Abstract
Background
Phenotypes of achalasia are based on esophageal body pressurization during swallow. The reasons that lead to pressurized waves are still unclear. This study aims to evaluate manometric parameters that may determine pressurized waves in patients with achalasia.
Methods
A total of 100 achalasia high-resolution manometry tests were reviewed. We measured before each swallow: upper esophageal sphincter (UES) basal pressure, esophageal length, lower esophageal sphincter (LES) basal pressure, LES length, gastric and thoracic pressure, transdiaphragmatic pressure gradient and the LES retention pressure (LES basal pressure-TPG); during swallow: UES pressure, UES residual pressure, UES recovery time, LES relaxation pressure, gastric and thoracic pressure, transdiaphragmatic pressure gradient and after swallow: esophageal length, LES length, wave pressure, gastric and thoracic pressure and transdiaphragmatic gradient pressure.
Results
Univariate analysis showed in pressurized waves before swallow: higher thoracic, UES and LES basal pressure, longer LES length and decrease in LES retention pressure; during swallow: higher thoracic, gastric and UES pressure, higher UES and LES relaxation pressure and after swallow: higher thoracic and gastric pressure. Multivariate analysis in pressurized waves showed as significant before swallow: thoracic and UES basal pressure; during swallow: thoracic, gastric and UES pressure, UES residual pressure and UES recovery time and after swallow: thoracic pressure.
Conclusions
Basal esophageal pressurization and the UES are independent variables that may be associated with pressurized waves.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Chicago Classification for esophageal motility disorders defined phenotypes of achalasia according to esophageal body contractility and pressurization [1]. Different studies showed that these phenotypes are of prognostic value for outcomes [2, 3] and may determine the best therapeutic approach according to subtypes [4]. Esophageal body pressurization is not, however, uniform in all swallows in the same individual and the reasons that lead to a pressurized wave or not are still unclear.
This study aims to evaluate manometric parameters that may determine pressurized waves in patients with achalasia.
Methods
A total of 100 consecutive high-resolution manometry (HRM) studies in patients with achalasia were reviewed.
Population
We studied 100 consecutive cases of adult patients with achalasia (51% females, mean age 53.70 ± 15.80 years).
Chagas disease esophagopathy was diagnosed in 71 (71%) patients based on positive serologic test for Chagas disease and/or manifestation of the disease in other target organs, such as colon or heart, in patients originated from endemic areas for Chagas disease.
Esophageal dilatation grades were: Grade I (<4 cm) in 4 (4.0%), Grade II (4–7 cm) in 39 (39.0%), Grade III (7–10 cm) in 16 (16.0%) and Grade IV (>10 cm) in 16 (16.0%). Esophagogram was not available to review in 25.0%.
All patients underwent upper digestive endoscopy to rule out pseudoachalasia. No patient had hiatal hernia.
Esophageal function test
All patients underwent HRM (Medtronic, Minneapolis, USA) after fasting for 8 h and discontinuation of medications that could interfere with esophageal motility. Data of ten 5 mL water swallows were acquired via the dedicated commercial software (ManoScan, Medtronic, Minneapolis, USA). All manometric studies were prospectively reviewed by two experienced researchers.
Automatic analysis of the manufacturer software (Manoview, Medtronic, Minneapolis, USA) was used but manually adjusted to different time intervals or areas according to necessity.
We recorded manometric parameters before each swallow [as defined by the time frame of 2 s immediately before the beginning of the upper esophageal sphincter (UES) relaxation], during swallowing [defined by the period of UES relaxation] and after each swallow [as defined by the time frame of 4 s immediately after UES relaxation].
Parameters before each swallow were: UES basal pressure, esophageal length (calculated from the lower border of the UES to the upper border of the LES), lower esophageal sphincter (LES) basal pressure, LES length, gastric and thoracic pressure, transdiaphragmatic pressure gradient (TPG) (thoracic–gastric pressure) and the LES retention pressure (LES basal pressure-TPG) according to the previous methodology [5].
Parameters during swallow were: UES nadir residual pressure, UES recovery time, UES post-relaxation contraction maximum pressure, LES integrated relaxation pressure (IRP), gastric and thoracic pressure and transdiaphragmatic pressure gradient.
Parameters after swallow were: esophageal length, LES length, wave pressure, gastric and thoracic pressure and transdiaphragmatic pressure gradient.
The threshold of 30 mmHg was used to classify individual waves as pressurized or not pressurized.
Statistical analysis
Shapiro–Wilk test was used to assess normal distribution of data. All variables had a non-normal distribution. Variables are expressed as median (interquartile range). Mann–Whitney and logistic regression with binary response variable tests were used for univariate and multivariate analysis, respectively. p < 0.05 was considered significant. Statistical analyses were performed using the R software version 3.6.1.
Ethics
The study protocol was approved by local Ethics Committee (CAAE 00493518.7.0000.5505). Informed consent was waived due to the retrospective nature of the study. There is no conflict of interest. All authors contributed sufficiently to be named as authors and are responsible for the manuscript. No professional or ghost writer was hired.
Results
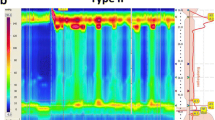
There were 70 (70%) achalasia type I and 30 (30%) type II patients. There was no type III. Waves were pressurized in 164 (16.4%) swallows and non-pressurized in 836 (83.6%) swallows (Fig. 1). Manometric parameters for pressurized versus non-pressurized waves are depicted in Table 1.
Univariate analysis showed that pressurized waves had before swallow: higher thoracic, UES and LES basal pressure, longer LES length and decrease in LES retention pressure. Pressurized waves had during swallow: higher thoracic, gastric and UES pressure, higher UES and LES relaxation pressure. Pressurized waves had after swallow: higher thoracic and gastric pressure. Intragroup analysis showed that thoracic pressure after swallow is higher compared to before swallow (p = 0.005) and there is no difference in gastric pressure (p = 0.373). For non-pressurized waves, thoracic pressure was higher after swallow compared to basal thoracic pressure (p < 0.001) and gastric pressure was lower than basal gastric pressure (p = 0.01).
Multivariate analysis is shown in Table 2. Independent variables found in pressurized waves before swallow were: thoracic and UES basal pressure; during swallow: thoracic, gastric and UES pressure, UES residual pressure and UES recovery time; and after swallow: thoracic pressure.
Discussion
The results of our study show that pressurized waves in patients with achalasia occur in the setting of a compartmentalized esophagus with increased basal pressure and hypertonic sphincters that relax poorly to keep a higher thoracic pressure. Multivariate analysis, however, showed that the basal esophageal pressurization and the UES are the independent variables associated with pressurized waves.
UES and wave pressurization
Achalasia affects the innervation of digestive smooth muscles. The UES is thus theoretically spared in this disease, and studies focusing on the UES in patients with achalasia were scarce until recently. Also, HRM surpassed some technical limitations of conventional manometry that did not allow an adequate evaluation of the UES and allowed the distinction of achalasia in subtypes. UES has a variable length of 3–4 cm at manometry, and during swallows, it moves upward of 1 to 1.5 cm: this means that sensors may drop in the esophagus during swallows and not record a relaxation but the intra-esophageal pressure. HRM compensates for this movement artifact since UES excursion is easily noticed and relaxation measured at the appropriate point as multiple sensors are available. Moreover, the pressures generated in the UOS are asymmetric, with the anterior–posterior pressures 60% to 70% higher than lateral pressures. HRM also compensates this fact using an average per sensor of 12 circumferential subsensors in the catheter in each centimeter.
We previously described that patients with achalasia have impaired UES relaxation that we described as a protective mechanism against aspiration in these patients [6]. This was even more noticeable in patients with achalasia type II. In fact, other studies supported the same finding [7,8,9,10,11]. The results of the current study show that the UES may also participate in the genesis of pressurized waves. UES basal pressure is hypertonic to sustain the higher thoracic pressure, and an effortful swallow with decreased relaxation increases even more the esophageal pressure. These unconscious reflexes may help to force the food into the stomach overcoming the non-functional barrier at the esophagogastric junction. Interestingly, UES residual pressure is reduced after endoscopic treatment for achalasia [8, 12, 13] proving that it is a reversible physiologic reflex and it seems to predict therapy outcomes [14, 15].
Esophageal body and wave pressurization
Pressurized waves after swallow in achalasia (previously known as simultaneous waves at the conventional manometry era) have been credited to muscular contraction associated with esophageal shortening [16]. We believe, for different reasons, that this pressurization is not caused by muscular contraction: (1) pressures are the same along the whole extension of the wave. Muscles are not able to contract with such precision; moreover, the transition zone—an area quiescent of contractions—is absent. These facts bear a resemblance to communicating vessel principle that may be applied to an esophagus replenish with fluids; and (2) the esophagus is denervated. The lack of innervation may promote tertiary spontaneous contractions; waves coordinated with deglutition are not feasible. Furthermore, even though idiopathic achalasia may conserve excitatory neurons allowing spastic contractions (achalasia type III), Chagasic achalasia does not [17] and spastic contractions are not seemed in this disease [18], but pressurized waves are present even though. (3) No manometric shortening of the esophagus was found in our study.
Pressurized waves occur in the setting of a higher thoracic pressure that progresses in intensity from before swallow to after swallow. This may suggest an increased retention of fluid inside the esophagus with the offered water to perform the test. Migration from one status to other (pressurized to non-pressurized or vice versa) as seen in type II patients may occur due to increased luminal content due to the offered water or sudden decrease caused by esophageal emptying when the pressure of the liquid column exceeds the LES retention pressure. Since this is dynamic, the comparison of the first and last swallow would not provide useful information.
Transdiaphragmatic pressure gradient and wave pressurization
TPG has been studied at light of HRM to the diagnosis of gastroesophageal reflux disease in some comorbidities which has demonstrated that it may surmount the esophagogastric barrier represented by LES and diaphragm [5, 19, 20]. However, to the best of our knowledge, no previous reports have dealt TGP as relevant play role in the mechanism of esophageal pressurization in achalasia.
Our results show that pressurized waves have a higher TGP in all studied moments (before, during and after swallows). Before swallows, TGP is higher thanks to an elevated basal thoracic pressure that may occur by esophageal liquid replenishment according to our theory. Furthermore, thoracic pressure presents significant ascension after swallow of water comparing with non-pressurized waves, reinforcing the liquid accumulation, as well as highlighting thoracic pressure as an important component to trespass the esophagogastric hurdle in achalasia patients.
Abdominal pressure raises during swallow in pressurized group. Notwithstanding achalasia patients can develop maneuvers to facilitate the passage of the bolus involving some grade of physical effort like to Valsalva maneuver which is known to increase intrathoracic and abdominal pressure by forcing expiratory effort against a closed glottis [21]. Thus, the higher gastric pressure seems to be intrinsically linked to effort made for the patient to swallow, contracting reflexively the abdominal wall.
LES and wave pressurization
LES is hypertonic in less than half of the patients with achalasia [22]. Even a hypotonic LES may be an obstacle to esophageal emptying if relaxation is impaired (one cannot pass through a door unless it is opened irrespective of whether it is ajar, closed or locked). Although our univariate analysis showed higher LES mean basal and IRP pressure in pressurized waves, logistic regression did not show LES manometric parameters as independent factors for pressurized waves. This may explain why basal LES pressure is not a predictor for surgical outcomes [22].
Study limitations, strengths and comparison to similar studies
Our study has some limitations. First, this is a retrospective study with the intrinsic limitations of this type of study even though all manometric studies were prospectively reviewed; as such, data as body mass index and waist circumference that may influence pressures were not available. Second, our clinical conclusions are based on manometric parameters but they are speculative. Third, the majority of patients had Chagas disease as the etiology for achalasia, although it is known that idiopathic achalasia and Chagasic achalasia seem to share more similarities than dissimilarities [17]. Chagas disease, however, is characterized by a total absence of excitatory and inhibitory neurons along the esophagus [17]. It is possible that this could explain the absence of shortening of the esophagus after swallowing. However, pressurized waves did occur in the absence of esophageal shortening, showing that it may not be necessary for the pressurization to happen. Forth, these patients have a decreased esophageal clearance. It is elusive if the esophagus was free of food residues after 8 h of fasting.
We theorized that esophageal liquid replenishment is causative for wave pressurization; however, we did not study this fact directly. Future studies with concomitant esophagram and manometry may elucidate this theory.
Clinical implications of the findings
We showed that the UES plays an important role in the genesis of pressurized waves (with denoted interaction with thoracic pressure allowing the pressurization and promoting esophageal emptying) and that the pressurization may be beneficial to promote esophageal emptying. It is uncertain if UES motility pattern (as well as the ascension of thoracic pressure) found in this study can be consciously learned and oral rehabilitation may provide better outcomes in patients with achalasia. It is interesting; however, to note that in patients with type II that manifested both types of waves (pressurized or not), there is a change in the UES pattern, probably related to unconscious swallowing maneuvers or effort.
Conclusions
We theorize that pressurized waves in patients with achalasia are not caused due to muscular contraction but caused by the lack of relaxation of the UES combined with increased thoracic pressure probably by reason of esophageal liquid replenishment. This theory must be confirmed by further studies since esophageal fluid volume was not actually measured.
References
Kahrilas PJ, Bredenoord AJ, Fox M, Pandolfino JE et al (2015) The Chicago classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil 27:160–174. https://doi.org/10.1111/nmo.12477
Pandolfino JE, Kwiatek MA, Nealis T, Bulsiewicz W, Post J, Kahrilas PJ (2008) Achalasia: a new clinically relevant classification by high-resolution manometry. Gastroenterology 135(5):1526–1533. https://doi.org/10.1053/j.gastro.2008.07.022(Epub 2008 Jul 22)
Rohof WO, Salvador R, Annese V, des Varannes SB, Chaussade S, Costantini M, Elizalde JI, Gaudric M, Smout AJ, Tack J, Busch OR, Zaninotto G, Boeckxstaens GE (2013) Outcomes of treatment for achalasia depend on manometric subtype. Gastroenterology 144(4):718–725
Andolfi C, Fisichella PM (2019) Meta-analysis of clinical outcome after treatment for achalasia based on manometric subtypes. Br J Surg 106(4):332–341. https://doi.org/10.1002/bjs.11049(Epub 2019 Jan 28)
Del Grande LM, Herbella FAM, Katayama RC, Schlottmann F, Patti MG (2018) The role of the transdiaphragmatic pressure gradient in the pathophysiology of gastroesophageal reflux. Arq Gastroenterol 55(Suppl 1):13–17. https://doi.org/10.1590/s0004-2803.201800000-39
Menezes MA, Herbella FA, Patti MG (2015) High-resolution manometry evaluation of the pharynx and upper esophageal sphincter motility in patients with achalasia. J Gastrointest Surg 19(10):1753–1757. https://doi.org/10.1007/s11605-015-2901-5
DeVault KR (1997) Incomplete upper esophageal sphincter relaxation: association with achalasia but not other esophageal motility disorders. Dysphagia 12(3):157–160
Yoneyama F, Miyachi M, Nimura Y (1998) Manometric findings of the upper esophageal sphincter in esophageal achalasia. World J Surg 22(10):1043–1046. https://doi.org/10.1007/s002689900514(discussion 1046-7)
Min M, Peng LH, Yang YS, Hou XH, Guo RB, Wang WF, Sun G, Wang XX (2012) Characteristics of achalasia subtypes in untreated Chinese patients: a high-resolution manometry study. J Dig Dis 13(10):504–509. https://doi.org/10.1111/j.1751-2980.2012.00622x
Blais P, Patel A, Sayuk GS, Gyawali CP (2017) Upper esophageal sphincter (UES) metrics on high-resolution manometry (HRM) differentiate achalasia subtypes. Neurogastroenterol Motil. https://doi.org/10.1111/nmo.13136
Triantafyllou T, Theodoropoulos C, Mantides A, Chrysikos D, Smparounis S, Filis K, Zografos G, Theodorou D (2018) Can the upper esophageal sphincter contractile integral help classify achalasia? Ann Gastroenterol 31(4):456–461. https://doi.org/10.20524/aog.2018.0270
Wauters L, Van Oudenhove L, Selleslagh M et al (2014) Balloon dilation of the esophago-gastric junction affects lower and upper esophageal sphincter function in achalasia. Neurogastroenterol Motil 26:69–76
Ren Y, Tang X, Chen F, Deng Z, Wu J, Nei S, Jiang B, Gong W (2016) Myotomy of distal esophagus influences proximal esophageal contraction and upper esophageal sphincter relaxation in patients with achalasia after peroral endoscopic myotomy. J Neurogastroenterol Motil 22(1):78–85. https://doi.org/10.5056/jnm15098
Chavez YH, Ciarleglio MM, Clarke JO, Nandwani M, Stein E, Roland BC (2015) Upper esophageal sphincter abnormalities: frequent finding on high-resolution esophageal manometry and association with poorer treatment response in achalasia. J Clin Gastroenterol 49:17–23
Mathews SC, Ciarleglio M, Chavez YH, Clarke JO, Stein E, Chander Roland B (2014) Upper esophageal sphincter abnormalities are strongly predictive of treatment response in patients with achalasia. World J Clin Cases 2(9):448–454. https://doi.org/10.12998/wjccv2.i9.448
Hong SJ, Bhargava V, Jiang Y, Denboer D, Mittal RK (2010) A unique esophageal motor pattern that involves longitudinal muscles is responsible for emptying in achalasia esophagus. Gastroenterology 139(1):102–111. https://doi.org/10.1053/j.gastro.2010.03.058
Herbella FA, Oliveira DR, Del Grande JC (2004) Are idiopathic and Chagasic achalasia two different diseases? