Abstract
Background
The rarity of esophageal achalasia has resulted in little being known about the characteristics of its three subtypes. The upper esophageal sphincter is considered one key factor to prevent aspiration pneumonia, a serious complication of esophageal achalasia. This study aimed to reveal the subtype characteristics of esophageal achalasia and how the upper esophageal sphincter functions and relates to other clinical parameters of the disease.
Methods
We retrospectively investigated the clinical records of patients diagnosed with esophageal achalasia. All participants underwent esophagogastroduodenoscopy and then, within 2 weeks, high-resolution manometry. Gastrointestinal symptoms were assessed using a previously validated self-reported questionnaire.
Results
A total of 110 patients with esophageal achalasia were enrolled: 50 with type I, 40 with type II, and 20 with type III. Mean age at diagnosis was 54.5, 50.4, and 66.1 years for types I, II, and III, respectively. Mean resting upper esophageal sphincter pressure was 28.0, 51.8, and 43.6 mmHg for patients with types I, II, and III, respectively (p < 0.01). Patients with type III esophageal achalasia more frequently reported stomachache than those with type I (p = 0.03). A negative correlation between resting upper esophageal sphincter pressure and age was observed in all subtypes.
Conclusions
A negative correlation was confirmed between resting upper esophageal sphincter pressure and age in all subtypes of esophageal achalasia. Type III patients were older at diagnosis, type II patients showed higher upper esophageal sphincter pressure, and type I patients showed a lower upper esophageal sphincter pressure at the early life stage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Esophageal achalasia is a rare refractory functional disorder presenting with aperistalsis in the esophageal body and impaired relaxation of the lower esophageal sphincter (LES) [1]. This disease can cause a wide variety of symptoms, including dysphagia, chest pain, regurgitation, and aspiration [2]. The assumed etiology is degeneration of neurons in the myenteric plexus ganglion leading to motor dysfunction, but its pathophysiology is currently not fully understood.
Recently, it has become possible to classify patients with esophageal achalasia into three subtypes (I, II, or III) according to the Chicago Classification of esophageal motility disorders, v3.0 (Chicago Classification) [3]. Classification is achieved using the findings of high-resolution manometry (HRM). Several studies have revealed characteristics of each subtype: symptom severity [2, 4, 5], degree of weight loss [6], HRM findings [7], histologic pattern [8], immunological status on a molecular basis detected by serum cytokines [2], and treatment response to peroral endoscopic myotomy [9] have been reported to be different among each subtype.
A theory has been proposed that each esophageal achalasia subtype is a consecutive status and constellation of the same disease, from type III to type II, and finally to type I [10]. However, as insufficient data have been accumulated regarding this disease—especially for type III esophageal achalasia—full understanding of the characteristics of each subtype is not possible. Furthermore, most reports are from Western countries [5, 6, 11, 12], with little data available on differences in esophageal achalasia subtypes in Asian populations [7, 13, 14].
One of the most serious complications of esophageal achalasia is aspiration pneumonia. The upper esophageal sphincter (UES) is a crucial barrier between the esophagus and pharynx, preventing aspiration. Dysfunction of this sphincter is associated with laryngeal symptoms [15], and UES dysfunction was proved to be associated with more frequent regurgitation and an elevated risk for aspiration [16, 17]. Although the UES consists of skeletal muscle and abnormality of the UES is not considered a criterion in the Chicago Classification [3], it has been proven that patients with esophageal achalasia also show abnormal function of the UES [18]. Currently, little is known about the factors connecting esophageal achalasia with UES functionality. Generally, older patients demonstrate lower UES pressures, which is related to a higher risk of aspiration pneumonia [19]. However, the influence of age on UES function in patients with esophageal achalasia remains unclear. Additionally, only a few reports have been published regarding the relationship between esophageal achalasia subtypes and UES pressure, and no study has reported on the disease activity in Asian countries.
This study aimed to elucidate the function of the UES and the clinical characteristics of each subtype of esophageal achalasia as well as investigate the relationship between UES pressure and other variables (including clinical backgrounds and symptoms) in the Japanese population.
