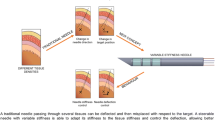
Abstract
Accurate needle targeting is critical for many clinical procedures, such as transcutaneous biopsy or radiofrequency ablation of tumors. However, targeting errors may arise, limiting the widespread adoption of these procedures. Advances in flexible needle (FN) steering are emerging to mitigate these errors. This review summarizes the state-of-the-art developments of FNs and addresses possible targeting errors that can be overcome with steering actuation techniques. FN steering techniques can be classified as passive and active. Passive steering directly results from the needle-tissue interaction forces, whereas active steering requires additional forces to be applied at the needle tip, which enhances needle steerability. Therefore, the corresponding targeting errors of most passive FNs and active FNs are between 1 and 2 mm, and less than 1 mm, respectively. However, the diameters of active FNs range from 1.42 to 12 mm, which is larger than the passive steering needle varying from 0.5 to 1.4 mm. Therefore, the development of active FNs is an area of active research. These active FNs can be steered using tethered internal direct actuation or untethered external actuation. Examples of tethered internal direct actuation include tendon-driven, longitudinal segment transmission and concentric tube transmission. Tendon-driven FNs have various structures, and longitudinal segment transmission needles could be adapted to reduce tissue damage. Additionally, concentric tube needles have immediate advantages and clinical applications in natural orifice surgery. Magnetic actuation enables active FN steering with untethered external actuation and facilitates miniaturization. The challenges faced in the fabrication, sensing, and actuation methods of FN are analyzed. Finally, bio-inspired FNs may offer solutions to address the challenges faced in FN active steering mechanisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Minimally invasive surgery (MIS) has the advantages of performing surgical procedures with minimal trauma and allowing patients to recover quickly, making it the preferred choice for clinical diagnosis and treatment.41 As one of the essential tools in MIS, needles are widely used in biopsy,55 brachytherapy,64 drug delivery,47 regional anesthesia,66 and the introduction of other instruments into the body, such as catheters and ablation electrodes.107 The procedural success rate dramatically relies on the precise placement in reaching the target region with a needle and without any targeting error.25 However, a needle can be misplaced by organ deformation and target displacement due to the tissue-needle interaction and physiologic properties of the respective organ.19,61,96 For example, the needle placement targeting error of 6–8 mm is common during surgical procedures. A follow-up study of needle biopsy reported that about 14% of biopsy results were invalid due to needle displacement.61 Once the needle deviates from the expected trajectory, the surgeon will remove and reinsert it back into the tissue, exposing the patient to additional risks and potential complications. In addition, poor tip steering will most likely lead to the risk of puncturing sensitive structures, sometimes even with fatal consequences.52
Using a flexible needle (FN) can reduce the targeting error and avoid damage to critical blood vessels and nerves between the entry point and target due to the enhanced maneuverability of the needle trajectory.26,104 The FN is usually made of a superelastic material (such as nitinol material that can be widely used as a solid stylet or in tubing form108). This super elasticity property allows the needle to be navigated with high accuracy. Therefore, the FN used in clinical procedures should be a highly versatile tool capable of reaching target locations deep within the human body. However, the utilization and actuation of an FN are limited in complex environments.5 The challenges are:
-
1.
The diameter of the FN should be thin enough to meet the function of limiting tissue damage.
-
2.
Good steerability should be achieved to move around obstructing anatomical structures and reach desired locations with low targeting errors.
-
3.
Difficulty in balancing the steerability of the FN with the choice of needle diameter. Steerability often relies on the complex steering actuation structure (often requiring a large diameter).
The reciprocal of the curvature or the minimum reachable radius of curvature is used to express the steerability of FN.78 Alternatively, steerability also has been described by the ratio of the maximum offset distance.79
FN steering actuation describes the research on how force can be transmitted to FNs to correct potential targeting errors and avoid obstacles during insertion, which has been one of the most focused research fields in the past decades.96 High targeting accuracy depends on improvements in FN steering actuation techniques. More dexterous control of the FN trajectory has been developed through robot-assisted needle insertion systems60 and with a beveled tip59. Recently, more and more attention has been paid to FN mechanical design, which mainly includes integrating sensors on FNs and FN actuation.2,11,84 Therefore, this review aims to classify and evaluate the current state of FN steering actuation from the targeting errors perspective, which can aid in developing high-precision steering actuation methods and functional prototypes suitable for clinical use.
Review Approach
Review Method
The literature search was conducted on the Web of Science and Google Scholar on the FN steering technique, with search queries that contain word combinations of the flexible needle, needle steering, steering actuation, tissue-needle interaction, and active needle. Search results were divided into fundamental studies (n = 52), FN mechanical design (n = 27), pre-puncture alignment techniques (n = 46), and needle steering actuation (n = 67). The underlying navigation and sensing could affect FN functionality and targeting accuracy, and the innovative active steering actuation design offers potential solutions. This review primarily extends to the research on achieving high targeting accuracy from the "hardware" of FNs, mainly including integrating sensors on the FN and designing various active steering actuation.
Focus
We have focused on articles published after 2010. It was found that 131 articles have reported the FN steering technique in detail. Furthermore, 41% (n = 53) was validated by placement accuracy experiments and reported the targeting errors. Of the experiments, 76% (n = 40) was validated in the artificial tissues, 22% (n = 12) in ex vivo biological tissue, and 2% (n = 1) in vivo biological tissue. The average targeting errors resulting in these studies are within [0.3 mm, 6.2 mm]. To the best of our knowledge, one magnetic FN (tip Ø = 1.3 mm, body Ø = 0.7 mm) study in neurosurgery reported that the measurement so far for the minimum targeting error in biological tissue (ex vivo pig brain) is 0.48 mm.38 The reasons for how FN steering actuations affect the targeting error results will be analyzed in the next section.
Steering Mechanisms and Targeting Error Results Analysis of FN
FN Steering Mechanisms and Category
Understanding the steering mechanisms of the FN is essential, especially when experiencing asymmetric forces. If FN has a symmetrical tip, without any force applying to the tip, it will enter the tissue in a straight line to insert the tissue like a rigid needle. According to the steering mechanisms, FN will be roughly classified as passive and active. Passive steering directly results from the needle-tissue interaction forces, and active steering utilizes additional forces applied at the needle tip.
With an asymmetric tip, the interaction between the needle tip and the tissues can cause an imbalance of force, and the passive FN deflects in the direction of this force. As shown in Figs. 1A–, B, and C, bevel-tip FNs, pre-curved FNs, and trocar FNs are passive steering techniques. In clinical practice, this passive FN steering actuation has been described by the basic rotations and translations of the needle base, commonly known as base manipulation.18 The constant radius of curvature is used to describe the FN movements because of the symmetry of curvature (the reachable workspace in Fig. 1D). A bevel-tip FN naturally bends when inserted into tissues.92 Precurves or prebends enhanced the steering behaviors of the bevel-tip FN.24,57 Asymmetry of the tip can be caused by a bevel-tip, a pre-curves segment.79 The trocar FN usually has a stylet forced through the cannula to approximate a straight configuration.52,106 When the stylet is moved out of the cannula, it resumes its original curved shape, resulting in a curve puncture trajectory.
Schematic diagram of FN passive steering mechanisms. (A) Bevel-tip FN, bevel tip is α, (B) Pre-curved needle, θ is bend angle, and L is length. The pre-curved needle may also have a beveled tip or not. (C) A trocar FN consists of a cannula and a stylet. The cannula base is used to drive the pre-curved stylet. (D) The reachable region of the FN is cone-shaped. (E) Conceptual diagram of FN trajectories, created by rotating the needle in different "duty-cycling". Fc is the interaction force between the soft tissue and needle tip that achieves passive steering. (F) Steer the FN inside the soft tissue.
Typically, the lateral displacement of the tissue and needle movements are induced by zero-velocity constraint (curvature \(\frac{1}{K}\)). A straight path will be obtained by spinning the FN all the time. The maximum bending degree of the FN can be achieved, when there is no spin during the process. Therefore, the trajectory of any bending degree between these two forms can be created by rotating the needle in different "duty-cycling",90 as shown in Fig. 1E. Furthermore, the needle tip can be actuated by rotation of the FN base to change the orientation of the needle tip so that it reaches the target point, as shown in Fig. 1F. However, it has been proven that if the FN is continuously rotated, a helical trajectory will be made in the soft tissues, which could increase needle twisting.52
Active needle steering actuation has garnered more attention in the past decade over the passive technique because the former can directly and responsively control needle tip orientation without tissue contact (can steer through open or liquid-filled cavities).68,96 The passive FN is driven by the insertion and rotation of the needle tail to drive the needle tip to rotate. Conversely, active FNs are the innovative design of directly driving the needle tip so the misalignments caused by unpredictable factors can be compensated during needle insertion. Active FNs can effectively avoid the error of needle body transmission, thereby reducing tissue damage and improving the accuracy of puncture surgery. The entire body of the active FN will naturally follow the tip, just like a train. The train will follow the locomotive direction, which has also been described as a "follow-the-leader".33 Active FNs usually can be steered by additional force application at the needle tip and thus increase the tip dexterity to control the swing angle of the tip to change the trajectory and reach the target.10 There are many different types of additional force supply, such as mechanical type,8,49,62,73 magnetically actuated type,38,103 J heating type,98 etc. However, we need to pay more attention to how the additional force is applied to the tip of the active FN, as this will affect its internal structure and size. Therefore, depending on whether the energy supply is tethered directly to the FN, the active FN steering actuation can be divided into two categories: FN steering with tethered internal direct actuation and FN steering with untethered external actuation.
Targeting Error Results and Analysis
The targeting error is one of the most important criteria for evaluating the performance of FNs. Higher targeting accuracy and tighter needle steering are important to reaching as many points as possible via one injection point, reducing the risk of hemorrhaging.6 Meanwhile, some surgical scenarios have additional requirements for the accuracy of FNs, such as the operation involving infants, eyes, and ears. These operations require much higher standards (submillimeter- to µm-grade) than common brachytherapy or biopsy for adults (mm-grade).115 Through the exploration of targeting errors, the premise of achieving lower targeting errors is found to guide the design and steering actuation. This paper will discuss two dimensions of targeting error and needle diameter (which may be related to the complexity of the needle structure), as shown in Fig. 2. Table 1 shows the research data sources for Fig. 2. Previous research with targeting errors of < 2 mm (acceptable in the field80) is presented in Table 2.
Current studies of FN steering. Targeting errors show how different FN steering systems with different needle diameters affect the steering of the FN to reach the desired target. Biological tissue experiments (the red circles) are distinguished, and different active FNs (the colored triangles) are distinguished from passive FNs (the black squares).
Typical clinical needle diameters range from 14 G (Ø 2.1 mm) for tissue biopsy74 to 25 G (Ø 0.5 mm) for spinal anesthesia or vaccination procedures.9 Smaller needles are.
Through collecting and analyzing the previous research results, we can draw the following key points about FN steering:
Corezone of Passive Steering
As shown in Fig. 2, the targeting errors of passive FNs and the needle diameters are concentrated on the circular area marked by blue, which we defined as the corezone of passive steering. The main feature of the corezone of passive steering is the small needle diameters that vary from 0.5 to 1.4 mm. A solid stylet with an asymmetric tip is often used as a passive FN, which is the direct reason for its small diameter. The targeting errors of passive FNs are from 0.5 to 3 mm, most of which are distributed between 1 and 2 mm.
The Diameter of the Active FN
The active FNs (the colored triangles) are almost entirely located on the right side of the circular area (corezone of passive steering) in Fig. 2. The diameter of active FNs is often much larger than that of passive FNs. Meanwhile, the targeting errors of many active FNs can be less than 1 mm. It can be illustrated that active FN has more potential to achieve lower targeting errors than passive FN. Good steerability is associated with complex steering actuation, but often at the expense of the larger needle diameters. We also noticed that among all kinds of active FNs in Fig. 2, the magnetic FNs seem to have a unique advantage. Anyhow, the needle diameter should be reduced to meet the needs of MIS.
Targeting Errors of Biological Tissue Experiment
The stiffness of the measured biological tissue is one important factor affecting the targeting error. Various biological tissues have different stiffness values, for example, the stiffness of porcine skin is 190 kPa, it is 52 kPa for porcine muscle,50 35 kPa for breast84, and 24.5 kPa for bovine tissue.44 Although the artificial tissues are made to have similar stiffness values to biological tissues, the targeting errors of biological tissue (e.g., bovine tissue,25,44 a prostate of a bull,59,60 chicken breast,2 the lungs of a human cadaver,84 brain,38 bovine liver,18 and porcine tissue50) experiments are usually higher than that in artificial tissue (gelatine phantom) experiments. The results primarily result from the homogeneity and predictable mechanical properties of the gelatine phantom. The steering model with artificial tissues is often accurate. However, the biological tissues are soft and viscoelastic materials and exhibit complex nonlinear and hypoplastic mechanical behaviors. Therefore, they are challenging to model accurately. Furthermore, in non-homogenous biological tissues, needle deflection and buckling could be caused when encountering stiffer tissues. Meanwhile, in vivo and ex vivo experiments represent two completely different conditions and unique challenges. It means that when putting the previous study in the clinic, the targeting errors will probably be higher than those reported so far or even the generally acceptable error of 2 mm.
In general, the FN targeting error results from the combined action of the steering strategy, algorithm and model used, tissue type and mechanical materials (e.g., stiffness), and design of the needles. From the summary results in Table 2, many outcomes on modeling needle-tissue interaction, kinematics, dynamics models, and path planning have been achieved. In addition, the closed-loop control method significantly reduces targeting errors, which can be achieved by medical image feedback or needle and sensor integration.
Closed-Loop Control Based on Sensor Integration
Closed-loop control is the most effective way that adjusts steering according to the position feedback of the FN to reduce targeting error. Integrating medical imaging into steering techniques is the most promising technological way of current FN position feedback.3
Currently, the artificial tissues used in some studies are transparent so that cameras can obtain the FN and target images. Furthermore, most of the spatial needle positioning depends on medical imaging, including ultrasound (US)6 and magnetic resonance images (MRI),83 and computed tomography (CT).84 However, the FN is sometimes too thin to obtain high-precision and stable information.61 At the same time, the current image guidance technology is often difficult to achieve high real-time requirements. Integrating the sensors into the needle can achieve real-time FN tip position feedback.59,85,106 Finding suitable sensing units placed on the FN with minimal geometric dimensions is the critical challenge in integrating the sensors.
Optical fiber has the characteristics of high-speed transmission. FBG (Fiber Bragg Grating) sensor is a kind of optical fiber sensor with a high frequency that can measure pressure, temperature, strain, shape, and force. Changes in mechanical strain may cause changes in reflected Bragg wavelengths.36 FBG sensors on the FN can reconstruct the multi-curvature shape.74,75 All Grating Fiber (AGF®), FemtoSecond Gratings (FSG®s), and Draw Tower Gratings (DTG®s) are the commercialized high-quality FBG sensing components. FBG sensors in the inner wall of the standard 18 G FN can effectively detect deviation during needle puncture.65 Three grooves with FBGs were made on the inner stylet, and each groove is 120° apart. The bent profile can be estimated by two sets of sensors placed at different positions on the needle, as shown in Fig. 3A. Although this detection range is limited, new ideas for FN sensing and self-identification have been proposed.1 Three optical fibers were integrated into the FN shaft. Four FBG sensors have been set on each fiber (Fig. 3B). Moreira et al.59 (Fig. 3C) and Roesthuis et al.74 (Fig. 3D) also reported similar configurations of FBG sensors on the FN. The number and location of FBG sensors are also proven to impact the results significantly. This integration of FBG sensors should balance sensing effects and needle size because the combined structure is more complex and difficult to manufacture for more than three optical fibers.
Configuration of FBG sensors on the FN. (A) Needle prototype design with inner stylet contained three optical fibers. Three same grooves are manufactured on the inner stylet. Each groove is 120° apart and embeds optical fibers with FBGs along the length of the needle.65 (B) Three optical fibers were integrated into the FN shaft. An array of four FBG sensors has been set on each fiber, and the numbers from 1 to 4 represent the sensor locations.1 (C) The FN contained three optical fibers, each with four FBG sensors.59 (D) The inner stylet consisted of a nitinol wire, which integrated three optical fibers. An array of four FBG sensors has also been set on each fiber.74
Strain gauges can adhere to the needle surface to collect the information and reconstruct the needle curve.111 In this way, a simple and inexpensive closed-loop control can be obtained. Electromagnetic (EM) sensors are also commonly used for needle position tracking and shape reconstruction based on the mutual induction of a field generator and multiple field sensors. Therefore, the pose of FNs can be determined by putting small EM sensors along the FN.1,18,47,73,84,106 EM sensors are vulnerable to magnetic fields from nearby devices.21 Meanwhile, in some fields that require higher accuracy, such as neurosurgery, the currently reported accuracy of the EM sensor has not yet met the accuracy requirements.38 At this point, the FBG sensor will be a magnet-free choice for fast shape reconstruction of flexible tools. However, the embedding of FBGs into small devices remains a challenge.
Active FN Steering
FNs with good steerability can accurately puncture small and deep targets in a small number of attempts by providing intraoperative compensation for tissue movement and targeting errors, thereby reducing trauma.49 As mentioned, active FNs usually have better steerability than passive FNs, but often at the expense of the larger needle diameters. An active FN can be considered a needle-shaped flexible surgical robot because it has a complex mechanical structure. The steerability largely depends on the effectiveness of the actuation method of the active FN, that is, how the force is applied or transmitted to the needle tip. However, the direct reason for the large diameter of active FNs is that the steering actuation usually takes up a portion of the space, which poses significant challenges. Therefore, depending on whether the energy supply is tethered directly to the FN, we propose to divide the active FN steering actuation into the following two categories: FN steering with tethered internal direct actuation and FN steering with untethered external actuation.
FN Steering with Tethered Internal Direct Actuation
Tethered internal direct actuation refers to using mediums to transmit forces directly onto the FN. The structure design of such an active FN, on its own, is a challenge. Because the higher steerability is typically associated with a more complex internal steering actuation design, and this actuation structure will take up much space inside the needle body, resulting in an increased needle diameter. Despite the challenges, researchers have been exploring potential solutions. Here, three standard methods for tethered internal direct actuation are introduced: tendon-driven, longitudinal segments transmission, and concentric tube transmission.
Tendon Driven
The tendon-driven FN is mechanically driven in which a control wire or rod deflects the needle. The tendons (cables) run along the FN body.108 Because the wire is attached eccentrically to the tip, the tension in a tendon deflects the tip, subject to the flexural rigidity of the FN itself. Certain manually operated, tendon-driven FNs have been commercialized,25 with designs consisting of a handle, tendons (cables), and flexible stylet. Kratchman et al. described the first robotically controlled tendon-driven FN system for transthoracic lung biopsy under CT guidance.49
The internal structure and constitution of the tendon-driven FNs are usually complex due to the use of tendon wires or rods. Figure 4 shows five main structure types of the tendon-driven FN according to different wire arrangements. The first type of tendon-driven FN has a flexible inner tube with grooves for the actuation cables and an outer tube (Fig. 4A).49,73 Based on this design, the tendons drive the inner tube to rotate and deploy in different directions and with different curvatures during insertion. Like the first type, the second kind of tendon-driven FN also consists of a stylet and a cannula. The apparent difference is that the outer cannula of the second type provides guideways to constrain the tendons.10,68,74 Based on this design, Berg et al. utilized an outer cannula with four grooves for actuation cables and an inner stylet with three grooves for optical fibers (Fig. 4B).10 Another tendon-driven FN was modeled with multi-flexible joints and rigid links for enhanced flexibility, and tendons were embedded to bend the needle (Fig. 4C).43,98,99 The tendon-driven FNs with a single hinge joint (Fig. 4D)6 and multi-DOF hinge joints (Fig. 4E)45,67 were developed.
Five structure types of tendon-driven FN. (A) A tendon-driven telescopic steerable needle and its working principle, including a flexible inner tube with a rectangular groove for the actuation cables and a rigid outer tube.73 (B) The tendon-driven needle comprises a stylet with three grooves for optical fibers and a cannula with four grooves for the actuation cables.10 (C) The FN includes rigid links and flexible joint(s) with embedded tendon actuators.99 (D). An example of a single hinge joint tendon-driven FN with an articulated tip. A single Nitinol pull wire articulates the needle tip.6 (E). Examples of multi-DOF hinge joint tendon-driven FNs, from the up, with 2–2, 3–3, and 1–1 orthogonal hinge joints.67
Similarly, Scali et al. proposed an ultra-thin FN prototype actuated by three exposed wires, and the needle can steer in different directions in 3D when pulling at different wires.81 Falco et al. have considered the differences in stiffness of biological tissues and proposed a tendon-driven FN with variable stiffness to solve the problem that the inhomogeneity of the tissue would cause the deflection of the needle tip and affect the targeting accuracy during the insertion of the FN into the tissue. Two antagonistic tendons simultaneously tensioned with the same load could compensate for changes in tissue stiffness during puncture, avoiding additional needle deflection (thus damage to the tissue) and achieving precise puncture.22 Although many types of tendon drive exist, the principle is essentially the same.
Innovative materials can be used as tendons. This kind of tendon-driven FNs has especially been proposed with particular actuation for the development of MRI-guided surgeries. The phase transition of SMA upon the electrical heating could be used for needle steering actuation under the MRI environment.78 However, the temperature of the wires can reach 80ºC for a complete phase transformation, which could cause damage to surrounding tissue. In addition, a low-transition-temperature shape memory alloy (LT SMA) wire actuator was used to insulate the FN and create a bend in the distal portion of the needle.77 Actuation is achieved by fiber-delivered laser light and internal optical heating side-coupled to the LT SMA, increasing the efficiency of optical heating for faster response and less tissue overheating.
The tendon-driven FN has provided many structural design possibilities and has already succeeded considerably. However, there are some problems: The accuracy of a tendon-driven FN may be limited due to the presence of actuation hysteresis and frictions.46 In addition, achieving a robust assembly with high stiffness and low cost is still challenging because of mechanical complexity and actuation. Conversely, scaling down these designs is challenging and future direction.116
Longitudinal Segment Transmission
To reduce tissue damage and achieve active steering, many scientists have found inspiration from the study of the ovipositor.15 Wasps use their ovipositors, consisting of three longitudinal segments called valves, to drill into stiff wood and lay their eggs.80 The secret of their success lies in the steering mechanism, as shown in Fig. 5A.52 Changing the asymmetry of the distal portion of the ovipositor enables steering, which can be achieved by lengthening one valve set relative to the other. This actuation method reduces tissue damage during insertion as the net pushing force can be limited. In addition, FNs with longitudinal segment transmission based on this structure can reduce tissue damage.
Examples of FN prototypes with longitudinal segment transmission inspired by the wasp ovipositor mechanism. (A) Schematic representation of a wasp that uses an ovipositor to transport eggs into the host material. (B) A two-segment FN with a diameter of 4 mm.48 (C) A 12 mm OD four-segment PBN4 (left yellow frame) and their latest 2.5 mm OD four-segment PBN82 (right red frame). (D) A 1.2 mm OD seven-segment ovipositor-needle prototype and its conceptual design process.80 (E) The flexible shaft of the transport mechanism and its conceptual design process.23
Principle and applications of concentric tube FNs. (A) The schematic diagram of concentric tube transmission. (B) The concentric tube robot is used for guiding the flexure tip steerable needle for transoral lung access surgery.92 (C) A compact concentric tube FN for transnasal procedures.106 (D) Two concentric tube FNs through one endoscope are used to reach a larger area without adjusting the endoscope for transurethral laser prostate surgery.35 (E) A multi-channel concentric tube transmission with various end effectors for transnasal procedures.102,110
Actuation principle and typical structure designs of magnetic FNs. (A) An alignment of the FN tip is caused by a torque T generated by the magnetic field B(P). (B) Scheme of the magnetic FN in neurosurgery. The permanent magnet is placed on the needle tip, connected with the needle shaft through a ball joint, and the entire structure is encased in a silicone tube.38 (C) Top: ten different shapes and diameters of needle tips have adhered with a cyanoacrylate adhesive to the magnet. Bottom: magnetic needle with a flexible shaft.40 (D) A variable stiffness magnetic FN is made of a conductive shape memory polymer (CSMP) for cardiac ablation.70
Five longitudinal segment transmission prototype designs are in Figs. 5B–5E. Meanwhile, the FNs were made out of different numbers of elements, two,48 four,47,82 seven,80 and much more,23 each of them interlocked along their length as an ovipositor. More specifically, researchers from Imperial College London have proposed the programmable bevel-tip needle (PBN) concept. The feasibility of this ovipositor-inspired design has been proved through experiments with their 12 mm in outer diameter (OD) prototype with four segments (Fig. 5C, left).47 Then, a 4-mm OD prototype with two-segment was manufactured to miniaturize this design. This prototype with a reduced diameter can steer around tight bends without protruding segment buckling. Meanwhile, the tracking performance of this two-segment FN is also substantially better (Fig. 5B).48 Most recently, a four-segment PBN with a diameter reduced to 2.5 mm was designed for localized drug delivery. It was the first in vivo deployment of an FN, and it was the first time that an FN was used as an implantable device (Fig. 5C, right).82 Since FNs with smaller diameters are needed for clinical use, Scali et al. developed a wasp ovipositor-inspired FN with a total diameter of 1.2 mm. However, it is challenging to manufacture long and well-aligned wedge-shaped sections of small size. Their solution is that these kinds of wedge-shaped sections can be approximated by a set of cylindrical sections (Fig. 5D).80 Adopting similar ideas, Kater et al. reported a flexible shaft of the transport mechanism for tissue (Fig. 5E).23 It has been proved that inserting an FN into a tissue phantom using this "push–pull" motion inspired by the ovipositor resulted in less tissue damage.
