Abstract
Endoscopic ultrasound (EUS) is an important modality for the evaluation of patients with a suspicion of intraductal papillary mucinous neoplasms (IPMNs) of the pancreas. EUS imaging from the stomach and duodenum can demonstrate the entire pancreatic gland with a high spatial resolution. It can distinguish IPMN from other cystic lesions, detect malignant degeneration in IPMN (IPMC), and is invaluable to follow up these patients. From a clinical viewpoint, the key issue is whether an individual patient with IPMN should undergo surgery or can be managed conservatively. EUS helps in this decision by demonstrating the presence or absence of “high-risk stigmata of malignancy” or “worrisome features,” as per the revised IPMN/MCN Consensus Guidelines 2012. It is important to detect mural nodules (MNs), which correspond to macroscopic papillary growth pattern of these tumors, and measure their precise diameter as an indicator of the malignant potential of BD- or mixed-type IPMN. EUS can depict MNs as slightly hyperechoic papillary projections. The differentiation between MNs and mucin plugs can be challenging, and contrast-enhanced EUS imaging may be needed to demonstrate enhancement of the former.
There are two echo patterns of pancreatic ductal adenocarcinoma (PDAC) derived from IPMN on EUS: mixed-echo pattern which is a feature of mucinous carcinoma usually derived from intestinal type and solid-echo pattern which is a feature of tubular adenocarcinoma usually derived from gastric type of IPMN. The latter is similar to the common PDAC. Since recent studies have shown that patients with IPMN have high risk for development of PDAC, it is vital to carefully evaluate the entire pancreas during follow-up.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Endoscopic ultrasonography (EUS)
- Intraductal papillary mucinous carcinoma (IPMC)
- Intraductal papillary mucinous neoplasm (IPMN)
- IPMN/MCN consensus guidelines
- Pancreatic ductal adenocarcinoma (PDAC)
1 Introduction
Endoscopic ultrasonography (EUS) includes probes with two methods of imaging: radial instruments with 360° imaging perpendicular to the long axis; and convex instruments with imaging plane parallel to the long axis of the instrument. The former only allows diagnostic imaging, whereas the latter was developed for fine-needle aspiration (FNA) (Inui et al. 2004; Yamao et al. 2007). EUS operates at a high ultrasound frequencies, with imaging from the stomach or duodenum, providing high-resolution, real-time imaging of the pancreas. This modality therefore plays an important role in the evaluation of pancreatic diseases.
In this chapter, we describe the diagnosis of intraductal papillary mucinous neoplasm (IPMN) using EUS with a special emphasis on (1) differentiation from other cystic lesions, (2) detailed morphologic description of IPMN, and (3) EUS-based follow-up protocol.
2 Differential Diagnosis of IPMN from Other Cystic Lesions
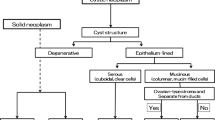
With advances in cross-sectional imaging techniques, IPMN and other pancreatic cysts are frequently detected by ultrasound (US), computed tomographic (CT) scanning, or magnetic resonance imaging (MRI). Although these imaging modalities are very sensitive for the detection of pancreatic cystic lesions, they are suboptimal for characterization of the cyst type. EUS remains an essential modality for differentiation of IPMN from other cystic lesions. There are many reports on EUS findings in cystic lesions of the pancreas (Sedlack et al. 2002; Song et al. 2003; Brugge 2000; Ahmad et al. 2003; Kim et al. 2010; Okabe et al. 2011; Sahani et al. 2013). Diagnosis based on EUS features requires close attention to the size and number of cysts, contour of the cystic lesion, morphology of the cyst wall, internal contents of the cysts, presence or absence of communication between the cyst and the pancreatic duct, as well as the coexistence of any other pancreatic pathology.
When the main pancreatic duct (MPD) is dilated along with the presence of multilocular cysts with typical “bunch of grapes” appearance, the diagnosis of IPMN is relatively easy. However, when mucinous secretions and hence ductal dilatation are minimal, IPMN can be difficult to differentiate from other cystic conditions, such as macrocystic serous cystic neoplasm (SCN) and retention cysts. In this situation, EUS depiction of communication between a cyst and the MPD is indicative of IPMN. Also use of sonographic contrast agents like Sonazoid® can help to distinguish debris in a retention cyst from mural nodules (MNs) in an IPMN cyst (Fig. 6.1).