Methods
Participants
This was a retrospective observational study conducted at the Kawasaki Medical School General Medical Center and Kawasaki Hospital in Japan. Throughout this study, we followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [20]. This study was reviewed and approved by the Research Ethics Committee of Kawasaki Medical School and Hospital (IRB No. 2010, 2061 and 3912).
We initially enrolled consecutive patients diagnosed with esophageal achalasia from March 2010 to April 2021. Patients were diagnosed by at least three members of the Japan Esophageal Society (RK, MA, and NM), two of whom are board-certified gastroenterologists of The Japanese Society of Gastroenterology (MA and NM). Patients were diagnosed using the Chicago Classification [3] and clinical findings, such as symptoms, endoscopic images, and upper gastrointestinal series, were investigated. Clinical data for age, sex, body mass index (BMI), alcohol intake (classified into two groups: none; drinker, > 12.6 g/day of ethanol), and smoking history (classified into three groups: never, ex-smoker, and current smoker) were gathered from medical records held in an electronic database.
All patients underwent an esophagogastroduodenoscopy after fasting for at least 8 h, and subsequently underwent an HRM examination to rule out organic disorders, including esophageal cancer, and we denied the presence of esophageal residue. The interval between the esophagogastroduodenoscopy and HRM examinations was 2 weeks at most. Endoscopies used in this study were conducted using either a GIF-260 series, GIF-290 series, GIF-1200 N (Olympus Medical Systems, Tokyo, Japan), or EG-L580NW (FUJIFILM Co., Tokyo, Japan).
The following were the exclusion criteria: (1) patients who underwent therapeutic procedures, such as endoscopic pneumatic dilation, peroral endoscopic myotomy, and surgical myotomy; and (2) patients who had a history of abdominal surgery.
HRM
We performed HRM with a Sandhill Scientific INSIGHT G3 with HRiM2 Prove (Diversatek Healthcare, Milwaukee, USA). The manometric probe adopted for use in this study was an HRiM2 high-resolution impedance manometry catheter with 32 circumferential pressure/16 impedance channels (model number UNI-ESO-WG1A1).
Manometric studies were performed with patients in a sitting position after they had fasted for at least 8 h. Patients taking a calcium blocker withdrew from using the drug at least 2 weeks prior to undergoing the examination. The calculated parameters in this study were the UES length, 4 s-integrated relaxation pressure (IRP), resting UES pressure, UES residual pressure (nadir UES pressure during relaxation), UES relaxation time, and distal contractile integral (DCI) as a parameter to esophageal body pressurization [11]. Resting UES pressure was defined as the average pressure of the UES between deglutition with resting breathing and without swallowing activity where we could observe stable UES pressure [21]. We assessed the pressure three times in each patient, and the average pressure was adopted as the resting UES pressure. UES relaxation time was evaluated using the tracing mode at the UES level based on a previous study [22]. If the evaluation of the UES was difficult owing to the short length of the esophagus, the catheter was pulled to assess only UES parameters by sensors located 1-cm apart before the end of manometric evaluations.
Symptom assessment
All patients answered a previously validated self-reported questionnaire regarding gastrointestinal symptoms [23] before any other examinations were conducted. We specifically analyzed those questions related to patients with esophageal achalasia and dysphagia [24]. Each symptom was rated on a Likert scale from 0 to 6 in accordance with the previously mentioned trial: 0 = absent; 1 = very rare; 2 = rare; 3 = a few; 4 = sometimes; 5 = often; 6 = always [24].
Endpoints and sample size
The primary endpoint was the difference in UES function among the three subtypes of esophageal achalasia, including resting UES pressure, and secondary endpoints were: differences in clinical parameters, such as age, sex, BMI, alcohol intake, and smoking history; manometric findings, including IRP; and correlation between resting UES pressure and other variables, such as age, BMI, and gastrointestinal symptoms. Based on previous studies, the sample size was set to a total of at least 100 patients, with at least 40 patients with types I and II, and 20 patients with type III esophageal achalasia to provide adequate statistical power [4, 5, 7, 11,12,13,14]
Statistical analysis
Continuous and normally distributed variables, such as age and BMI, were expressed as means and standard deviations (SDs). Non-normally distributed continuous variables, such as HRM findings and categorical data, were expressed as medians and interquartile ranges. Frequencies were described as percentages. The Spearman test was used to demonstrate correlations between UES pressure and other variables, such as age and BMI. We used analysis of variance (ANOVA) (normally distributed continuous variables), Chi-square test (frequencies), and Kruskal–Wallis test (non-normally distributed continuous variables and categorical data) to compare the variables among the three subtypes. Statistical significance was set at p < 0.05. All statistical analyses and visualizations were performed using SPSS software version 27.0 (IBM Corp., Armonk, NY, USA).