Concentric Tube Transmission
Concentric tube transmission is another example of active needle steering with tethered internal direct actuation. The schematic diagram of concentric tube transmission is shown in Fig. 6A. Typically, the concentric tube FNs, also called concentric tube robots, are composed of several hyperelastic tubes (usually two or three), and the relative translation and rotation of the tubes can control their shape.91 With relative translation and rotation, different curvatures can be achieved, and coupled motion can further increase the workspace.
As mentioned, FN steering entirely depends on the needle-tissue interaction at the early development stage of the FNs. Given the surgical manipulation in natural orifices such as the vagina and urethra, using FNs in this area may also have potential. Concentric tube FNs demonstrated steerability from soft tissues to liquid-filled cavities or air.33 A period of rapid development in modeling research of concentric tube needles contributed to the current commonly used model.27
As a surgical instrument for MIS,105 concentric tube FN is widely used for endoscopic or medical image-guided natural orifice surgeries, including transoral central airway puncture,31 transoral lung access system,91,92 transnasal surgery12,102,106,110, transurethral laser prostate surgery,35 and endoscopic full-thickness resection.71 Among them, a single concentric tube FN can be used directly in transoral lung access surgery (Fig. 6B) and transnasal procedures (Fig. 6C). Moreover, the multi-channel concentric tube transmission system is developed to collaborate on tasks within a confined space. For example, for transurethral laser prostate surgery, two concentric tube FNs reach a larger area without adjusting the endoscope (Fig. 6D).35 A multi-channel concentric tube robot system was proposed and expected to fill the gap in treatment tools for MIS-based nasopharyngeal carcinoma treatment (Fig. 6E).102,110 Meanwhile, the concentric tube FNs can achieve different operations with various end-effectors, such as forceps, scissors, and electric coagulators.
With curvilinear ability and maneuverability to avoid collisions, concentric tube FNs offer a novel solution to the challenge in narrow and complex natural orifice environments.106 The immediate advantages of concentric tube transmission are highlighted in these applications. However, their path-following capability is affected by torsional instability and the pre-curvature of tubes.21,71 Meanwhile, the concentric tube FNs only can provide a limited payload, which limits their current interventional capacity. For example, a survey has found that clinically typical needle-tissue interaction forces range from 1 to 10 N.97 The average puncture force value for human skin, fat, and muscle is 6.0 N, 2.0 N, and 4.4 N, respectively.20 However, it is reported that the maximum payload can be supported by a concentric tube FN with a diameter of 25 G (0.5 mm) is only around 24.50 mN,30, which is challenging to meet the requirements of clinical operations.
Several designs on the traditional tethered internal direct actuation have been proposed, such as tendon-driven, longitudinal segments transmission, and concentric tube transmission mentioned above. Because the actuation will take up much space in the human body, the sizes of active FNs are too challenging to be miniaturized to the prescribed scale. Therefore, most active FNs with tethered internal direct actuation are usually very complex and bulky. A large needle diameter can result in great puncture force, aggravating patient pain. Due to the miniaturization requirements in MIS, FN steering with untethered external actuation has raised the attention of researchers.
FN Steering with Untethered External Actuation
In recent years, FN steering with untethered external actuation has drawn significant attention due to its small size and the ability to exert forces without the need for tethers. Advances in the untethered external actuation rely on the development of magnetic insertions. Magnetic insertions provide a tetherless method to implant flexible percutaneous instruments, such as needles, probes, or catheters, into soft tissue.29 The magnetic FNs are usually designed and manufactured by adding a permanent magnet on the needle tips38,39,40,72 (basic steering mechanism in Fig. 7A). The neodymium-iron-boron (Nd-Fe-B) alloy is widely utilized because of its strong magnetization and biocompatibility, making it a candidate for magnetic FNs.37 In general, the design of magnetic FNs can be divided into three categories: ball joint-based, adhesives-based, and special materials-based. The magnetic FN was proposed in neurosurgery, where a permanent magnet is placed on the needle tip, connected with the needle shaft through a ball joint, and the entire structure is encased in a silicone tube (Fig. 7B).38 Adhesives adhere to the sharp tip, permanent magnet, and flexible shaft to fabricate magnetic FNs, as shown in Fig. 7C.40 A variable stiffness magnetic FN can be made of a conductive phase-change polymer for MIS (Fig. 7D).70
Due to their complex structures, it is typically tough to balance steerability and needle diameter for active FN steering with tethered internal direct actuation. Recent advances in untethered external actuation methods have addressed the miniaturization requirements in MIS because no physical connection between the actuator and the FN is required, significantly simplifying the active FN structure.70 The magnetic FN can be magnetically guided to enable sharp bending and multiple targeting.32 It is particularly beneficial for steering and small diameter requirements, making it an up-and-coming option for further research into active steering techniques.
The first proposal to use magnetic interaction for remotely steering continuum manipulators has been postulated for over 70 years.95 For more than ten years, Tang et al. pioneered an FN design with a magnetic head and a compliant hinge to orient the head using magnetic fields.93 The needle tip with a permanent magnet was steered by applying torques generated by a clinically-sized magnetic manipulation system.38,69 The magnetic needle steering technique allows for flexible shafts because the needle was pulled by magnetic force during insertion. The magnetic FN removes physical connections between the needles and actuators. These ultraminimally invasive surgical tools offer considerable advantages in medical applications with good biocompatibility and excellent payload capacities.
The proper and effective actuation system is necessary to control the magnetic FN and improve clinical outcomes. As an easily generated physical field, the external magnetic field does not cause harm to the human body and has no side effects. Therefore, it is very suitable for the wireless power supply of magnetic FNs. We provide an overview of two main categories: stationary electromagnets and mobile permanent magnets, as summarized in Table 3.
Both permanent magnets and electromagnets can produce equivalent magnetic fields. The most significant advantage of electromagnets is their ability to generate a steerable magnetic field, which depends on the current flowing through the coil windings. Multiple electromagnets can provide the actuation in different directions. In contrast, permanent magnets have inherent field distributions and can be controlled by changing their position and posture. However, complex motion control algorithms are required for successful operation due to the nonlinear relationship between the magnetic needle and the magnetic field.76,93 Magnetic FN poses challenges as each steering method is highly device-specific. However, the issue is their scalability for clinical use due to the workspace and forced limitations of the electromagnetic systems that involve necessary high currents. When the magnetic FN works, the safety of the electromagnetic field and the effectiveness of the cooling system must be maintained. Meanwhile, the magnetic FN may exclude its use in patients with pacemakers. In addition, frequently used magnetic actuation methods using permanent magnets are unavailable in an MRI scanner.89 Applying an MRI scanner for magnetic actuation is a recent study that has the potential to actuate, position and image simultaneously without introducing additional hardware.109
Opportunities
The FN can avoid obstacles and deal with the tissue that cannot be reached by ordinary steel needles, making essential contributions to MIS. Such comprehensive and great clinical application demand prompt researchers to continuously pursue innovation. Compared with passive FNs, active FNs are undoubtedly technological advancements. Through the collation of many existing positive reported research articles, we highlight two critical issues that limit the clinical application of active FNs: lack of structural design for various requirements and difficulty in miniaturizing. Furthermore, the development of active FNs suffers from trade-offs in size and steerability. Many of the proposed active FNs achieve high levels of steerability. However, larger diameters are typically necessary. Moreover, the current thin active FN can only provide a minimal payload. Here, we mainly discuss several methods as potential opportunities to address the issues that need to be considered in the future development of active FNs.
Biomimetic technology usually provides practical, safe, elegant, and efficient ideas for unsolved challenges in science and engineering.114 Taking inspiration from nature to design modern medical instruments has ever had significant precedence in soft medical robotics.112 Scientists can learn from the principle of the structures, behaviors, and functions of biological organisms to invent new tools. For example, many bio-inspired rigid needles have been developed and they mainly focus on reducing puncture force and tissue deformations during percutaneous therapies, such as various mosquito proboscis-inspired needles,54 cicada-inspired needles,101 and bee-inspired needles.19 Moreover, many bio-inspired microneedles with high tissue adhesion have been proposed for transdermal drug delivery.34,113 In the previous section, we introduced longitudinal segment transmission, an FN steering strategy with tethered internal direct actuation inspired by a wasp ovipositor. It is a typical example of biomimetic technology that can help improve the design of FNs.