The typical microcystic SCNs have a honeycomb-like aggregation of tiny cysts, and this appearance on EUS is a characteristic (Fig. 6.2). Differentiating branch duct IPMN (BD-IPMN) from mucinous cystic neoplasms (MCN) can be sometimes problematic. MCN are typically round to ovoid tumor, having a typical cyst-in-cyst pattern, with a common external thick wall (Fig. 6.3). Differentiating MCN from IPMN therefore relies on whether the cyst structure is directed inwards or outwards. Retention cysts and pseudocysts are formed when pancreatic duct is obstructed by a solid tumor such as PDAC. These cysts can be misdiagnosed as IPMNs, particularly when the obstructing solid component is small. Thus, meticulous EUS observation is essential to look for any solid lesion near the cysts.
Ahmad et al. (2003) reported that the EUS diagnosis was correct in 40–93 % cases, among eight endoscopists, depending on their experience in terms of number of cases that they had performed and also on their technical skills. Thus EUS is an operator-dependent examination, and there may be considerable variability in the ability to correctly differentiate between benign and malignant lesions (Sedlack et al. 2002; Hernandez et al. 2002; Canto et al. 2004; Brugge et al. 2004; Ahmad et al. 2003; Khalid and Brugge 2007).
For the differential diagnosis of pancreatic cystic lesions and the grading of tumors, cyst fluid cytology, and measurements of pancreatic enzymes (amylase, lipase) and tumor markers like carcinoembryonic antigen (CEA), carbohydrate antigen (CA19-9, CA125, etc.) in the cyst fluid is widely used (Brugge et al. 2004). One of the noticeable differences in the diagnostic approach to pancreatic cystic neoplasms between Japan and other countries is the use of pancreatic cyst aspiration. Because of the presence of a case report of post-EUS-FNA tumor seeding (Hirooka et al. 2003), current Japanese consensus is that aspiration of pancreatic cystic lesions should be avoided when an MCN is suspected (Yamao et al. 2009).
3 Detailed Morphological Examination of IPMN
3.1 Imaging BD-Type and Mixed-Type IPMN (Figs. 6.4 and 6.5)
One of the key features of IPMN is dilatation of a branch duct (BD) or main duct (MD) due to proliferative papillary tumors themselves or large amounts of secreted intraductal mucin. Accordingly, the size of IPMN depends on the diameter of the dilated BD, MD, and MNs. An accurate measurement of the dilated BD and the MD diameters is important for defining BD- and mixed-type IPMN. The diameter of dilated ducts can be measured by either MDCT or MRCP, but only EUS is sufficiently accurate for measuring the size of MNs. Presence of MNs is considered to be the most reliable indicator of whether an IPMN tumor is benign or malignant, and this issue has been the subject of numerous studies. However, a cutoff diameter for differentiating benign from malignant nodules has been controversial and ranges between 3 and 10 mm.
The revised international guidelines 2012 (Tanaka et al. 2012) recommend that cysts with worrisome features should undergo a detailed evaluation by EUS. Surgery is indicated if EUS reveals obvious MNs (Fig. 6.4a), or main duct lesions (Fig. 6.4b), and when cyst fluid cytology reveals malignancy. These guidelines define high-risk stigmata in BD- and mixed-type IPMN as the presence of obstructive jaundice, an enhancing solid component (Fig. 6.5e), and a main duct diameter ≥10 mm. A notable change from the previous guidelines is that side-branch dilatation of ≥3 cm in BD-IPMN, which was an indication for surgery earlier (Tanaka et al. 2006), is now considered a worrisome feature. These lesions should be carefully assessed for the presence of MNs by EUS. In all of these situations, EUS is a key modality for risk stratification and classification of IPMN lesions.
MNs appear as hyperechoic wall-based structures on EUS, because the papillary structures comprising the MN scatter the ultrasound waves (Fig. 6.6). It is important to distinguish MNs from protein plaques, viscous mucin, or debris. Protein plaques can be differentiated by their characteristic annular hyperechoic appearance with a low echoic central part (Fig. 6.7), whereas discriminating mucin from MNs is difficult by B-mode imaging. Caution is needed in this regard, because misdiagnosis of mucin as a nodule will lead to an overdiagnosis of malignancy. The use of ultrasound contrast agents, such as Sonazoid®, can rule out MNs by the absence of blood flow signals in the intra-cystic structure (Fig. 6.8), thus increasing the diagnostic precision of EUS (Ohno et al. 2009).
3.2 Imaging of MD-Type IPMN
Main duct IPMN (MD-IPMN) is defined by segmental or diffuse MD dilatation to ≥6 mm, without branch duct dilatation >5 mm (Tanaka et al. 2006). Furthermore, an MD diameter ≥10 mm is considered as high-risk stigmata, as per the international consensus guidelines (Tanaka et al. 2012), and resection is recommended in such cases. It is important to observe the entire pancreatic duct till the ampulla of Vater, to rule out upstream ductal dilatation due to chronic pancreatitis or obstruction by a PDAC.
Large papillary projections in a dilated MD can be evaluated using CT or MRCP, but EUS may be the most suitable investigation for smaller nodules (Fig. 6.9). MD-IPMN has a tendency for superficial intraductal extension. Hence, an accurate preoperative assessment of the longitudinal extent of the disease is important to decide the magnitude of pancreatic resection, such as total pancreatectomy or partial pancreatectomy. Intraductal ultrasound (IDUS) and peroral pancreatoscopy (POPS) are other useful modalities for determining the extent of intraductal superficial lesions (Fig. 6.10).
3.3 Imaging of PDAC Derived from IPMN
The Japan Pancreas Society (JPS) formed a committee to resolve the clinical and pathological issues associated with PDAC derived from IPMN and PDAC concomitant with IPMN. This committee proposed new definitions of three categories based on the topological relationship of two conditions and presence or absence of a histological transition between these conditions (Yamaguchi et al. 2011):
-
(a)
PDAC derived from IPMN (PDAC is clearly derived from IPMN.)
-
(b)
PDAC concomitant with IPMN (PDAC is obviously different from the IPMN lesions.)
-
(c)
PDAC of undetermined relationship with IPMN (whether PDAC was derived from IPMN or whether PDAC was concomitant with IPMN could not be determined, because there was no histological transition between the two diseases).
With regard to the histological subtypes, approximately one-third of PDAC derived from IPMN (41/122) were mucinous carcinomas, while most of PDAC concomitant with IPMN (28/31) were tubular adenocarcinomas, similar to the usual PDAC. Accordingly mucinous carcinoma was more frequently seen as the histological subtype when the PDAC was derived from IPMN, than when PDAC occurred either alone or concomitantly with IPMN (Yamaguchi et al. 2011). During EUS evaluation of PDAC derived from IPMN, two echo patterns can be observed: Mucinous carcinoma derived from intestinal type usually shows a mixed-echo pattern (Fig. 6.11). On the other hand, tubular adenocarcinoma, which is similar to common PDAC and is usually derived from gastric type, shows a solid-echo pattern (Kobayashi et al. 2005) (Fig. 6.12).
4 Protocol for Follow-Up of Patients with IPMN
When both high-risk stigmata and worrisome features are absent, no MNs are detected by EUS examination, lesions localized in the BD, and pancreatic juice cytology findings are negative, the revised international guidelines specify the follow-up protocol depending on the cyst size (1–2 cm or 2–3 cm). The recommended imaging modalities for follow-up of these patients are CT/MRI and EUS (Tanaka et al. 2012).
A large natural history study of BD-IPMN from Japan (Maguchi et al. 2011), based on a nationwide survey, found that disease progression rate was 18 %, whereas stable disease was seen in 82 % of 349 patients without MNs at the initial diagnosis, over a mean observation period of 3.7 years. The rate of IPMC occurring in these patients was 2.5 % (Fig. 6.13).
Recently high rates of PDAC concomitant with IPMN have been reported (2.0–9.3 %). Hence, patients with IPMN should be regarded as a high-risk group for developing PDAC (Fig. 6.14).
These observations highlight the importance of not only evaluating the IPMN lesions but also carefully observing the entire pancreas during the follow-up EUS studies, so as not to miss PDAC. Regular EUS evaluations can allow early detection of PDAC in such cases.