Results
Clinical features and HRM findings
In total, 503 patients underwent HRM during the study period, 178 of whom were diagnosed with esophageal achalasia. After excluding 68 patients according to our criteria, we eventually enrolled 110 patients with esophageal achalasia at initial diagnosis in our hospital: 50 with type I, 40 with type II, and 20 with type III esophageal achalasia. Table 1 shows the patients’ characteristics, including clinical features and HRM findings.
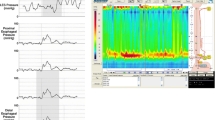
Patients with type III achalasia had a significantly higher mean age at initial diagnosis than those with types I and II (66.1 years compared with 54.5 and 50.4, respectively, p < 0.01), although the BMI and ratios for male/female, alcohol drinker/non-drinker, and smoker/non-smoker were similar among the subtypes. Resting UES pressure was highest in patients with type II esophageal achalasia and lowest in patients with type I (51.8 mmHg and 28.0 mmHg, respectively, p < 0.01); patients with types II and III had a higher IRP than those with type I (Fig. 1).
Gastrointestinal symptoms
A comparison of the gastrointestinal symptom frequencies is shown in Table 2. The frequency of experience of stomachache was significantly different between patients with types I and III, with type III patients complaining of having stomachache more often (0 vs. 1.0 vs. 2.0, p = 0.03). Symptoms related to dysphagia, such as a stuck feeling during swallowing, were not significantly different among the subtypes.
Correlation between HRM and other variables
Resting UES pressure was negatively correlated with age at diagnosis in all patients and for each subtype (Fig. 2). This pressure measured below 40 mmHg—the average UES pressure of patients with aspiration pneumonia reported in a previous study [25]—at approximately 40, 70, and 60 years of age, for patients with types I, II, and III achalasia, respectively. No significant differences in the rate of decline in UES pressure among the three groups were noted. Upon comparing the percentage of patients with UES pressure < 40 mmHg, the percentage was significantly higher in patients with type I. There were 36 (72%), 8 (20%), and 7 (35%) cases in types I, II, and III, respectively (Table 1). No correlation was found between UES pressure and BMI across all patients and subtypes.
Correlations between resting upper esophageal sphincter pressure and age at diagnosis in total esophageal achalasia patients and each of the three subtypes: a in all esophageal achalasia patients (n = 110); b in type I esophageal achalasia patients (n = 50); c in type II esophageal achalasia patients (n = 40); d in type III esophageal achalasia patients (n = 20)
Discussion
In this study, we confirmed differences in UES function among the three subtypes of esophageal achalasia. A higher resting UES pressure was found among Japanese patients with type II esophageal achalasia, which is consistent with the previous reports based on other population groups. Furthermore, to the best of our knowledge, this is the first study to reveal a negative correlation between UES pressure and age in patients with esophageal achalasia, and the different potential risk for aspiration pneumonia according to subtypes. This subtype-specific correlation may contribute to individualized treatment management that achieves optimal patient outcomes. Moreover, we confirmed that type III esophageal achalasia patients are older at diagnosis and experience severe symptoms.
In terms of the etiology of this disease, there is a prevailing hypothesis that the subtypes defined in the Chicago Classification represent different stages of the same disorder, and that type III achalasia is the first phase in the development of the disease, progressing to type II and then I [10]. Our data showed that elderly subjects with type III were dominant compared with other subtypes, contradicting this theory. Our findings are consistent with the previous studies conducted in European [12] and Asian countries [7, 14]. The reason for this distribution is unknown: perhaps, age-related ganglion loss that occurs in the myenteric plexus of the esophagus [19] is related to the onset of type III esophageal achalasia only.