Flexible biological organisms can likely use some steering strategies that differ from those applied in current FNs to insert hard wood, earth, or skin. Many natural structures are inherently flexible, such as stingers of the honeybee, wasp, scorpion, mosquito, bed bug, and leech. Integration and combination of these biological steering strategies could contribute to the design of active FNs. Insight into the inserting and steering strategies found in nature may give essential clues about minimizing the size of active FNs while facilitating their performance. The idea of biomimetics accompanied the development process of FNs, and there is reason to expect more surprises from bio-inspired FN research.
Research on magnetic FNs is a considerably newer area, and the structure design of the magnetic needle is relatively simple. However, magnetic FNs have shown immense potential as minimally invasive interventional tools because of their untethered actuation. Therefore, the various designs of magnetic FNs are worthy of being created to serve this field.
Zhang et al. have presented a mantis-inspired microneedle, and different sizes and angles for microneedles can be manufactured easily based on the flexibility and versatility of ferrofluids.113 However, to the best of our knowledge, there is no report of biomimetic FNs with magnetic actuation. Therefore, developing active FNs by combining magnetic actuation with biomimetic technology must be exciting and meaningful. With the help of magnetic actuation, biological behaviors of inserting and steering can be better realized. As the technology matures, the magnetization techniques and fabrication strategies will enable greater steerability and decreased needle sizes. Based on advanced technologies, significant opportunities for increased function within FNs will be offered.
Conclusions
Current MIS procedures contain a straight and rigid needle, which limits the surgical trajectory. Targeting errors arise when the needle tip is placed in soft tissues because of unpredictable factors, such as intra-operative tissue motion, that complicate accurate needle tip placement, stimulating the development of FN steering actuation. We undertook a comparative review of publications describing targeting errors and focused on the actuation of FNs, which is directly related to needle performance. In addition, reviewing a body of work spanning the last decade suggests the current research status, unique technical challenges, and opportunities.
The implementation of needle steering distinguishes passive FNs and active FNs. The passive steering mechanisms depend on the needle-tissue interaction to control needle-path curvature. Active FN steering actuation involves an additional force applied at the needle tip, which should be a concern because the actuation methods determine the structures of the active FN. Targeting errors and needle diameters have been investigated to analyze and compare the current status of FNs. Passive FNs have thin diameters ranging from 0.5 mm to 1.4 mm, as complex internal structures are not required. However, the diameter of the active FN, which requires higher steering performance, is generally larger than that of the passive FN, ranging from 1.42 mm to 12 mm, due to the complex internal actuation structure. The targeting errors of passive FNs are distributed primarily between 1 and 2 mm. Due to the better achieving extensive needle steerability, the targeting errors of many active FNs can be less than 1 mm. Therefore, the active FN has more potential to achieve lower targeting errors than the passive FN, but often at the expense of the larger needle diameters. A needle with a too-large diameter is not conducive to clinical MIS applications.
Different FN steering actuation methods have also induced their diverse structural designs. Depending on whether the energy supply is tethered directly to the FN, we propose to divide the active FN steering actuation into tethered internal direct actuation and untethered external actuation. Tethered internal direct actuation refers to using mediums to transmit forces directly within the FN. We introduced three active FN steering actuation with tethered internal direct actuation: tendon-driven, longitudinal segment transmission, and concentric tube transmission. Five main structure types of tendon-driven FNs have been discussed according to different wire arrangement methods. Longitudinal segment transmission needles could be adapted to reduce tissue damage, and the concentric tube needles have immediate advantages in natural orifices.
The advantages of FN steering with tethered internal direct actuation are that the design is diversified and the needle function is comprehensive. However, the disadvantage is that the complex actuation structure will occupy a large amount of space within the needle, which is not conducive to miniaturization.
The limitation in FN steering with tethered internal direct actuation is expected to be overcome by magnetic actuation in an untethered way. Magnetic insertion uses an external magnetic field to drive a magnetic FN inside the patient to reach the target position along a preset path. It simplifies the design by eliminating the need for a drive mechanism within the FN. In addition, magnetic actuation provides a contactless in-air actuation approach and allows for its miniaturization.
Closed-loop control is the most effective way that adjusts steering according to the position feedback of the FN to reduce targeting error. However, the poses of the FN in practical clinical applications are difficult to predict accurately due to the diversity of the tissues.88 It is now widely recognized that integrating sensors into the FN is key to realizing real-time needle position feedback. FBG and EM sensors are widely used to provide FNs with accurate and quick shape information. However, until now, the integration into small devices using embedded sensors to steer a needle has remained an open research challenge. A practical approach to improving needle-sensor integration is to reduce the sensor footprint and mechanically-compliant designs. Optimizing the placement of the sensors on the needle is also the basis for meeting both low-cost and high-precision requirements.
The targeting errors of biological tissue experiments are higher than those of artificial tissue experiments. Furthermore, in non-homogenous biological tissues, needle deflection and even buckling could be caused when encountering stiffer tissues. Therefore, it is necessary to know to what extent soft tissue experimental measurement can simulate the in vivo puncture.
Taking further inspiration from nature may offer solutions to various challenges in active FN steering mechanisms. Future research will progress on the FN structure miniaturized and integrated design, manufacturing processes, and regular biological tissue experiments, even clinical experiments.
References
Abayazid, M., M. Kemp, and S. Misra. 3D flexible needle steering in soft-tissue phantoms using fiber bragg grating sensors. in Proc. IEEE Int. Conf. Robot. Autom., Karlsruhe, Germany, 2013, pp. 5843–5849.
Abayazid, M., P. Moreira, N. Shahriari, S. Patil, R. Alterovitz, and S. Misra. Ultrasound-guided three-dimensional needle steering in biological tissue with curved surfaces. Med. Eng. Phys. 37:145–150, 2015
Abayazid, M., R. J. Roesthuis, R. Reilink, and S. Misra. Integrating deflection models and image feedback for real-time flexible needle steering. IEEE Trans. Robot. 29:542–553, 2012
Adagolodjo, Y., L. Goffin, M. De Mathelin, and H. Courtecuisse. Robotic insertion of flexible needle in deformable structures using inverse finite-element simulation. IEEE Trans. Robot. 35:697–708, 2019
Adebar, T., A. Fletcher, and A. Okamura. 3D ultrasound-guided robotic needle steering in biological tissue. IEEE Trans. Biomed. Eng. 61:2899–2910, 2014
Adebar, T. K., J. D. Greer, P. F. Laeseke, G. L. Hwang, and A. M. Okamura. Methods for improving the curvature of steerable needles in biological tissue. IEEE Trans. Biomed. Eng. 63:1167–1177, 2015
Aggravi, M., D. A. Estima, A. Krupa, S. Misra, and C. Pacchierotti. Haptic teleoperation of flexible needles combining 3d ultrasound guidance and needle tip force feedback. IEEE Robot. Autom. Lett. 6:4859–4866, 2021
Amanov, E., T.-D. Nguyen, and J. Burgner-Kahrs. Tendon-driven continuum robots with extensible sections - A model-based evaluation of path following motions. Int. J. Robot. Res. 40:7–23, 2021
Beirne, P. V., S. Hennessy, S. L. Cadogan, F. Shiely, T. Fitzgerald, and F. MacLeod. Needle size for vaccination procedures in children and adolescents. Cochrane Database Syst. Rev. 2018. https://doi.org/10.1002/14651858.CD010720.pub3
van de Berg, N. J., J. Dankelman, and J. J. van den Dobbelsteen. Design of an actively controlled steerable needle with tendon actuation and fbg-based shape sensing. Med. Eng. Phys. 37:617–622, 2015
Bernardes, M. C., B. V. Adorno, P. Poignet, and G. A. Borges. Robotassisted automatic insertion of steerable needles with closed-loop imaging feedback and intraoperative trajectory replanning. Mechatronics. 23:630–645, 2013
Burgner, J., D. C. Rucker, H. B. Gilbert, P. J. Swaney, P. T. Russell, K. D. Weaver, and R. J. Webster III. A telerobotic system for transnasal surgery. IEEE Trans. Mechatron. 19:996–1006, 2014
Cai, C., C. Sun, Y. Han, and Q. Zhang. Clinical flexible needle puncture path planning based on particle swarm optimization. Comput. Meth. Prog. Biol.193:105511, 2020
Carpi, F., and C. Pappone. Stereotaxis niobe® magnetic navigation system for endocardial catheter ablation and gastrointestinal capsule endoscopy. Expert. Rev. Med. Devic. 6:487–498, 2014
Cerkvenik, U., B. Van de Straat, S. W. Gussekloo, and J. L. Van Leeuwen. Mechanisms of ovipositor insertion and steering of a parasitic wasp. Proc. Natl. Acad. Sci. U.S.A. 114:E7822–E7831, 2017
Chautems, C., A. Tonazzini, Q. Boehler, S. H. Jeong, D. Floreano, and B. J. Nelson. Magnetic continuum device with variable stiffness for minimally invasive surgery. Adv. Intell. Syst. 2:1–9, 2020
Chautems, C., A. Tonazzini, D. Floreano, and B. J. Nelson. A variable stiffness catheter controlled with an external magnetic field. in Proc. IEEE/RSJ Int. Conf. Intell. Robots Syst., Vancouver, BC, Canada, 2017, pp. 181–186.
Chevrie, J., N. Shahriari, M. Babel, A. Krupa, and S. Misra. Flexible needle steering in moving biological tissue with motion compensation using ultrasound and force feedback. IEEE Robot. Autom. Lett. 3:2338–2345, 2018
Chiroiu, V., N. Nedelcu, D. Pisla, L. Munteanu, and C. Rugina. On the flexible needle insertion into the human liver. Sci. Rep. 11:1–14, 2021
Conci, A., Brazil, A. L., Popovici, D., Jiga, G., and Lebon, F. Modeling the behavior of human body tissues on penetration. AIP Conf. Proc. 1932: 020006-1–020006-6, 2018.
da Veiga, T., J. H. Chandler, P. Lloyd, G. Pittiglio, N. J. Wilkinson, A. K. Hoshiar, R. A. Harris, and P. Valdastri. Challenges of continuum robots in clinical context: a review. P Biomed. Eng. 2:032003, 2020
De Falco, I., C. Culmone, A. Menciassi, J. Dankelman, and J. J. van Den Dobbelsteen. A variable stiffness mechanism for steerable percutaneous instruments: integration in a needle. Med. Biol. Eng. Comput. 56:2185–2199, 2018
de Kater, E. P., A. Sakes, J. Bloemberg, D. J. Jager, and P. Breedveld. Design of a flexible wasp-inspired tissue transport mechanism. Front. Bioeng. Biotechnol. 2021. https://doi.org/10.3389/fbioe.2021.782037
de Ruiter, Q. M., J. R. Fontana, W. F. Pritchard, M. Mauda-Havakuk, I. Bakhutashvili, J. A. Esparza-Trujillo, N. A. Varble, M. Verstege, S. Xu, R. Seifabadi, et al. Endovascular steerable and endobronchial precurved guiding sheaths for transbronchial needle delivery under augmented fluoroscopy and cone beam CT image guidance. Transl. Lung Cancer Res. 10:3627–3644, 2021
De Vries, M., J. Sikorski, S. Misra, and J. van den Dobbelsteen. Axially rigid steerable needle with compliant active tip control. PLoS ONE. 16:1–18, 2021
DiMaio, S. P., and S. E. Salcudean. Needle insertion modeling and simulation. IEEE Trans. Robot. Autom. 19:864–875, 2003
Dupont, P. E., J. Lock, B. Itkowitz, and E. Butler. Design and control of concentric-tube robots. IEEE Trans. Robot. 26:209–225, 2010
Edelmann, J., A. J. Petruska, and B. J. Nelson. Magnetic control of continuum devices. Int. J. Robot. Res. 36:68–85, 2017
Limpabandhu, C., Y. Hu, H. Ren, W. Song, and Z. Tse. Towards catheter steering using magnetic tractor beam coupling. Proc. IMechE Part H: J Eng. Med. 236:583–591, 2022.
Farooq, M. U., B. Xu, and S. Y. Ko. A concentric tube-based 4-DOF puncturing needle with a novel miniaturized actuation system for vitrectomy. Biomed. Eng. Online. 18:1–16, 2019
Gafford, J. B., S. Webster, N. Dillon, E. Blum, R. Hendrick, F. Maldon-ado, E. A. Gillaspie, O. B. Rickman, S. D. Herrell, and R. J. Webster. A concentric tube robot system for rigid bronchoscopy: a feasibility study on central airway obstruction removal. Ann. Biomed. Eng. 48:181–189, 2020
Gang, E. S., B. L. Nguyen, Y. Shachar, L. Farkas, L. Farkas, B. Marx, D. Johnson, M. C. Fishbein, C. Gaudio, and S. J. Kim. Dynamically shaped magnetic fields: initial animal validation of a new remote electrophysiology catheter guidance and control system. Circ- Arrhythmia Electr. 4:770–777, 2011
Gilbert, H. B., J. Neimat, and R. J. Webster. Concentric tube robots as steerable needles: achieving follow-the-leader deployment. IEEE Trans. Robot. 31:246–258, 2015
Han, D., R. S. Morde, S. Mariani, A. L. Mattina, E. Vignali, C. Yang, G. Barillaro, and H. Lee. 4D Printing of a bioinspired microneedle array with backward-facing barbs for enhanced tissue adhesion. Adv. Funct. Mater. 30:1–12, 2020
Hendrick, R. J., S. D. Herrell and R. J. Webster III. A multi-arm hand-held robotic system for transurethral laser prostate surgery. in Proc. IEEE Int. Conf. Robot. Autom., Hong Kong, China, 2014, pp. 2850–2855.
Henken, K., D. Van Gerwen, J. Dankelman, and J. Van Den Dobbelsteen. Accuracy of needle position measurements using fiber bragg gratings. Minim. Invasiv. Ther. 21:408–414, 2012
Heunis, C., J. Sikorski, and S. Misra. Flexible instruments for endovascular interventions: Improved magnetic steering, actuation, and image-guided surgical instruments. IEEE Robot. Autom. Mag. 25:71–82, 2018
Hong, A., A. J. Petruska, A. Zemmar, and B. J. Nelson. Magnetic control of a flexible needle in neurosurgery. IEEE Trans. Biomed. Eng. 68:616–627, 2020
Hu, X., A. Chen, Y. Luo, C. Zhang, and E. Zhang. Steerable catheters for minimally invasive surgery: a review and future directions. Comput. Assist. Surg. 23:21–41, 2018
Ilami, M., R. J. Ahmed, A. Petras, B. Beigzadeh, and H. Marvi. Magnetic needle steering in soft phantom tissue. Sci. Rep. 10:1–11, 2020
Issatayeva, A., A. Amantayeva, W. Blanc, D. Tosi, and C. Molardi. Design and analysis of a fiber-optic sensing system for shape reconstruction of a minimally invasive surgical needle. Sci. Rep. 11:1–12, 2021
Jiang, S., B. Jiang, P. Fang, and Z. Yang. Pre-operative motion planner for steerable needles using cost map based on repulsive field and empirical model of needle deflection. J. Med. Devices. 16:021004, 2022
Karimi, S., and B. Konh. Self-sensing feedback control of multiple interacting shape memory alloy actuators in a 3d steerable active needle. J. Intel. Mat. Syst. Str. 31:1524–1540, 2020
Khadem, M., C. Rossa, N. Usmani, R. S. Sloboda, and M. Tavakoli. A two-body rigid/flexible model of needle steering dynamics in soft tissue. IEEE/ASME Trans. Mech. 21:2352–2364, 2016
Khashei Varnamkhasti, Z., and B. Konh. Cable-driven 3d steerable surgical needle for needle-based procedures. in Proc. Conf Des Med Dev., Minneapolis, MN, USA, 2020, pp. V001T06A008-1-5.
Kimura, T., S. Takatsuki, A. Oishi, M. Negishi, S. Kashimura, Y. Katsumata, T. Nishiyama, N. Nishiyama, Y. Tanimoto, Y. Aizawa, et al. Operator-blinded contact force monitoring during pulmonary vein isolation using conventional and steerable sheaths. Int. J. Cardiol. 177:970–976, 2014
Ko, S. Y., L. Frasson, and F. R. y Baena. Closed-loop planar motion control of a steerable probe with a “programmable bevel” inspired by nature. IEEE Trans. Robot. 27:970–983, 2011
Ko, S., and Y. B. F. Rodriguez. Toward a miniaturized needle steering system with path planning for obstacle avoidance. IEEE Trans. Biomed. Eng. 60:910–917, 2013
Kratchman, L. B., M. M. Rahman, J. R. Saunders, P. J. Swaney, and R. J. Webster III. Toward robotic needle steering in lung biopsy: a tendon-actuated approach. Proc. SPIE, Med. Imag. Vis., Image-Guided Procedures, Model. 7964:79641I-1–79641I-8, 2011.
Lee, H., and J. Kim. Estimation of flexible needle deflection in layered soft tissues with different elastic moduli. Med. Biol. Eng. Comput. 52:729–740, 2014
Lee, W., J. Nam, J. Kim, E. Jung, N. Kim, and G. Jang. Steering, tunneling, and stent delivery of a multifunctional magnetic catheter robot to treat occlusive vascular disease. IEEE Trans. Ind. Electron. 68:391–400, 2020
Lee, J., J. Wang, and W. Park. Efficient mechanism design and systematic operation planning for tube-wire flexible needles. J. Mech. Robot. 10:1–9, 2018
Li, M., D. Gao, Y. Lei, and T. Xu. Dynamic path planning for beveltip flexible needle insertion into soft tissue based on a real-time finite element model. Math. Probl. Eng. 1–13:2020, 2020
Li, A. D., K. B. Putra, L. Chen, J. S. Montgomery, and A. Shih. Mosquito proboscis-inspired needle insertion to reduce tissue deformation and organ displacement. Sci Rep. 10:1–14, 2020
Lu, M., Y. Zhang, and H. Du. Design and control of a novel magnetic resonance imaging-compatible breast intervention robot. Int. J. Adv. Robot. Sysm. 17:1–14, 2020
Mahoney, A. W., and J. J. Abbott. 5-DOF manipulation of an untethered magnetic device in fluid using a single permanent magnet. in Proc. Conf. Robotics: Sci. Syst. 2014.
Majewicz, A., T. R. Wedlick, K. B. Reed, and A. M. Okamura. Evaluation of robotic needle steering in ex vivo tissue. in Proc. IEEE Int. Conf. Robot. Autom., Anchorage, Alaska, USA. 2010, pp. 2068–2073.
Matheson, E., and F. Rodriguez y Baena. Biologically inspired surgical needle steering: technology and application of the programmable beveltip needle. Biomimetics. 5:1–23, 2020
Moreira, P., K. J. Boskma, and S. Misra. Towards MRI-guided flexible needle steering using fiber bragg grating-based tip tracking. in Proc. IEEE Int. Conf. Robot. Autom., Singapore, 2017, pp. 4849–4854.
Moreira, P., and S. Misra. Biomechanics-based curvature estimation for ultrasound-guided flexible needle steering in biological tissues. Ann. Biomed. Eng. 43:1716–1726, 2015
Neubach, Z., and M. Shoham. Ultrasound-guided robot for flexible needle steering. IEEE Trans. Biomed. Eng. 57:799–805, 2010
Neumann, M., and J. Burgner-Kahrs. Considerations for follow-the -leader motion of extensible tendon-driven continuum robots. in Proc. IEEE Int. Conf. Robot. Autom., 2016, pp. 917–923.
Norton, J. C., P. R. Slawinski, H. S. Lay, J. W. Martin, B. F. Cox, G. Cummins, M. P. Desmulliez, R. E. Clutton, K. L. Obstein, S. Cochran, et al. Intelligent magnetic manipulation for gastrointestinal ultrasound. Sci. Robot. 4:eaav7725, 2019
Padasdao, B., and B. Konh. Shape memory alloy actuators in an active needle—modeling, precise assembly, and performance evaluation. J. Manuf. Sci. Eng. 143:1–10, 2021
Park, Y. L., S. Elayaperumal, B. Daniel, S. C. Ryu, M. Shin, J. Savall, R. J. Black, B. Moslehi, and M. R. Cutkosky. Real-time estimation of 3-D needle shape and deflection for MRI-guided interventions. IEEE/ASME Trans. Mech. 15:906–915, 2010
Patil, J., S. Ford, C. Egeler, and D. Williams. The effect of needle dimensions and infusion rates on injection pressures in regional anaesthesia needles: a bench-top study. Anaesthesia. 70:183–189, 2015
Pattanshetti, S., and S. C. Ryu. Design and fabrication of lasermachined hinge joints on miniature tubes for steerable medical devices. J. Mech. Robot. 10:011002, 2018
Pattanshetti, S., R. Sandström, A. Kottala, N. M. Amato, and S. C. Ryu. Feasibility Study of Robotic Needles with a Rotational Tip-Joint and Notch Patterns. in Proc. IEEE Int. Conf. Robot. Autom., 2019, pp. 1534–1540.
Petruska, A. J., F. Ruetz, A. Hong, L. Regli, O. Surucu, A. Zemmar, and B. J. Nelson. Magnetic needle guidance for neurosurgery: Initial design and proof of concept. in Proc. IEEE Int. Conf. Robot. Autom., Stockholm, Sweden, 2016, pp. 4392–4397.
Piskarev, Y., J. Shintake, C. Chautems, J. Lussi, Q. Boehler, B. J. Nelson, and D. Floreano. A variable stiffness magnetic catheter made of a conductive phase-change polymer for minimally invasive surgery. Adv. Funct. Mater. 32:2107662, 2022
Ponten, R., C. B. Black, A. J. Russ, and D. C. Rucker. Analysis of a concentric-tube robot design and feasibility for endoscopic deployment. in Medical Imaging 2017: Image-Guided Procedures, Robotic Interventions, and Modeling.10135. SPIE, pp. 290–300, 2017.
Pratt, R. L., and A. J. Petruska. Empirically comparing magnetic needle steering models using expectation-maximization. Robotics. 11:1–18, 2022
Qi, B., Z. Yu, Z. K. Varnamkhasti, Y. Zhou, and J. Sheng. Toward a telescopic steerable robotic needle for minimally invasive tissue biopsy. IEEE Robot. Autom. Let. 6:1989–1996, 2021
Roesthuis, R. J., N. J. van de Berg, J. J. van den Dobbelsteen, and S. Misra. Modeling and steering of a novel actuated-tip needle through a soft-tissue simulant using fiber bragg grating sensors. in Proc. IEEE Int. Conf. Robot. Autom., Washington, USA, 2015, pp. 2283–2289.
Roesthuis, R. J., M. Kemp, J. J. van den Dobbelsteen, and S. Misra. Three-dimensional needle shape reconstruction using an array of fiber bragg grating sensors. IEEE/ASME Trans. Mechatron. 19:1115–1126, 2014
Ryan, P., and E. Diller. Magnetic actuation for full dexterity microrobotic control using rotating permanent magnets. IEEE Trans. Robot. 33:1398–1409, 2017
Ryu, S. C., Z. F. Quek, J.-S. Koh, P. Renaud, R. J. Black, B. Moslehi, B. L. Daniel, K.-J. Cho, and M. R. Cutkosky. Design of an optically controlled MR-compatible active needle. IEEE Trans. Robot. 31:1–11, 2014
Ryu, S. C., Z. F. Quek, P. Renaud, R. J. Black, B. L. Daniel, and M. R. Cutkosky. An optical actuation system and curvature sensor for a MRcompatible active needle. in Proc. IEEE Int. Conf. Robot. Autom., Saint Paul, MN, USA, 2012, pp. 1589–1594.
Scali, M., D. Kreeft, P. Breedveld, and D. Dodou. Design and evaluation of a wasp-inspired steerable needle. Proc. SPIE 10162, Bioinspiration, Biomimetics, and Bioreplication 2017, 10162:34–46, 2017.
Scali, M., T. Pusch, P. Breedveld, and D. Dodou. Ovipositor-inspired steerable needle: design and preliminary experimental evaluation. Bioinspir Biomim. 13:016006, 2017
Scali, M., P. A. Veldhoven, P. W. Henselmans, D. Dodou, and P. Breedveld. Design of an ultra-thin steerable probe for percutaneous interventions and preliminary evaluation in a gelatine phantom. PLoS ONE. 14:e0221165, 2019
Secoli, R., E. Matheson, M. Pinzi, S. Galvan, A. Donder, T. Watts, M. Riva, D. D. Zani, L. Bello, and F. R. Baena. Modular robotic platform for precision neurosurgery with a bio-inspired needle: system overview and first in-vivo deployment. PloS one. 17:e0275686, 2022
Seifabadi, R., E. E. Gomez, F. Aalamifar, G. Fichtinger, and I. Iordachita. Real-time tracking of a bevel-tip needle with varying insertion depth: Toward teleoperated MRI-guided needle steering. in Proc. IEEE/RSJ Int. Conf. Intell. Robots Syst., Tokyo, Japan. 2013, pp 469–476.
Shahriari, N., J. R. Georgiadis, M. Oudkerk, and S. Misra. Hybrid control algorithm for flexible needle steering: demonstration in phantom and human cadaver. PLoS ONE. 13:e0210052, 2018
Shi, C., X. Luo, P. Qi, T. Li, S. Song, Z. Najdovski, T. Fukuda, and H. Ren. Shape sensing techniques for continuum robots in minimally invasive surgery: a survey. IEEE Trans. Biomed. Eng. 64:1665–1678, 2017
Sikorski, J., I. Dawson, A. Denasi, E. E. Hekman, and S. Misra. Introducing BigMag—a novel system for 3d magnetic actuation of flexible surgical manipulators. in Proc. IEEE Int. Conf. Robot. Autom., Singapore, 2017, pp. 3594–3599.
Sikorski, J., A. Denasi, G. Bucchi, S. Scheggi, and S. Misra. Visionbased 3-D control of magnetically actuated catheter using BigMag—An array of mobile electromagnetic coils. IEEE/ASME Trans. Mech. 24:505–516, 2019
Song, S., Z. Li, H. Yu, and H. Ren. Electromagnetic positioning for tip tracking and shape sensing of flexible robots. IEEE Sens J. 15:4565–4575, 2015
Su, H., K.-W. Kwok, K. Cleary, I. Iordachita, M. C. Cavusoglu, J. P. Desai, and G. S. Fischer. State of the art and future opportunities in MRI-guided robot-assisted surgery and interventions. Proc. IEEE. 110:968–992, 2022
Swaney, P. J., J. Burgner, H. B. Gilbert, and R. J. Webster. A flexurebased steerable needle: high curvature with reduced tissue damage. IEEE Trans. Biomed. Eng. 60:906–909, 2012
Swaney, P. J., A. W. Mahoney, B. I. Hartley, A. A. Remirez, E. Lamers, R. H. Feins, R. Alterovitz, and R. J. Webster III. Toward transoral peripheral lung access: Combining continuum robots and steerable needles. J. Med. Robot. Res. 2:1–14, 2017
Swaney, P. J., A. W. Mahoney, A. A. Remirez, E. Lamers, B. I. Hartley, R. H. Feins, R. Alterovitz, and R. J. Webster. Tendons, concentric tubes, and a bevel tip: Three steerable robots in one transoral lung access system. in Proc. IEEE Int. Conf. Robot. Autom., 2015, pp. 5378–5383.
Tang, L. B., Y. H. Chen, and X. J. He. Magnetic force aided compliant needle navigation and needle performance analysis. in Proc. IEEE Int. Conf. Robot. Biomi., Sanya, China, 2007, pp. 612–616.
Thakur Singh, R. R., I. Tekko, K. McAvoy, H. McMillan, D. Jones, and R. F. Donnelly. Minimally invasive microneedles for ocular drug delivery. Expert Opin. Drug Del. 14:525–537, 2017
Tillander, H. Magnetic guidance of a catheter with articulated steel tip. Acta Radiol. 35:62–64, 1951
Van de Berg, N. J., D. J. van Gerwen, J. Dankelman, and J. J. van den Dobbelsteen. Design choices in needle steering—a review. IEEE/ASME Trans. Mech. 20:2172–2183, 2015
Van, G., J. Dennis, D. Jenny, and J. John. Needle-tissue interaction forces—a survey of experimental data. Med. Eng. Phys. 34:665–680, 2012
Varnamkhasti, Z. K., and B. Konh. Compact 3D-printed active flexible needle for percutaneous procedures. Surg. Innov. 27:402–405, 2020
Varnamkhasti, Z. K., and B. Konh. Design, fabrication, and testing of a flexible three-dimensional printed percutaneous needle with embedded actuators. J. Med. Devices. 15:021007, 2021
Vrooijink, G. J., M. Abayazid, S. Patil, R. Alterovitz, and S. Misra. Needle path planning and steering in a three-dimensional non-static environment using two-dimensional ultrasound images. Int. J. Robot. Res. 33:1361–1374, 2014
Wang, J., Q. Cong, X. Qi, and Y. Zhang. Optimum structural design and analysis of drag reduction mechanism of bionic needles inspired by cicada stylet. J. Jilin Univ. (Eng. Technol. Ed.). 44:696–700, 2014
Wang, J., X. Yang, P. Li, S. Song, L. Liu, and M.Q.-H. Meng. Design of a multi-arm concentric-tube robot system for transnasal surgery. Med. Biol. Eng. Comput. 58:497–508, 2020
Wang, Y. Z., Z. G. Zhou, Y. H. Chen, and H. F. Huang. Towards a magnetic articulated needle. Biotechnol. Chem. Mater. Eng. Adv. Mater. Res. 393:1060–1063, 2012
Webster, R. J., III., J. S. Kim, N. J. Cowan, G. S. Chirikjian, and A. M. Okamura. Nonholonomic modeling of needle steering. Int. J. Robot. Res. 25:509–525, 2006
Webster, R. J., A. M. Okamura, and N. J. Cowan. Toward active cannulas: Miniature snake-like surgical robots. in Proc. IEEE Conf. RSJ Intell. Robot. Syst., pp. 2857–2863, 2006.
Wu, L., S. Song, K. Wu, C. M. Lim, and H. Ren. Development of a compact continuum tubular robotic system for nasopharyngeal biopsy. Med. Biol. Eng. Comput. 55:403–417, 2017
Xiao, X., H. Poon, C. M. Lim, M.Q.-H. Meng, and H. Ren. Pilot study of trans-oral robotic-assisted needle direct tracheostomy puncture in patients requiring prolonged mechanical ventilation. Front. Robot. AI. 7:1–10, 2020
Yamada, A., S. Naka, N. Nitta, S. Morikawa, and T. Tani. A Loop -Shaped Flexible Mechanism for Robotic Needle Steering. IEEE Robot. Autom. Lett. 3:648–655, 2018
Yang, Z., and L. Zhang. Magnetic actuation systems for miniature robots: a review. Adv. Intell. Syst. 2:2000082, 2020
Yu, H., L. Wu, K. Wu, and H. Ren. Development of a multi-channel concentric tube robotic system with active vision for transnasal nasopharyngeal carcinoma procedures. IEEE Robot. Autom. Lett. 1:1172–1178, 2016
Zhang, B., F. Chen, M. Yang, L. Huang, Z. Du, L. Sun, and W. Dong. Real-time curvature detection of a flexible needle with a bevel tip. Sensors. 18:1–15, 2018
Zhang, Y., and M. Lu. A review of recent advancements in soft and flexible robots for medical applications. Int. J. Med. Robot. Comp. 16:e2096, 2020
Zhang, X., F. Wang, Y. Yu, G. Chen, L. Shang, L. Sun, and Y. Zhao. Bio-inspired clamping microneedle arrays from flexible ferrofluid -configured moldings. Sci. Bull. 64:1110–1117, 2019
Zhang, W., Y. Zhang, and Y. Liu. Design and control of a bionic needle puncture robot. Int. J. Med. Robot. Comp. 17:e2200, 2021
Zhao, S., D. Gao, M. Zhao, and J. Fu. Trajectory estimation of flexible needle using PVA tissue material. in IOP Conf. Series: Materials Sci Eng. 646:2019, p. 012063.
Zhong, Y., L. Hu, and Y. Xu. Recent advances in design and actuation of continuum robots for medical applications. Actuators. 9(4):142, 2020
Acknowledgments
The authors gratefully acknowledge funding support from the National Medical Research Council Clinician Scientist grant (NMRC CSA-MOH-000326), the Hong Kong Research Grants Council (RGC) Collaborative Research Fund (CRF C4026-21GF: 2300075; C4063-18G: 2300056), General Research Fund (GRF Ref. No.: N_CUHK.420/22 and 14216022) and (GRS) #3110167#3110137, the Reserve Leader Funding Project of Leading Talent Echelon of Heilongjiang Province of China (No. 2501050628), and China Scholarship Council under CSC 202108230187.
Conflict of interest
The authors declare that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Stefan M. Duma oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lu, M., Zhang, Y., Lim, C.M. et al. Flexible Needle Steering with Tethered and Untethered Actuation: Current States, Targeting Errors, Challenges and Opportunities. Ann Biomed Eng 51, 905–924 (2023). https://doi.org/10.1007/s10439-023-03163-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-023-03163-8