, TBNA reduces the median time-to-treatment decision [145] and reduces the risk of pneumothorax [146]. In addition, for mediastinal disease diagnosis, TBNA is also a primary part [147].
Needle steering techniques can improve the current TBNA mainly in two aspects: increasing the targeting accuracy and extending the territory of diagnosis. First, the movement of the lung tumor during respiration can reach up to 30 mm, which seriously affects the success rate of targeting [148]. With the help of steerable needles, the targeting accuracy can be intraoperatively compensated [149]. Second, steerable needles enable more complex motion to reach deeply into the bronchus, as well as extend the aspiration territory after puncture through the tissue [150]. Remarkably, the TBNA is not the only approach that combines the endoscope with needles. However, in comparison with other endoscope-needle investigations such as pancreatic cancer detection [151], TBNA is representative due to the complex and narrow environment in the bronchus with considerable motion planning problems [152, 153].
Liver
Hepatocellular carcinoma (HCC, the most common liver cancer) is one of the leading causes of cancer deaths worldwide [154]. The low five-year survival rate and the similarity between the incidence and mortality both indicate the significance of diagnosis and prognosis [144].
Liver biopsy is an interventional approach to the HCC diagnosis. Although validated imaging criteria have been developed for diagnosis, the biopsy is still required when the imaging criteria for diagnosis are not met or are not applicable [155]. Moreover, a prospective multicenter audit shows that up to 9% of patients are failing to detect HCC when adopting the imaging criteria [156]. Thermal ablation, most commonly radiofrequency ablation (RFA), is recommended to treat early-stage HCC with the tumor site less than 2 mm [154]. More recently, microwave ablation (MWA) and cryoablation are also used in early-stage HCC treatment. In comparison with RFA, MWA shortens the ablation time and is more applicable to the tumor near large vessels and relatively large (3–4 cm) [157]. Cryoablation has also occasionally been used in high-resource settings in HCC, its effectiveness, and safety in HCC are also proved [158].
Steerable needles provide ways to handle several major problems in HCC. First, needle steering techniques can raise the targeting accuracy of needle tips, which is both significant in biopsy and ablation. Due to the softness of liver tissue, large deformation occurs when the liver contact with the diaphragm during the respiratory motion (5–25 mm [77]). Since flexible needle insertion with tissue deformation remains an open question, it is hard to accurately target the lesion [159, 160]. Second, needle steering techniques can avoid obstacles (blood vessels, bile ducts, or lungs) in the liver to reach some lesions that would otherwise be inaccessible. [161, 162]. Third, a 3D steering curve extends the current surgical procedure, enabling multi-target ablation [41] or large liver tumor ablation [127] by puncturing the liver capsule only once. These procedures are not recommended before because multiple puncturing of the liver capsule would increase the risk of hemorrhage.
Kidneys
Kidney stones are a common clinical problem worldwide that can eventually induce chronic kidney disease and renal function decline [163]. Percutaneous nephrolithotomy (PCNL) is the standard treatment to remove large (\(\ge\)2 cm) stones by a small puncture wound through the skin, associated with higher stone-free rates at the expense of higher complication rates, blood loss, and admission times [164]. Although invasiveness treatment such as mini-PCNL is implemented with smaller instruments and wounds, strategical adjustment remains an open question [165].
Needle steering techniques can reduce complications and blood loss in PCNL by deploying suitable and accurate percutaneous renal access [166]. Technically, PCNL is very difficult because the kidney is a very vascular organ, and delicate surgical techniques are required to avoid bleeding as much as possible. Meanwhile, the stones also need to be removed clearly. By introducing needle steering techniques, the needle can well follow the predefined trajectory to avoid unexpected injury [167]. Meanwhile, a safer curved path may be findable to reduce the side effects of PCNL [168, 169].
Prostate
Prostate cancer is common cancer worldwide and is the first leading cancer in the United States [144]. BT, with any form such as permanent BT, is very positive in high-risk prostate and is the most effective radiotherapy treatment for localized tumor [170]. This procedure places radioactive seeds inside or next to the prostate by means of long hollow needles to perform radiation therapy [171].
Needle steering techniques can not only increase the accuracy of seed implantation [172,173,174,175] but also enable the accessibility of large (\(\ge\) 50 cm\(^3\)) prostates with the contraindication known as pubic arch interference (PAI) [53, 176, 177]. Manual placement of the seed could lead to a position error of ±5 mm by an expert practitioner and is hard to compensate for intraoperatively [40]. Controlled robotically with needle steering techniques, a 0.5 mm error can be reached [178]. PAI leads to the target being unreachable with a straight-line insertion path, which excludes approximately 10% of patients from the BT treatment. Thanks to the steering techniques, a curved path can be achieved to place the seed even with interference [53].
Future Development
Miniaturization of Needle Structure
Active needles increase their steerability at the expanse of complex mechanical structures, which results in concerned structure parameters (especially the maximum needle diameter) exceeding the medical grades. Currently, most of the active needles fail to reduce their diameter smaller than 18-gauge (18 G) grade, which is contrary to the current momentum of high-gauge needles. Some promising clinical applications, including lung aspiration, lumbar puncture, prostate biopsy, etc., usually require needles with diameters smaller than 18 G grade. Additionally, reducing the needle diameter within a certain range shows positive effects on some surgery. Notably, structure miniaturization not only shows advantages except for invasiveness but also reduces preoperative fear for the children.
Integration of Steering Approaches
In “Overview and Hierarchical Classification” section, the functional sections (i.e., needle base, needle body, and needle tip) have been defined. Remarkably, most of the current steering approaches usually involve the designs and manipulations of only one functional section. This enables the possibility of the integration of several needle steering approaches. To this end, the advantages of the selected steering approaches can be combined to break through the limitations in some current surgical procedures. For example, the integration of base manipulation and bevel-tip needles can modify the steerability of passive needles in deep tissue. Integrating CTNs and bendable joint-body needles can “program” the degree of pre-bending of each tube of CTN. Although integration of steering approaches can achieve larger steerability, the drawbacks of the integrated approaches may also be concentrated, and the integrated approach often leads to more complex design and control issues.
Intellectualization Toward Needle-Like Robots
After twenty years of development, steerable needles are no longer simply passive medical tools to provide access to the inner target. They become a kind of needle-like continuum or hyper-redundant robots equipped with sensors, actuators, and medical-related matters. As for the needle body, remarkable actuators including SMA wires and sensors including fiber Bragg grating (FBG) are integrated into needles to actuate the needle body and reconstruct the needle shape respectively. Integrated FBG sensors within sensory bundles are a promising way to sense the curvature and shape of steerable needles, which is crucial for achieving accurate and complex steering trajectories as well as providing the clinician with a graphical visualization of the robot shape inside the body. However, problems with the current techniques of FBG sensors, including limited strain tolerance and temperature sensitivity, limit the ability to sense large steering degrees as well as the sensing accuracy. These issues require further solutions. As for the needle tip, the piezoelectric microsystem is integrated into needle tips as mechanical sensors to detect tumor tissue. Some clinical applications involved with ablation or DBS require the needle tip as the generator to perform the treatment. We believe that needle steering techniques based on intelligent needles will become the development direction of needle intervention in the future.
Conclusion
Needle steering techniques can conduct needle insertion with a curved path by means of extracorporeal manipulations and mechanical designs of needles, which improves and extends the current modalities of MIS. This survey summarizes the design choices and clinical considerations of current needle steering approaches to answer the open question “How to choose an applicable needle steering approach for a specific clinical application?” from the perspective of mechanical designs. For this purpose, this survey proposes a definite taxonomy with a hierarchical structure to give an insight into the steering mechanism, working principle, and medical influence of needle steering approaches with different mechanical designs. The discussion section fully summarizes the clinical considerations of the classified needle steering approaches with their suitable clinical applications. As a newly developing technique developed for two decades, needle steering has shown great potential in some promising applications. We demonstrate this point and discuss the challenges in mechanical design for the future developments of next-generation steerable needles.
Abbreviations
- BT:
-
Brachytherapy
- CED:
-
Convection-enhanced delivery
- CT:
-
Computed tomography
- CTN:
-
Concentric tube needle
- DBS:
-
Deep brain stimulation
- DOF:
-
Degree of freedom
- G:
-
Gauge
- HCC:
-
Hepatocellular carcinoma
- TBNA:
-
Transbronchial needle aspiration
- ICH:
-
Intracranial hemorrhage
- MIS:
-
Minimally invasive surgery
- MRI:
-
Magnetic resonance imaging
- MWA:
-
Microwave ablation
- PAI:
-
Pubic arch interference
- PBN:
-
Programmable bevel-tip needle
- PCNL:
-
Percutaneous nephrolithotomy
- RFA:
-
Radiofrequency ablation
- ROC:
-
Radius of curvature
- SMA:
-
Shape memory alloy
- US:
-
Ultrasound
- 3D:
-
Three dimensional
References
Ayvali, E., and J. P. Desai. Optical flow-based tracking of needles and needle-tip localization using circular hough transform in ultrasound images. Ann. Biomed. Eng. 43(8):1828–1840, 2015.
Pratt, R. L., and A. J. Petruska. Empirically comparing magnetic needle steering models using expectation-maximization. Robotics. 11(2):49, 2022.
Berg, N. J., J. Dankelman, and J. J. Dobbelsteen. Design of an actively controlled steerable needle with tendon actuation and fbg-based shape sensing. Med. Eng. Phys. 37(6):617–622, 2015.
Matheson, E., and F. R. Y. Baena. Biologically inspired surgical needle steering: technology and application of the programmable bevel-tip needle. Biomimetics. 5(4):68, 2020.
Glozman, D., and M. Shoham. Flexible needle steering and optimal trajectory planning for percutaneous therapies. In: International Conference on Medical Image Computing and Computer-Assisted Intervention. New York: Springer, pp. 137–144.
Farooq, M. U., B. Xu, and S. Y. Ko. A concentric tube-based 4-dof puncturing needle with a novel miniaturized actuation system for vitrectomy. Biomed. Eng. Online. 18(1):1–16, 2019.
Berg, N. J., F. C. Meeuwsen, M. Doukas, G. Kronreif, A. Moelker, and J. J. Dobbelsteen. Steerable needles for radio-frequency ablation in cirrhotic livers. Sci. Rep. 11(1):309, 2021.
Rox, M., M. Emerson, T. E. Ertop, I. Fried, M. Fu, J. Hoelscher, A. Kuntz, J. Granna, J. E. Mitchell, M. Lester, F. Maldonado, E. A. Gillaspie, J. A. Akulian, R. Alterovitz, and I. J. RobertWebster. Decoupling steerability from diameter: helical dovetail laser patterning for steerable needles. IEEE Access. 8:181411–181419, 2020.
Schlich, T., and C. L. Tang. Patient choice and the history of minimally invasive surgery. The Lancet. 388(10052):1369–1370, 2016.
Topal, H., R. Aerts, A. Laenen, A. Collignon, J. Jaekers, J. Geers, and B. Topal. Survival after minimally invasive vs open surgery for pancreatic adenocarcinoma. JAMA Netw. Open. 5(12):2248147–2248147, 2022.
Tsumura, R., and H. Iwata. Trajectory planning for abdominal fine needle insertion based on insertion angles. IEEE Robot. Autom. Lett. 2(2):1226–1231, 2017.
Kim, Y.-J., S. B. Park, C.-H. Yoon, Y. Kim, H.-S. Kang, and Y.-H. Jo. Robust deflected path planning method for superelastic nitinol coaxial biopsy needle: application to an automated magnetic resonance image-guided breast biopsy robot. IEEE Trans. Robot. 38(4):2220–2237, 2022.
Berg, N. J., D. J. Gerwen, J. Dankelman, and J. J. Dobbelsteen. Design choices in needle steering—a review. IEEE/ASME Trans. Mechatron. 20(5):2172–2183, 2015.
Rossa, C., and M. Tavakoli. Issues in closed-loop needle steering. Control Eng. Pract. 62:55–69, 2017.
Scali, M., T. P. Pusch, P. Breedveld, and D. Dodou. Needle-like instruments for steering through solid organs: a review of the scientific and patent literature. Proc. Inst. Mech. Eng. 231(3):250–265, 2017.
Li, P., Z. Yang, and S. Jiang. Needle-tissue interactive mechanism and steering control in image-guided robot-assisted minimally invasive surgery: a review. Med. Biol. Eng. Comput. 56(6):931–949, 2018.
Audette, M. A., S. P. A. Bordas, and J. E. Blatt. Robotically steered needles: a survey of neurosurgical applications and technical innovations. Robot. Surg. 7:1–23, 2020.
Babaiasl, M., F. Yang, and J. P. Swensen. Robotic needle steering: state-of-the-art and research challenges. Intell. Serv. Robot. 15(5):679–711, 2022.
Wu, K., B. Li, Y. Zhang, and X. Dai. Review of research on path planning and control methods of flexible steerable needle puncture robot. Comput. Assist. Surg. 27(1):91–112, 2022.