There is a wide range of differing gastrointestinal symptoms reported among esophageal achalasia subtypes. In this study, type III patients reported experiencing stomachache more frequently than the other subtypes. Some studies have claimed that type III patients reported chest pain more frequently [2, 4]. Even though the symptom we detected was stomachache, which is less common in achalasia, past research proved that patients with achalasia experienced epigastric pain [26]. Based on the fact that higher pressure in the LES was found to be correlated with esophageal achalasia symptoms [27], our result that type III showed the symptom more frequently was generally reasonable. However, other investigations reported that symptom frequency was not different among subtypes [7, 12], and HRM findings were not related to gastrointestinal symptoms in esophageal achalasia patients [28]. Self-reported gastrointestinal symptoms in esophageal achalasia can be modified by various factors, including hypersensitivity and central factors [29]. Further accumulation of clinical data is required to better understand the clinical features of each subtype.
In addition to clinical parameters, HRM findings differed according to the subtypes. In our study, higher IRPs were confirmed in patients with types II and III achalasia, which is consistent with the previous studies in Asian populations [7]. This seems to reflect the influences of pan-esophageal pressurization and spastic waves in types II and III, respectively [3]. On the contrary, some reports from Western countries described indifferent IRPs and pressures of the LES [11, 12]. Therefore, ethnic differences may exist in this area. Notably, in this study, resting UES pressure was highest in patients with type II esophageal achalasia, which is comparable to results reported from the United States [11]. The underlying mechanism of this tendency is that pan-esophageal pressurization and higher pressure in the esophageal body might elevate UES pressure [11]. However, we could not demonstrate a correlation between resting UES pressure and DCI, as a substitutive parameter of esophageal body pressurization. Additionally, the residual UES pressure was similar among the three subtypes in our data, which is inconsistent with the past study [11]. Data accumulation and additional investigations are needed to identify the factors associated with UES function in esophageal achalasia.
Of note, we detected a correlation between resting UES pressure and age in patients with esophageal achalasia. UES pressure is negatively correlated with age in the general population [30], and this dysfunction is believed to be a critical factor that causes aspiration pneumonia in senior citizens [19] as well as the impaired reflex relaxation and opening of UES [16, 17]. Thus, to prevent aspiration pneumonia from developing in esophageal achalasia patients, treatment should be initiated at an early stage. Interestingly, although significant negative correlations were confirmed in all subtypes, the degree of decline was different. Patti et al. showed that subjects with pulmonary aspiration showed lower mean UES pressure (44 ± 23 mmHg) than those without (74 ± 38 mmHg) [25]. In our study, the median UES pressure for type I esophageal achalasia patients was below this value. A previous histopathological study indicated that the most severe ganglion loss was found in type I among the three subtypes, which might be associated with lower UES pressure [8]. Moreover, type I patients presented with the critical UES pressure at an earlier age than types II and III patients: type I patients were approximately 40 years old when their UES pressure fell below the value, compared with 70 and 60 years old in types II and III, respectively. Based on these findings, we hypothesized a higher risk of aspiration pneumonia in type I patients even at the early life stage and proposed that early initiation of treatment for esophageal achalasia to prevent aspiration pneumonia could be more beneficial for type I patients, although further large-scale studies will be necessary to confirm this.
