Abstract
We present the case of a 45-year-old man with an aberrant pancreas in the duodenum. He was referred to our hospital for gastric cancer screening. On upper gastrointestinal endoscopy, a submucosal tumor was noted in the second portion of the duodenum; it was 10 mm in diameter, with a smooth surface and bridging fold. Endoscopic ultrasonography (EUS) showed a hypoechoic lesion with small anechoic areas located in the third sonographic layer of the duodenum wall. To confirm the exact diagnosis, endoscopic resection was performed. The histological diagnosis was aberrant pancreas, Heinrich type II. The hypoechoic lesion and anechoic areas on EUS findings clearly corresponded with pancreatic acinus cells and duct dilation on histological findings, respectively. EUS findings are useful to diagnosis a duodenal aberrant pancreas that has ductal structures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aberrant pancreas is a congenital malformation found in areas such as the gastrointestinal tract, biliary duct, liver, spleen, or mediastinum [1]; however, the majority is located in the stomach, duodenum, or small intestine. The frequency of aberrant pancreas in the population is reportedly as high as 2 % [2], and it is present in approximately 1 in every 500 abdominal laparotomies [3]. This condition is usually discovered as a submucosal tumor (SMT) of the gastrointestinal (GI) tract by endoscopy; however, its differential diagnosis is difficult. Here, we describe a patient with a duodenal aberrant pancreas diagnosed by endoscopic ultrasonography (EUS) and pathological examination of an endoscopically resected specimen.
Case report
A 45-year-old Japanese man was referred to our hospital for gastric cancer screening. On esophagogastroduodenoscopy, an SMT was noted in the second portion of the duodenum; it was approximately 10 mm in diameter with a smooth surface and bridging fold (Fig. 1). The SMT appeared as two humps without a central umbilication. It was hard and negative for the “cushion” sign.
Based on these endoscopic findings, the differential diagnoses included benign and malignant tumors: aberrant pancreas, Brunner’s gland hyperplasia, neuroendocrine tumor (NET) G1 (carcinoid tumor), or gastrointestinal stromal tumor (GIST).
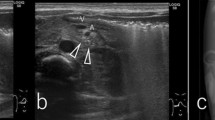
Endoscopic ultrasonography was performed with a 20-MHz probe inserted through an endoscope (GIF Q260, Olympus) after the duodenum was filled with water. EUS findings showed a heterogeneous and hypoechoic lesion with small anechoic spots mainly located in the third sonographic layer of the duodenum wall (Fig. 2). The tumor margin was unclear. From the EUS findings, aberrant pancreas was strongly suspected because the lesion was located in the third layer and small anechoic areas within the lesion were scattered, suggesting ductal structures. However, mucosal biopsy specimens obtained with standard forceps were not sufficient for definitive diagnosis.
The patient requested an exact histological diagnosis of the tumor. To histologically diagnose an SMT, the options include endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA), endoscopic submucosal dissection, or endoscopic submucosal–mucosal resection (ESMR) [4–6]. We obtained informed consent for ESMR because the lesion was only 10 mm in diameter.
To confirm the exact diagnosis, endoscopic resection was performed using an electrosurgical snare. No complications, such as hemorrhage or perforation, were encountered.
Histologically, pancreatic tissue was also situated in the duodenal submucosa and appeared as a broad-based, smooth nodule (Fig. 3). The nodule was composed of pancreatic ducts and acini. No islets of Langerhans were found in any sections. We compared the EUS findings with the histological findings (Fig. 4). The hypoechoic mass on EUS findings corresponded with pancreatic acinus cells on histological findings, and the anechoic lesion on EUS findings corresponded with duct dilation on histological findings. Therefore, the EUS findings clearly corresponded with the histological findings. Two years of patient follow-up have been uneventful.
Schematic drawing of the endoscopic ultrasonography (EUS) finding (left) and microscopy of the specimen (right). Yellow circles point to the hypoechoic mass on EUS findings and pancreatic acinus cells on histological findings. Green circles point to the anechoic lesion on the EUS picture and duct dilation on histological findings. The same colored circles point to the same structures
Discussion
Submucosal tumors of the GI tract are frequently found during endoscopic examination. However, a histological diagnosis of SMTs is difficult based on endoscopic forceps biopsy alone, because an SMT is covered with normal mucosa. However, EUS findings of SMTs may lead to a more definitive diagnosis, because they correlate with the histological findings. EUS findings alone have a sensitivity of 64 % and specificity of 80 % in the diagnosis of malignant subepithelial lesions [7]. Moreover, it has been previously reported that EUS and histological findings agree in 74.1 % of cases [6].
Of the SMTs in the duodenum, NET G1 and GIST have malignant potential, often requiring surgical therapy. Thus, differential diagnoses of SMTs are important.
In the case of NET G1, the internal echo is generally hypoechoic and homogeneous. The margins are clearly described, the contour is somewhat smooth, and the tumors are primarily located in the third layer [8]. On the other hand, GIST is also usually homogeneous, with a well-defined margin. GIST typically appears as hypoechoic mass lesions located in the fourth layer. EUS findings of GIST that are associated with malignancy include an irregular extraluminal border, presence of cystic spaces (which represent areas of necrosis), and echogenic foci within the tumor [9]. Meanwhile, GIST <30 mm in diameter with regular margins and a homogeneous echo pattern are usually benign [9]. In the present case, NET G1 and GIST were distinguishable by the origin, the margin of the tumor, and an internal echo on EUS.
There have been few reports of duodenal aberrant pancreas, compared with those for the stomach. Ran Wei et al. compared duodenal aberrant pancreas with gastric ones by computed tomography, and reported that the former tends to be small and round [10]. In the present case, the lesion was almost round and approximately 10 mm in diameter. Regarding endosonographic findings, Shim et al. [11] reported that the EUS findings of aberrant pancreas in the stomach were primarily heterogeneous (84.6 %), intermediate internal echoic (100 %), and with an unclear boundary (82.9 %). In gastric aberrant pancreas without central umbilication, 40 % of the lesions appear within the internal cystic area [9]. Moreover, Park et al. [12] reported that the characteristic EUS features of ectopic pancreas in the stomach are indistinct borders, lobulated margins, presence of anechoic duct-like structures, mural growth pattern, and localization within the second layer or deeper. Anechoic duct-like structures were observed in 65.4 % of gastric aberrant pancreas. Matsushita et al. [13] also reported that an anechoic area (duct dilatation) (80 %) was commonly visualized in gastric aberrant pancreas. The ducts in the aberrant pancreas on the EUS image were described as an anechoic, cystic area. Focal, anechoic duct-like structures in an aberrant pancreas are a characteristic finding of duct dilation [13]. Microscopically, aberrant pancreas has been classified into three types by von Heinrich [14]. Type I shows the presence of ducts, acini, and islets of Langerhans cells; Type II shows only a few acini and multiple ducts; and Type III has only ducts. Ducts exist in every type. Therefore, anechoic duct-like structures can be observed in aberrant pancreas not only in the stomach but also in the duodenum. Based on the comparison between the specimen and EUS image, we obtained the specimen and corresponding results from the duodenum, not the stomach.
In conclusion, the detection of anechoic, duct-like structures inside the SMT on EUS is helpful for the diagnosis of aberrant pancreas not only in the stomach but also in the duodenum.
References
Rosai JR. Pancreas and periampullary region. In: Rosai JR, editor. Ackerman’s surgical pathology. St Louis: CV Mosby; 1989. p. 757–88.
Lai EC, Tompkins RK. Heterotopic pancreas. Review of a 26 year experience. Am J Surg. 1986;151:697–700.
de Castro Barbosa JJ, Dockerty MB, Waugh JM. Pancreatic heterotopia; review of the literature and report of 41 authenticated surgical cases, of which 25 were clinically significant. Surg Gynecol Obstet. 1946;82:527–42.
Mekky MA, Yamao K, Sawaki A, et al. Diagnostic utility of EUS-guided FNA in patients with gastric submucosal tumors. Gastrointest Endosc. 2010;71:913–9.
Matsumoto S, Miyatani H, Yoshida Y. Endoscopic submucosal dissection for duodenal tumors: a single-center experience. Endoscopy. 2013;45:136–7.
Kojima T, Takahashi H, Parra-Blanco A, et al. Diagnosis of submucosal tumor of the upper GI tract by endoscopic resection. Gastrointest Endosc. 1999;50:516–22.
Hunt GC, Smith PP, Faigel DO. Yield of tissue sampling for submucosal lesions evaluated by EUS. Gastrointest Endosc. 2003;57:68–72.
Yoshikane H, Tsukamoto Y, Niwa Y, et al. Carcinoid tumors of the gastrointestinal tract: evaluation with endoscopic ultrasonography. Gastrointest Endosc. 1993;39:375–83.
Palazzo L, Landi B, Cellier C, et al. Endosonographic features predictive of benign and malignant gastrointestinal stromal cell tumours. Gut. 2000;46:88–92.
Wei R, Wang QB, Chen QH, et al. Upper gastrointestinal tract heterotopic pancreas: findings from CT and endoscopic imaging with histopathologic correlation. Clin Imaging. 2011;35:353–9.
Shim CS, Hong SJ, Cho YD, et al. Endoscopic ultrasonographic features of gastric aberrant pancreas without central dimpling. Ultrasound Med Biol. 1997;23:42.
Park SH, Kim GH, do Park Y, et al. Endosonographic findings of gastric ectopic pancreas: a single center experience. J Gastroenterol Hepatol. 2011;26:1441–6.
Matsushita M, Hajiro K, Okazaki K, et al. Gastric aberrant pancreas: EUS analysis in comparison with the histology. Gastrointest Endosc. 1999;49:493–7.
von Heinrich H. Ein Beitrag zur Histologie des sogen: akzessorischen Pankreas. Virchows Arch A Pathol Anat Histopathol. 1909;198:392–401.
Conflict of interest
None.
Ethical standard
All procedures were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Declaration of Helsinki of 1975, as revised in 2008 (5). Informed consent was obtained from the patient. Additional informed consent was obtained from the patient regarding the identifying information that is included in this article.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Watanabe, T., Aoyagi, K., Tomioka, Y. et al. Endoscopic ultrasonography of duodenal aberrant pancreas: comparison with histology after endoscopic resection. J Med Ultrasonics 42, 277–280 (2015). https://doi.org/10.1007/s10396-014-0592-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10396-014-0592-2