Lu, M., Y. Zhang, C. M. Lim, and H. Ren. Flexible needle steering with tethered and untethered actuation: current states, targeting errors, challenges and opportunities. Ann. Biomed. Eng. 51(5):905–924, 2023.
Su, H., K. W. Kwok, K. Cleary, I. Iordachita, M. C. Cavusoglu, J. P. Desai, and G. S. Fischer. State of the art and future opportunities in mri-guided robotassisted surgery and interventions. Proc. IEEE. 110(7):968–992, 2022.
Omisore, O. M., S. Han, J. Xiong, H. Li, Z. Li, and L. Wang. A review on flexible robotic systems for minimally invasive surgery. IEEE Trans. Syst. Man Cybern. 52(1):631–644, 2022.
Yaniv, Z., P. Cheng, E. Wilson, T. Popa, D. Lindisch, E. Campos-Nanez, H. Abeledo, V. Watson, K. Cleary, and F. Banovac. Needle-based interventions with the image-guided surgery toolkit (igstk): from phantoms to clinical trials. IEEE Trans. Biomed. Eng. 57(4):922–933, 2010.
Yu, X., H. Wang, X. Ning, R. Sun, H. Albadawi, M. Salomao, A. C. Silva, Y. Yu, L. Tian, A. Koh, C. M. Lee, A. Chempakasseril, P. Tian, M. Pharr, J. Yuan, Y. Huang, R. Oklu, and J. A. Rogers. Needle-shaped ultrathin piezoelectric microsystem for guided tissue targeting via mechanical sensing. Nat. Biomed. Eng. 2(3):165–172, 2018.
Li, A. D. R., Y. Liu, J. Plott, L. Chen, J. S. Montgomery, and A. Shih. Multi-bevel needle design enabling accurate insertion in biopsy for cancer diagnosis. IEEE Trans. Biomed. Eng. 68(5):1477–1486, 2021.
McDermott, S., and D. A. Gervais. Radiofrequency ablation of liver tumors. Semin. Interv. Radiol. 30(1):49–55, 2013.
Ramakonar, H., B. C. Quirk, R. W. Kirk, J. Li, A. Jacques, C. R. Lind, and R. A. McLaughlin. Intraoperative detection of blood vessels with an imaging needle during neurosurgery in humans. Sci. Adv. 4(12):4992, 2018.
Gilbert, H. B., J. Neimat, I. Robert, and J. Webster. Concentric tube robots as steerable needles: achieving follow-the-leader deployment. IEEE Trans. Robot. 31(2):246–258, 2015.
Ahn, W., J. Bahk, Y. Lim, and Y. Kim. The effect of introducer gauge, design and bevel direction on the deflection of spinal needles. Anaesthesia. 57(10):1007–1011, 2002.
Bernardes, M. C., B. V. Adorno, P. Poignet, N. Zemiti, and G. A. Borges. Adaptive path planning for steerable needles using duty-cycling. In: 2011 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), pp. 2545–2550.
Girerd, C., A. V. Kudryavtsev, P. Rougeot, P. Renaud, K. Rabenorosoa, and B. Tamadazte. Automatic tip-steering of concentric tube robots in the trachea based on visual slam. IEEE Trans. Med. Robot. Bionics. 2(4):582–585, 2020.
Fu, M., A. Kuntz, I. Webster, J. Robert, and R. Alterovitz. Safe motion planning for steerable needles using cost maps automatically extracted from pulmonary images. In: 2018 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), pp. 4942–4949, 2018.
Webster, R. J., J. S. Kim, N. J. Cowan, G. S. Chirikjian, and A. M. Okamura. Nonholonomic modeling of needle steering. Int. J. Robot. Res. 25(5–6):509–525, 2006.
Hoelscher, J., I. Fried, M. Fu, M. Patwardhan, M. Christman, J. Akulian, I. Webster, J. Robert, and R. Alterovitz. A metric for finding robust start positions for medical steerable needle automation. In: 2022 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), pp. 9526–9533, 2022.
Cowan, N. J., K. Goldberg, G. S. Chirikjian, G. Fichtinger, R. Alterovitz, K. B. Reed, V. Kallem, W. Park, S. Misra, and A. M. Okamura. Chapter 23. Robotic needle steering: Design, modeling, planning, and image guidance, pp. 557–582, 2011.
Northcutt, B. G., A. A. Shah, Y. R. Sheu, and L. Carmi. Wires, catheters, and more: a primer for residents and fellows entering interventional radiology. RadioGraphics. 35(5):1621–1622, 2015.
Jeong, S., Y. Chitalia, and J. P. Desai. Design, modeling, and control of a coaxially aligned steerable (coast) guidewire robot. IEEE Robot. Autom. Lett. 5(3):4947–4954, 2020.
Ali, A., D. H. Plettenburg, and P. Breedveld. Steerable catheters in cardiology: classifying steerability and assessing future challenges. IEEE Trans. Biomed. Eng. 63(4):679–693, 2016.
Abolhassani, N., R. Patel, and M. Moallem. Needle insertion into soft tissue: a survey. Med. Eng. Phys. 29(4):413–431, 2007.
Lehmann, T., R. Sloboda, N. Usmani, and M. Tavakoli. Model-based needle steering in soft tissue via lateral needle actuation. IEEE Robot. Autom. Lett. 3(4):3930–3936, 2018.
Gerboni, G., J. D. Greer, P. F. Laeseke, G. L. Hwang, and A. M. Okamura. Highly articulated robotic needle achieves distributed ablation of liver tissue. IEEE Robot. Autom. Lett. 2(3):1367–1374, 2017.
Zhao, Y.-J., Z.-H. Liu, Y.-D. Zhang, and Z.-Q. Liu. Kinematic model and its parameter identification for cannula flexible needle insertion into soft tissue. Adv. Mech. Eng. 11(6):1687814019852185, 2019.
Yamada, A., N. Nitta, S. Naka, D. Khiem Tran, S. Morikawa, and T. Tani. Design and implementation of loop shaped steering mechanisms for flexible needles. In: 7th International Conference on the Development of Biomedical Engineering in Vietnam (BME7) Translational Health Science and Technology for Developing Countries 7, vol. 69, pp. 15–19.
Okamura, A. M., C. Simone, and M. D. O’Leary. Force modeling for needle insertion into soft tissue. IEEE Trans. Biomed. Eng. 51(10):1707–1716, 2004.
Zhao, B., L. Lei, L. Xu, S. Li, Y. Hu, J. Zhang, X. Yang, and Y. Zhang. Needle deflection modeling and preoperative trajectory planning during insertion into multilayered tissues. IEEE/ASME Trans. Mechatron. 26(2):943–954, 2021.
Khadem, M., C. Rossa, R. S. Sloboda, N. Usmani, and M. Tavakoli. Mechanics of tissue cutting during needle insertion in biological tissue. IEEE Robot. Autom. Lett. 1(2):800–807, 2016.
Webster, R. J., and B. A. Jones. Design and kinematic modeling of constant curvature continuum robots: a review. Int. J. Robot. Res. 29(13):1661–1683, 2010.
Wedlick, T. R., and A. M. Okamura. Characterization of pre-curved needles for steering in tissue. In: 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, pp. 1200–1203.
Majewicz, A., T. R. Wedlick, K. B. Reed, and A. M. Okamura. Evaluation of robotic needle steering in ex vivo tissue. In: 2010 IEEE International Conference on Robotics and Automation (ICRA), pp. 2068–2073.
Majewicz, A., S. P. Marra, M. G. V. Vledder, M. Lin, M. A. Choti, D. Y. Song, and A. M. Okamura. Behavior of tip-steerable needles in ex vivo and in vivo tissue. IEEE Trans. Biomed. Eng. 59(10):2705–2715, 2012.
Karimi, S., and B. Konh. Kinematics modelling and dynamics analysis of an smaactuated active flexible needle for feedback-controlled manipulation in phantom. Med. Eng. Phys.107:103846, 2022.
Burgner, J., P. J. Swaney, T. L. Bruns, M. S. Clark, D. C. Rucker, E. C. Burdette, and R. J. Webster. An autoclavable steerable cannula manual deployment device: design and accuracy analysis. J. Med. Devices.6(4):041007, 2012.
Konh, B., B. Padasdao, Z. Batsaikhan, and J. Lederer. Steering a tendon-driven needle in high-dose-rate prostate brachytherapy for patients with pubic arch interference. In: 2021 International Symposium on Medical Robotics (ISMR), pp. 1–7.
Yamada, A., S. Naka, N. Nitta, S. Morikawa, and T. Tani. A loop-shaped flexible mechanism for robotic needle steering. IEEE Robot. Autom. Lett. 3(2):648–655, 2018.
Lindenroth, L., S. Bano, A. Stilli, J. G. Manjaly, and D. Stoyanov. A fluidic soft robot for needle guidance and motion compensation in intratympanic steroid injections. IEEE Robot. Autom. Lett. 6(2):871–878, 2021.
Gilbert, H. B., and R. J. Webster. Can concentric tube robots follow the leader? In: 2013 IEEE International Conference on Robotics and Automation (ICRA), pp. 4881–4887.
Swaney, P. J., J. Burgner, H. B. Gilbert, and R. R. J. Webster. A flexure-based steerable needle: high curvature with reduced tissue damage. IEEE Trans. Biomed. Eng. 60(4):906–909, 2013.
Lehmann, T., C. Rossa, N. Usmani, R. Sloboda, and M. Tavakoli. Deflection modeling for a needle actuated by lateral force and axial rotation during insertion in soft phantom tissue. Mechatronics. 48:42–53, 2017.
A. Krupa. A new duty-cycling approach for 3d needle steering allowing the use of the classical visual servoing framework for targeting tasks. In: 2014 5th IEEE RAS/EMBS International Conference for Biomedical Robotics and Biomechatronics (BioRob), pp. 301–307.
Moreira, P., and S. Misra. Biomechanics-based curvature estimation for ultrasoundguided flexible needle steering in biological tissues. Ann. Biomed. Eng. 43(8):1716–1726, 2015.
Khadem, M., C. Rossa, N. Usmani, R. S. Sloboda, and M. Tavakoli. A two-body rigid/flexible model of needle steering dynamics in soft tissue. IEEE/ASME Trans. Mechatron. 21(5):2352–2364, 2016.
Khadem, M., C. Rossa, N. Usmani, R. S. Sloboda, and M. Tavakoli. Geometric control of 3d needle steering in soft-tissue. Automatica. 101:36–43, 2019.
Reed, K. B., A. M. Okamura, and N. J. Cowan. Controlling a robotically steered needle in the presence of torsional friction. In: 2009 IEEE International Conference on Robotics and Automation (ICRA), pp. 3476–3481.
Khadem, M., C. Rossa, N. Usmani, R. S. Sloboda, and M. Tavakoli. Feedbacklinearization-based 3d needle steering in a frenet-serret frame using a reduced order bicycle model. In: 2017 American Control Conference (ACC), pp. 1438–1443.
Reed, K. B., A. M. Okamura, and N. J. Cowan. Modeling and control of needles with torsional friction. IEEE Trans. Biomed. Eng. 56(12):2905–2916, 2009.
K. B. Reed. Compensating for torsion windup in steerable needles. In: 2008 2nd IEEE RAS/EMBS International Conference on Biomedical Robotics and Biomechatronics (BioRob), pp. 936–941.
Swensen, J. P., M. Lin, A. M. Okamura, and N. J. Cowan. Torsional dynamics of steerable needles: modeling and fluoroscopic guidance. IEEE Trans. Biomed. Eng. 61(11):2707–2717, 2014.
Berg, N. J., T. L. Jong, D. J. Gerwen, J. Dankelman, and J. J. Dobbelsteen. The influence of tip shape on bending force during needle insertion. Sci. Rep. 7:40477, 2017.
Engh, J. A., G. Podnar, D. Kondziolka, and C. N. Riviere. Toward effective needle steering in brain tissue. In: 2006 International Conference of the IEEE Engineering in Medicine and Biology Society, pp. 559–562.
Qi, Z., Q. Luo, and H. Zhang. A tube-based robust mpc for duty-cycled rotation needle steering systems with bounded disturbances. Trans. Inst. Meas. Control. 44(4):960–970, 2021.
Engh, J. A., D. S. Minhas, D. Kondziolka, and C. N. Riviere. Percutaneous intracerebral navigation by duty-cycled spinning of flexible bevel-tipped needles. Neurosurgery. 67(4):1117–1122, 2010.
Lin, C. L., and Y. A. Huang. Simultaneously reducing cutting force and tissue damage in needle insertion with rotation. IEEE Trans. Biomed. Eng. 67(11):3195–3202, 2020.
Tsumura, R., Y. Takishita, Y. Fukushima, and H. Iwata. Histological evaluation of tissue damage caused by rotational needle insertion. In: 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), pp. 5120–5123.
Majewicz, A., J. J. Siegel, A. A. Stanley, and A. M. Okamura. Design and evaluation of duty-cycling steering algorithms for robotically-driven steerable needles. In: 2014 IEEE International Conference on Robotics and Automation (ICRA), pp. 5883–5888.
Konh, B., D. Sasaki, T. K. Podder, and H. Ashrafiuon. 3d manipulation of an active steerable needle via actuation of multiple sma wires. Robotica. 38(3):410–426, 2020.
Adebar, T. K., J. D. Greer, P. F. Laeseke, G. L. Hwang, and A. M. Okamura. Methods for improving the curvature of steerable needles in biological tissue. IEEE Trans. Biomed. Eng. 63(6):1167–1177, 2016.
Berg, N. J., J. Dankelman, and J. J. Dobbelsteen. Endpoint accuracy in manual control of a steerable needle. J. Vasc. Interv. Radiol. 28(2):276–283, 2017.
Scali, M., P. A. H. Veldhoven, P. W. J. Henselmans, D. Dodou, and P. Breedveld. Design of an ultra-thin steerable probe for percutaneous interventions and preliminary evaluation in a gelatine phantom. PLoS ONE. 14(9):0221165, 2019.
De Falco, I., C. Culmone, A. Menciassi, J. Dankelman, and J. J. Dobbelsteen. A variable stiffness mechanism for steerable percutaneous instruments: integration in a needle. Med. Biol. Eng. Comput. 56(12):2185–2199, 2018.
Henken, K. R., P. R. Seevinck, J. Dankelman, and J. J. Dobbelsteen. Manually controlled steerable needle for MRI-guided percutaneous interventions. Med. Biol. Eng. Comput. 55(2):235–244, 2017.
Varnamkhasti, Z. K., and B. Konh. Design and performance study of a novel minimally invasive active surgical needle. J. Med. Devices.13(4):041006, 2019.
Datla, N. V., and P. Hutapea. Flexure-based active needle for enhanced steering within soft tissue. J. Med. Devices.9(4):041005, 2015.
Ayvali, E., C. P. Liang, M. Ho, Y. Chen, and J. P. Desai. Towards a discretely actuated steerable cannula for diagnostic and therapeutic procedures. Int. J. Robot. Res. 31(5):588–603, 2012.
Pratt, R. L., and A. J. Petruska. Magnetic needle steering model identification using expectation-maximization. In: 2019 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), pp. 5432–5437.
Petruska, A. J., F. Ruetz, A. Hong, L. Regli, O. Sürücü, A. Zemmar, and B. J. Nelson. Magnetic needle guidance for neurosurgery: initial design and proof of concept. In: 2016 IEEE International Conference on Robotics and Automation (ICRA), pp. 4392–4397.
Hong, A., A. J. Petruska, A. Zemmar, and B. J. Nelson. Magnetic control of a flexible needle in neurosurgery. IEEE Trans. Biomed. Eng. 68(2):616–627, 2021.
Hong, A., Q. Boehler, R. Moser, A. Zemmar, L. Stieglitz, and B. J. Nelson. 3d path planning for flexible needle steering in neurosurgery. Int. J. Med. Robot. Comput. Assist. Surg. 15(4):1998, 2019.
Babaiasl, M., F. Yang, S. Boccelli, and J. P. Swensen. Fracture-directed waterjet needle steering: Design, modeling, and path planning. In: 2020 8th IEEE RAS/EMBS International Conference for Biomedical Robotics and Biomechatronics (BioRob), pp. 1166–1173.
Babaiasl, M., F. Yang, and J. P. Swensen. Duty cycling of waterjet can improve steerability and radius-of-curvature (roc) for waterjet steerable needles. In: 2020 International Symposium on Medical Robotics (ISMR), pp. 50–56, 2020.
Frasson, L., S. Y. Ko, A. Turner, T. Parittotokkaporn, J. F. Vincent, and F. Baena. Sting: a soft-tissue intervention and neurosurgical guide to access deep brain lesions through curved trajectories. Proc. Inst. Mech. Eng. Part H. 224(6):775–788, 2010.
Ko, S. Y., B. L. Davies, and F. R. Y. Baena. Two-dimensional needle steering with a “programmable bevel” inspired by nature: Modeling preliminaries. In: 2010 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), pp. 2319–2324.
Watts, T., R. Secoli, and F. R. Y. Baena. A mechanics-based model for 3-d steering of programmable bevel-tip needles. IEEE Trans. Robot. 35(2):371–386, 2019.
Favaro, A., R. Secoli, F. Baena, and E. De Momi. Model-based robust pose estimation for a multi-segment, programmable bevel-tip steerable needle. IEEE Robot. Autom. Lett. 5(4):6780–6787, 2020.
Donder, A., and F. R. Y. Baena. Kalman-filter-based, dynamic 3-d shape reconstruction for steerable needles with fiber bragg gratings in multicore fibers. IEEE Trans. Robot. 38(4):2262–2275, 2022.
Lee, J., J. Wang, and W. Park. Efficient mechanism design and systematic operation planning for tube-wire flexible needles. J. Mech. Robot.10(6):065001, 2018.
Okazawa, S., R. Ebrahimi, J. Chuang, S. E. Salcudean, and R. Rohling. Handheld steerable needle device. IEEE/ASME Trans. Mechatron. 10(3):285–296, 2005.
Bui, V. K., S. Park, J.-O. Park, and S. Y. Ko. A novel curvature-controllable steerable needle for percutaneous intervention. Proc. Inst. Mech. Eng. 230(8):727–738, 2016.
Torabi, M., K. Hauser, R. Alterovitz, V. Duindam, and K. Goldberg. Guiding medical needles using single-point tissue manipulation. In: 2009 IEEE International Conference on Robotics and Automation (ICRA), pp. 2705–2710.
Mallapragada, V. G., N. Sarkar, and T. K. Podder. Robot-assisted real-time tumor manipulation for breast biopsy. IEEE Trans. Robot. 25(2):316–324, 2009.
DiMaio, S. P., and S. E. Salcudean. Needle steering and model-based trajectory planning. In: International Conference on Medical Image Computing and Computer-Assisted Intervention. New York: Springer, 2003, pp. 33–40.
DiMaio, S. P., and S. E. Salcudean. Needle steering and motion planning in soft tissues. IEEE Trans. Biomed. Eng. 52(6):965–974, 2005.
Glozman, D., and M. Shoham. Image-guided robotic flexible needle steering. IEEE Trans. Robot. 23(3):459–467, 2007.
Neubach, Z., and M. Shoham. Ultrasound-guided robot for flexible needle steering. IEEE Trans. Biomed. Eng. 57(4):799–805, 2010.
Chevrie, J., N. Shahriari, M. Babel, A. Krupa, and S. Misra. Flexible needle steering in moving biological tissue with motion compensation using ultrasound and force feedback. IEEE Robot. Autom. Lett. 3(3):2338–2345, 2018.
Chevrie, J., A. Krupa, and M. Babel. Needle steering fusing direct base manipulation and tip-based control. In: 2016 IEEE International Conference on Robotics and Automation (ICRA), pp. 4450–4455. IEEE.
Shahriari, N., J. R. Georgiadis, M. Oudkerk, and S. Misra. Hybrid control algorithm for flexible needle steering: demonstration in phantom and human cadaver. PLoS ONE. 13(12):0210052, 2018.
Duan, Y., Y. Zhang, Y. Shen, J. Ling, and Y. Zhu, A three-rhombus configured remote center of motion mechanism for robot-assisted surgery. In: 2022 37th Youth Academic Annual Conference of Chinese Association of Automation (YAC), pp. 930–934.
Varnamkhasti, Z. K., and B. Konh. Design, fabrication, and testing of a flexible three-dimensional printed percutaneous needle with embedded actuators. J. Med. Devices.15(2):021007, 2021.
Varnamkhasti, Z. K., and B. Konh. Cable-driven 3d steerable surgical needle for needle-based procedures. In: Proceedings of the 2020 Design of Medical Devices Conference (DMD2020), vol. 83549, pp. 001–06008.
Padasdao, B., and B. Konh. Shape memory alloy actuators in an active needlemodeling, precise assembly, and performance evaluation. J. Manuf. Sci. Eng.143(2):021003, 2021.
Konh, B., M. Honarvar, and P. Hutapea. Simulation and experimental studies of the sma-activated needle behavior inside the tissue. In: Active and Passive Smart Structures and Integrated Systems 2015, vol. 9431, pp. 693–697.
Ayvali, E., and J. P. Desai. Towards a discretely actuated steerable cannula. In: 2012 IEEE International Conference on Robotics and Automation (ICRA), pp. 1614–1619.
Ryu, S. C., Z. F. Quek, J.-S. Koh, P. Renaud, R. J. Black, B. Moslehi, B. L. Daniel, K.-J. Cho, and M. R. Cutkosky. Design of an optically controlled mr-compatible active needle. IEEE Trans. Robot. 31(1):1–11, 2015.
Ryu, S .C., P. Renaud, R. J. Black, B. L. Daniel, and M. R. Cutkosky. Feasibility study of an optically actuated mr-compatible active needle. In: 2011 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), pp. 2564–2569.
Ryu, S. C., Z. F. Quek, P. Renaud, R. J. Black, B. L. Daniel, and M. R. Cutkosky. An optical actuation system and curvature sensor for a mr-compatible active needle. In: 2012 IEEE International Conference on Robotics and Automation (ICRA), pp. 1589–1594.
Black, R. J., S. Ryu, B. Moslehi, and J. M. Costa. Characterization of optically actuated mri-compatible active needles for medical interventions. In: Behavior and Mechanics of Multifunctional Materials and Composites 2014, vol. 9058, pp. 160–167.
Mitros, Z., S. M. H. Sadati, R. Henry, L. Da Cruz, and C. Bergeles. From theoretical work to clinical translation: progress in concentric tube robots. Annu. Rev. Control Robot. Autonom. Syst. 5:335–359, 2022.
Webster, R. J., A. M. Okamura, and N. J. Cowan. Toward active cannulas: Miniature snake-like surgical robots. In: 2006 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), pp. 2857–2863.
Garriga-Casanovas, A., and F. Baena. Complete follow-the-leader kinematics using concentric tube robots. Int. J. Robot. Res. 37(1):197–222, 2017.
Bergeles, C., A. H. Gosline, N. V. Vasilyev, P. J. Codd, P. J. Del Nido, and P. E. Dupont. Concentric tube robot design and optimization based on task and anatomical constraints. IEEE Trans. Biomed. Eng. 31(1):67–84, 2015.
Qi, B., Z. Yu, Z. K. Varnamkhasti, Y. Zhou, and J. Sheng. Toward a telescopic steerable robotic needle for minimally invasive tissue biopsy. IEEE Robot. Autom. Lett. 6(2):1989–1996, 2021.
Swaney, P. J., A. W. Mahoney, A. A. Remirez, E. Lamers, B. I. Hariley, R. H. Feins, R. Alterovitz, I. Webster, and J. Robert. Tendons, concentric tubes, and a bevel tip: three steerable robots in one transoral lung access system. In: 2015 IEEE International Conference on Robotics and Automation (ICRA), pp. 5378–5383.
Khadem, M., J. O’Neill, Z. Mitros, L. Cruz, and C. Bergeles. Autonomous steering of concentric tube robots for enhanced force/velocity manipulability. In: 2019 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), pp. 2197–2204.
Khadem, M., J. O’Neill, Z. Mitros, L. Cruz, and C. Bergeles. Autonomous steering of concentric tube robots via nonlinear model predictive control. IEEE Trans. Robot. 36(5):1595–1602, 2020.
Roesthuis, R. J., N. J. V. D. Berg, J. J. V. D. Dobbelsteen, and S. Misra. Modeling and steering of a novel actuated-tip needle through a soft-tissue simulant using fiber bragg grating sensors. In: 2015 IEEE International Conference on Robotics and Automation (ICRA), pp. 2283–2289.
Shahriari, N., R. J. Roesthuis, N. J. Berg, J. J. Dobbelsteen, and S. Misra. Steering an actuated-tip needle in biological tissue: Fusing fbg-sensor data and ultrasound images. In: 2016 IEEE International Conference on Robotics and Automation (ICRA), pp. 4443–4449.
Burdette, E. C., D. C. Rucker, P. Prakash, C. J. Diederich, J. M. Croom, C. Clarke, P. Stolka, T. Juang, E. M. Boctor, I. Webster, and J. Robert. The acusitt ultrasonic ablator: The first steerable needle with an integrated interventional tool. In: Medical Imaging 2010: Ultrasonic Imaging, Tomography, and Therapy, vol. 7629, pp. 283–292.
Vries, M., J. Sikorski, S. Misra, and J. Dobbelsteen. Axially rigid steerable needle with compliant active tip control. PLoS ONE. 170:0261089, 2022.
Watts, T., R. Secoli, F. R. Y. Baena. Needle steerability measures: definition and application for optimized steering of the programmable bevel-tip needle. In: 2018 IEEE International Conference on Robotics and Biomimetics (ROBIO), pp. 59–64.
Arif, M., A. Moelker, and T. Walsum. Needle tip visibility in 3d ultrasound images. Cardiovasc. Interv. Radiol. 41:145–152, 2018.
Berg, N. J., J. A. Sanchez-Margallo, T. Lango, and J. J. Dobbelsteen. Compliant joint echogenicity in ultrasound images: towards highly visible steerable needles. In: Medical Imaging 2018: Image-Guided Procedures, Robotic Interventions, and Modeling, vol. 10576, 2018, pp. 219–224.
Li, M., B. Gonenc, K. Kim, W. Shang, and I. Iordachita. Development of an MRI-compatible needle driver for in-bore prostate biopsy. In: 2015 International Conference on Advanced Robotics (ICAR), pp. 130–136, 2015.
Su, H., M. Zervas, G. A. Cole, C. Furlong, and G. S. Fischer. Real-time mri-guided needle placement robot with integrated fiber optic force sensing. In: 2011 IEEE International Conference on Robotics and Automation (ICRA), pp. 1583–1588.
Reisch, R., A. Stadie, R. A. Kockro, and N. Hopf. The keyhole concept in neurosurgery. World Neurosurg. 79(2 Suppl):17–913, 2013.
Lan, Q. Clinical application of keyhole techniques in minimally invasive neurosurgery. Chin. Med. J. 119(16):1327–1330, 2006.
Van Niekerk, M., P. Goussard, R. Van Toorn, and R. Solomons. Giant cerebral tuberculoma mimicking a high-grade tumour in a child. BMJ Case Rep.15(4):248545, 2022.
Roediger, J., T. A. Dembek, J. Achtzehn, J. L. Busch, A. P. Kramer, K. Faust, G. H. Schneider, P. Krause, A. Horn, and A. A. Kuhn. Automated deep brain stimulation programming based on electrode location: a randomised, crossover trial using a data-driven algorithm. Lancet Digital Health. 5(2):59–70, 2023.
Zhan, W., and C. H. Wang. Convection enhanced delivery of chemotherapeutic drugs into brain tumour. J. Controlled Release. 271:74–87, 2018.
Virdyawan, V., and F. Baena. Vessel pose estimation for obstacle avoidance in needle steering surgery using multiple forward looking sensors. In: 2018 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), pp. 3845–3852, 2018.
Pinzi, M., T. Watts, R. Secoli, S. Galvan, and F. R. Y. Baena. Path replanning for orientation-constrained needle steering. IEEE Trans. Biomed. Eng. 68(5):1459–1466, 2021.
Segato, A., M. Di Marzo, S. Zucchelli, S. Galvan, R. Secoli, and E. De Momi. Inverse reinforcement learning intra-operative path planning for steerable needle. IEEE Trans. Biomed. Eng. 69(6):1995–2005, 2022.
Pinzi, M., S. Galvan, and Y. B. F. Rodriguez. The adaptive hermite fractal tree (AHFT): a novel surgical 3d path planning approach with curvature and heading constraints. Int. J. Comput. Assist. Radiol. Surg. 14(4):659–670, 2019.
Caborni, C., S. Y. Ko, E. De Momi, G. Ferrigno, and F. Baena. Risk-based path planning for a steerable flexible probe for neurosurgical intervention. In: 4th IEEE RAS and EMBS International Conference on Biomedical Robotics and Biomechatronics (BioRob)/Symposium on Surgical Robotics, pp. 866–871, 2012.
Siegel, R. L., K. D. Miller, H. E. Fuchs, and A. Jemal. Cancer statistics, 2022. CA: A Cancer Journal for Clinicians. 72(1):7–33, 2022.
Navani, N., M. Nankivell, D. R. Lawrence, S. Lock, H. Makker, D. R. Baldwin, R. J. Stephens, M. K. Parmar, S. G. Spiro, S. Morris, S. M. Janes, and B. T. I. Lung. Lung cancer diagnosis and staging with endobronchial ultrasound-guided transbronchial needle aspiration compared with conventional approaches: an open-label, pragmatic, randomised controlled trial. Lancet Respir. Med. 3(4):282–289, 2015.
Memoli, J. S. W., P. J. Nietert, and G. A. Silvestri. Meta-analysis of guided bronchoscopy for the evaluation of the pulmonary nodule. Chest. 142(2):385–393, 2012.
Fan, Y., A.-M. Zhang, X.-L. Wu, Z.-S. Huang, K. Kontogianni, K. Sun, W.-L. Fu, N. Wu, W. M. Kuebler, and F. J. F. Herth. Transbronchial needle aspiration combined with cryobiopsy in the diagnosis of mediastinal diseases: a multicentre, open-label, randomised trial. Lancet Respir. Med. 11(3):256–264, 2023.
Shahriari, N., W. Heerink, T. Katwijk, E. Hekman, M. Oudkerk, and S. Misra. Computed tomography (CT)-compatible remote center of motion needle steering robot: fusing CT images and electromagnetic sensor data. Med. Eng. Phys. 45:71–77, 2017.
Moreira, P., M. Abayazid, and S. Misra. Towards physiological motion compensation for flexible needle interventions. In: 2015 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), pp. 831–836, 2015.
Fried, I., J. Hoelscher, M. Fu, M. Emerson, T. E. Ertop, M. Rox, J. Granna, A. Kuntz, J. A. Akulian, I. Webster, J. Robert, and R. Alterovitz. Design considerations for a steerable needle robot to maximize reachable lung volume. In: 2021 IEEE International Conference on Robotics and Automation (ICRA), pp. 1418–1425, 2021.
Lee, S. Y., J. M. Pakela, K. Na, J. Shi, B. J. McKenna, D. M. Simeone, E. Yoon, J. M. Scheiman, and M.-A. Mycek. Needle-compatible miniaturized optoelectronic sensor for pancreatic cancer detection. Sci. Adv. 6(47):1746, 2020.
Fu, M., K. Solovey, O. Salzman, and R. Alterovitz. Resolution-optimal motion planning for steerable needles. In: 2022 IEEE International Conference on Robotics and Automation (ICRA), vol. 2022, pp. 9652–9659.
Kuntz, A., L. G. Torres, R. H. Feins, I. Webster, J. Robert, and R. Alterovitz, Motion planning for a three-stage multilumen transoral lung access system. In: 2015 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), pp. 3255–3261, 2015.
Vogel, A., T. Meyer, G. Sapisochin, R. Salem, and A. Saborowski. Hepatocellular carcinoma. The Lancet. 400(10360):1345–1362, 2022.
Yang, J. D., P. Hainaut, G. J. Gores, A. Amadou, A. Plymoth, and L. R. Roberts. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 16(10):589–604, 2019.
Childs, A., N. Zakeri, Y. T. Ma, J. O’Rourke, P. Ross, E. Hashem, R. A. Hubner, K. Hockenhull, C. Iwuji, S. Khan, D. H. Palmer, J. Connor, D. Swinson, S. Darby, C. Braconi, T. Roques, D. Yu, T. V. Luong, and T. Meyer. Biopsy for advanced hepatocellular carcinoma: results of a multicentre uk audit. Br. J. Cancer. 125(10):1350–1355, 2021.
Izzo, F., V. Granata, R. Grassi, R. Fusco, R. Palaia, P. Delrio, G. Carrafiello, D. Azoulay, A. Petrillo, and S. A. Curley. Radiofrequency ablation and microwave ablation in liver tumors: an update. Oncologist. 24(10):990–1005, 2019.
Kim, G. M., J. Y. Won, M. D. Kim, S. I. Park, Y. do Lee, W. Shin, M. Shin, K. H. Han, Y. do Kim, and S. U. Kim. Cryoablation of hepatocellular carcinoma with high-risk for percutaneous ablation: safety and efficacy. Cardiovasc. Interv. Radiol. 39(10):1447–1454, 2016.
Konh, B., B. Padasdao, Z. Batsaikhan, and S. Y. Ko. Integrating robot-assisted ultrasound tracking and 3d needle shape prediction for real-time tracking of the needle tip in needle steering procedures. Int. J. Med. Robot. Comput. Assist. Surg. 17(4):e2272, 2021.
Baksic, P., H. Courtecuisse, C. Duriez, and B. Bayle. Robotic needle insertion in moving soft tissues using constraint-based inverse finite element simulation. In: 2020 IEEE International Conference on Robotics and Automation (ICRA), pp. 2407–2413, 2020.
Adebar, T. K., A. E. Fletcher, and A. M. Okamura. 3-d ultrasound-guided robotic needle steering in biological tissue. IEEE Trans. Biomed. Eng. 61(12):2899–2910, 2014.
Rucker, D. C., J. Das, H. B. Gilbert, P. J. Swaney, M. I. Miga, N. Sarkar, I. Robert, and J. Webster. Sliding mode control of steerable needles. IEEE Trans. Robot. 29(5):1289–1299, 2013.
Alexander, R. T., B. R. Hemmelgarn, N. Wiebe, A. Bello, C. Morgan, S. Samuel, S. W. Klarenbach, G. C. Curhan, and M. Tonelli. Kidney stones and kidney function loss: a cohort study. BMJ. 345:5287, 2012.
De, S., R. Autorino, F. J. Kim, H. Zargar, H. Laydner, R. Balsamo, F. C. Torricelli, C. Di Palma, W. R. Molina, M. Monga, and M. De Sio. Percutaneous nephrolithotomy versus retrograde intrarenal surgery: A systematic review and meta-analysis. Eur. Urol. 67(1):125–137, 2015.
Zhu, W., Z. Huang, and F. Zeng. Miniaturization in percutaneous nephrolithotomy: what is new? Asian J. Urol. 10(3):275–280, 2023.
Knoll, T., F. Daels, J. Desai, A. Hoznek, B. Knudsen, E. Montanari, C. Scoffone, A. Skolarikos, and K. Tozawa. Percutaneous nephrolithotomy: technique. World J. Urol. 35:1361–1368, 2017.
Zhang, D., Z. Li, K. Chen, J. Xiong, X. Zhang, and L. Wang. An optical tracker based robot registration and servoing method for ultrasound guided percutaneous renal access. Biomed. Eng. Online. 12:1–16, 2013.
Wood, N. A., K. Shahrour, M. C. Ost, and C. N. Riviere. Needle steering system using duty-cycled rotation for percutaneous kidney access, 2010.
Wilz, O., B. Kent, B. Sainsbury, and C. Rossa. Multiobjective trajectory tracking of a flexible tool during robotic percutaneous nephrolithotomy. IEEE Robot. Autom. Lett. 6(4):8110–8117, 2021.
Gomez-Iturriaga, A., M. Keyes, J. Martin, and D. E. Spratt. Should brachytherapy be added to external beam radiotherapy for prostate cancer? Lancet Oncol. 23(1):23–25, 2022.
Maghsoudi, A., and M. Jahed. Needle dynamics modelling and control in prostate brachytherapy. IET Control Theory Appl. 6(11):1671–1681, 2012.
Varnamkhasti, Z. K., and B. Konh. Compact 3d-printed active flexible needle for percutaneous procedures. Surg. Innov. 27(4):402–405, 2020.
Lehmann, T., R. Sloboda, N. Usmani, and M. Tavakoli. Human-machine collaboration modalities for semi-automated needle insertion into soft tissue. IEEE Robot. Autom. Lett. 3(1):477–483, 2018.
Carriere, J., M. Khadem, C. Rossa, N. Usmani, R. Sloboda, and M. Tavakoli. Surgeon-in-the-loop 3-d needle steering through ultrasound-guided feedback control. IEEE Robot. Autom. Lett. 3(1):469–476, 2018.
Rossa, C., N. Usmani, R. Sloboda, and M. Tavakoli. A hand-held assistant for semiautomated percutaneous needle steering. IEEE Trans. Biomed. Eng. 64(3):637–648, 2017.
Vries, M., S. L. Wilby, A. L. Palmer, W. Polak, I. O’Hea, D. Hodgson, and J. J. Dobbelsteen. Overcoming pubic arch interference in prostate brachytherapy using steerable needles. J. Contemp. Brachyther. 14(5):495–500, 2022.
Khadem, M., C. Rossa, N. Usmani, R. S. Sloboda, and M. Tavakoli. Roboticassisted needle steering around anatomical obstacles using notched steerable needles. IEEE J. Biomed. Health Inform. 22(6):1917–1928, 2018.
Fallahi, B., M. Waine, C. Rossa, R. Sloboda, N. Usmani, and M. Tavakoli. An integrator-backstepping control approach for three-dimensional needle steering. IEEE/ASME Trans. Mechatron. 24(5):2204–2214, 2019.
Acknowledgements
This work was supported by the Natural Science Foundation of Jiangsu Province [Grant No. BK20210294], the Fundamental Research Funds for the Central Universities [Grant No. NS2022051], Knowledge Innovation Program of Wuhan-Shuguang Project [Grant No. 2023010201020252], and Guangdong Basic and Applied Basic Research Foundation [Grant No. 2023A1515110156].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare and the authors also declare no compensation from any commercial entity that has been obtained for this work.
Additional information
Associate Editor Lyndia (Chun) Wu oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Duan, Y., Ling, J., Feng, Z. et al. A Survey of Needle Steering Approaches in Minimally Invasive Surgery. Ann Biomed Eng 52, 1492–1517 (2024). https://doi.org/10.1007/s10439-024-03494-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-024-03494-0