This study had several limitations. First, we retrospectively collected data of patients who had symptoms, such as dysphagia, which could have led to information bias: the history of prescribed medication, which may have affected the HRM findings and gastrointestinal symptoms, could not be analyzed. In addition, we could not collect enough number of healthy subjects and patients with non-major esophageal motility disorders. Second, since our study was conducted at a single institution and we enrolled a large number of referral patients, selection bias should be considered. Nevertheless, this study aimed to compare the characteristics among the achalasia subtypes; moreover, given the rarity of the disease, it is feasible to publish these data as an initial step toward understanding esophageal achalasia [2]. To compensate for these two biases, an international multicenter prospective large survey is needed in the future, and further dynamic evaluation of UES function, including the assessment of UES response to deglutition and esophago-UES contractile reflex during reflux events, can lead to a better understanding of the UES function. Third, the questionnaire we adopted did not specifically evaluate achalasia symptoms; several crucial symptoms, such as chest pain, were not assessed. Fourth, we did not evaluate the clinical course, including disease duration and treatment response. Past studies have reported weight loss during the observation period [6] and outcome of therapy [9] according to subtypes. Owing to feasibility concerns, only the data collected at the initial diagnosis were used in this study. Fifth, the method used in this study was not completely equivalent to previous studies. For instance, unlike our method, 3D catheter [21], water perfused method, and pull-through technique [25] were used in the previous studies. The difference made it harder to directly compare the results; however, we could discuss the tendency in each research, not the actual value itself. Furthermore, we did not measure UES pressure using e-sleeve function, which could lead to inadequate evaluation due to alteration of UES location by deglutition. Finally, pathological evaluation was not performed in all participants. Endoscopists performed biopsy examinations based on clinical judgment, mainly to exclude esophageal cancer and eosinophilic esophagitis. Subtype classification was performed according to the Chicago Classification in this study, which is recognized as the gold standard for esophageal achalasia subtyping. Since a previous study showed differences in the pathology of each esophageal achalasia subtype [8], pathological examination in all participants would have provided further insights into the pathophysiology of the disease and helped to comprehend the factors causing different UES functions among subtypes.
In conclusion, we elucidated the features of each of the three esophageal achalasia subtypes. These include older age at diagnosis and greater frequency of symptoms in type III patients and higher UES pressure in type II. Although resting UES pressure was negatively correlated with age in esophageal achalasia patients, type I patients reached a risk level for aspiration pneumonia at a younger age than other subtypes.
Our findings may contribute to a deeper understanding of the pathogenesis and personalized therapeutic strategies for esophageal achalasia.
References
Pandolfino JE, Gawron AJ. Achalasia: a systematic review. JAMA. 2015;313:1841–52.
Patel DA, Lappas BM, Vaezi MF. An overview of achalasia and its subtypes. Gastroenterol Hepatol (NY). 2017;13:411–21.
Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil. 2015;27:160–74.
Khan MQ, AlQaraawi A, Al-Sohaibani F, et al. Clinical, endoscopic, and radiologic features of three subtypes of achalasia, classified using high-resolution manometry. Saudi J Gastroenterol. 2015;21:152–7.
Surdea-Blaga T, David L, Pop A, et al. Clinical and manometric characteristics of patients with achalasia: one disease with three presentations or three diseases with one presentation? J Gastrointestin Liver Dis. 2020;29:501–8.
Patel DA, Naik R, Slaughter JC, et al. Weight loss in achalasia is determined by its phenotype. Dis Esophagus. 2018;31:1–8.
Lee JY, Kim N, Kim SE, et al. Clinical characteristics and treatment outcomes of 3 subtypes of achalasia according to the Chicago classification in a tertiary institute in Korea. J Neurogastroenterol Motil. 2013;19:485–94.
Sodikoff JB, Lo AA, Shetuni BB, et al. Histopathologic patterns among achalasia subtypes. Neurogastroenterol Motil. 2016;28:139–45.
Andolfi C, Fisichella PM. Meta-analysis of clinical outcome after treatment for achalasia based on manometric subtypes. Br J Surg. 2019;106:332–41.
Salvador R, Voltarel G, Savarino E, et al. The natural history of achalasia: evidence of a continuum—“The evolutive pattern theory.” Dig Liver Dis. 2018;50:342–7.
Blais P, Patel A, Sayuk GS, et al. Upper esophageal sphincter (UES) metrics on high-resolution manometry (HRM) differentiate achalasia subtypes. Neurogastroenterol Motil. 2017;29: e131136.
Roman S, Zerbib F, Quenehervé L, et al. The Chicago classification for achalasia in a French multicentric cohort. Dig Liver Dis. 2012;44:976–80.
Min M, Peng LH, Yang YS, et al. Characteristics of achalasia subtypes in untreated Chinese patients: a high-resolution manometry study. J Dig Dis. 2012;13:504–9.
Yamashita H, Ashida K, Fukuchi T, et al. Predictive factors associated with the success of pneumatic dilatation in Japanese patients with primary achalasia: a study using high-resolution manometry. Digestion. 2013;87:23–8.
Yadlapati R, Craft J, Adkins CJ, et al. The upper esophageal sphincter assist device is associated with symptom response in reflux-associated laryngeal symptoms. Clin Gastroenterol Hepatol. 2018;16:1670–2.
Cook IJ. Clinical disorders of the upper esophageal sphincter. GI Motility Online. 2006. https://doi.org/10.1038/gimo37.
Babaei A, Venu M, Naini SR, et al. Impaired upper esophageal sphincter reflexes in patients with supraesophageal reflux disease. Gastroenterology. 2015;149:1381–91.
Anefalos A, Herbella FAM, Patti MG. Upper esophageal sphincter motility and thoracic pressure are determinants of pressurized waves in achalasia subtypes according to the Chicago Classification. World J Surg. 2020;44:1932–8.
Cock C, Omari T. Systematic review of pharyngeal and esophageal manometry in healthy or dysphagic older persons (>60 years). Geriatrics (Basel). 2018;3:67.
von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–7.
Chitose SI, Shin Y, Sato K, et al. Three-dimensional imaging of upper esophageal sphincter resting pressure. Laryngoscope Investig Otolaryngol. 2019;4(6):645–52.
Manabe N, Haruma K, Nakato R, et al. New ultrasonographic screening method for oropharyngeal dysphagia: tissue Doppler imaging. Am J Physiol Gastrointest Liver Physiol. 2018;314:G32–8.
Manabe N, Haruma K, Hata J, et al. Clinical characteristics of Japanese dyspeptic patients: Is the Rome III classification applicable? Scand J Gastroenterol. 2010;45:567–72.
Tsukamoto M, Manabe N, Kamada T, et al. Number of gastrointestinal symptoms is a useful means of identifying patients with cancer for dysphagia. Dysphagia. 2016;31:547–54.
Patti MG, Debas HT, Pellegrini CA. Esophageal manometry and 24-hour pH monitoring in the diagnosis of pulmonary aspiration secondary to gastroesophageal reflux. Am J Surg. 1992;163:401–6.
Ahmed WU, Qureshi H, Maher M, et al. Achalasia in a gastroenterology unit of Karachi. J Pak Med Assoc. 2008;58:661–4.
Yaghoobi M, Mikaeli J, Montazeri G, et al. Correlation between clinical severity score and the lower esophageal sphincter relaxation pressure in idiopathic achalasia. Am J Gastroenterol. 2003;98:278–83.
Xiao Y, Kahrilas PJ, Nicodème F, et al. Lack of correlation between HRM metrics and symptoms during the manometric protocol. Am J Gastroenterol. 2014;109:521–6.
Ponds FA, Oors JM, Smout AJPM, et al. Reflux symptoms and oesophageal acidification in treated achalasia patients are often not reflux related. Gut. 2021;70:30–9.
Shaker R, Ren J, Podvrsan B, et al. Effect of aging and bolus variables on pharyngeal and upper esophageal sphincter motor function. Am J Physiol. 1993;264:427–32.
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Funding
None.
Author information
Authors and Affiliations
Contributions
Conceptualization: NM and KH; data curation: RK, NM, and MA; formal analysis: RK and NM; investigation: RK, NM, and KH; methodology: RK, NM, MF, MA, YS, HS, KH, and KH; project administration: NM; resources: RK, NM, MF, MA, YS, HS, KH, HK, and KH; software: RK, NM, and HK; supervision: NM and KH; validation: NM; visualization: RK and NM; writing—original draft: RK; writing—review and editing: NM; approval of the final manuscript: all authors.
Corresponding author
Ethics declarations
Ethics approval
This study was reviewed and approved by the Research Ethics Committee of Kawasaki Medical School and Hospital (IRB numbers 2010, 2061, and 3912).
Conflict of interest
The authors have no conflicts of interest to declare.
Human rights statement and informed consent
All procedures were in accordance with the ethical standards of the responsible committee on institutional human experimentation and with the Helsinki Declaration of 1964 and later versions. Informed consent or substitute for it was obtained from all patients for being included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Katsumata, R., Manabe, N., Ayaki, M. et al. Differences in upper esophageal sphincter function and clinical characteristics among the three subtypes of Japanese patients with esophageal achalasia. Esophagus 19, 316–323 (2022). https://doi.org/10.1007/s10388-021-00897-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10388-021-00897-z