References
Ahmad NA, Kochman ML, Brensinger C, et al. Interobserver agreement among endosonographers for the diagnosis of neoplastic versus non-neoplastic pancreatic cystic lesions. Gastrointest Endosc. 2003;58:59–64.
Brugge WR. The role of EUS in the diagnosis of cystic lesions of the pancreas. Gastrointest Endosc. 2000;52:18.
Brugge WR, Lewandrowski K, Lee-Lewandrowski E, et al. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330–6.
Canto MI, Goggins M, Yeo CJ, et al. Screening for pancreatic neoplasia in high-risk individuals: an EUS-based approach. Clin Gastroenterol Hepatol. 2004;2:606–21.
Hernandez LV, Mishra G, Forsmark C, et al. Role of endoscopic ultrasound (EUS) and EUS-guided fine needle aspiration in the diagnosis and treatment of cystic lesions of the pancreas. Pancreas. 2002;25:222–8.
Hirooka Y, Goto H, Itoh A, et al. Case of intraductal papillary mucinous tumor in which endosonography‐guided fine‐needle aspiration biopsy caused dissemination. J Gastroenterol Hepatol. 2003;18:1323–4.
Inui K, Kida M, Fujita N, et al. Standard imaging techniques in the pancreatobiliary region using radial scanning endoscopic ultrasonography. Dig Endosc. 2004;16:S118–33.
Khalid A, Brugge W. ACG practice guidelines for the diagnosis and management of neoplastic pancreatic cysts. Am J Gastroenterol. 2007;102:2339–49.
Kim YC, Choi JY, Chung YE, et al. Comparison of MRI and endoscopic ultrasound in the characterization of pancreatic cystic lesions. AJR Am J Roentgenol. 2010;195:947–52.
Kobayashi G, Fujita N, Noda Y, et al. Mode of progression of intraductal papillary-mucinous tumor of the pancreas: analysis of patients with follow-up by EUS. J Gastroenterol. 2005;40:744–51.
Maguchi H, Tanno S, Mizuno N, et al. Natural history of branch duct intraductal papillary mucinous neoplasms of the pancreas: a multicenter study in Japan. Pancreas. 2011;40:364.
Ohno E, Hirooka Y, Itoh A, et al. Intraductal papillary mucinous neoplasms of the pancreas: differentiation of malignant and benign tumors by endoscopic ultrasound findings of mural nodules. Ann Surg. 2009;249:628–34.
Okabe Y, Kaji R, Ishida Y, et al. The management of the pancreatic cystic neoplasm: the role of the EUS in Japan. Dig Endosc. 2011;23:39–42.
Sahani DV, Kambadakone A, Macari M, et al. Diagnosis and management of cystic pancreatic lesions. AJR Am J Roentgenol. 2013;200:343–54.
Sedlack R, Affi A, Vazquez-Sequeiros E, et al. Utility of EUS in the evaluation of cystic pancreatic lesions. Gastrointest Endosc. 2002;56:543–7.
Song MH, Lee SK, Kim MH, et al. EUS in the evaluation of pancreatic cystic lesions. Gastrointest Endosc. 2003;57:891–6.
Tanaka M, Chari S, Adsay V, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17–32.
Tanaka M, Castillo CF, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183–97.
Yamaguchi K, Kanemitsu S, Hatori T, et al. Pancreatic ductal adenocarcinoma derived from IPMN and pancreatic ductal adenocarcinoma concomitant with IPMN. Pancreas. 2011;40:571–80.
Yamao K, Irisawa A, Inoue H, et al. Standard imaging techniques of endoscopic ultrasound‐guided fine‐needle aspiration using a curved linear array echoendoscope. Dig Endosc. 2007;19:S180–205.
Yamao K, Mizuno N, Takagi T, et al. How I do it and when I use (and do not use) EUS-FNA. Gastrointest Endosc. 2009;69.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Japan
About this chapter
Cite this chapter
Hijioka, S., Bhatia, V., Yamao, K. (2014). Endosonography. In: Tanaka, M. (eds) Intraductal Papillary Mucinous Neoplasm of the Pancreas. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54472-2_6
Download citation
DOI: https://doi.org/10.1007/978-4-431-54472-2_6
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-54471-5
Online ISBN: 978-4-431-54472-2
eBook Packages: MedicineMedicine (R0)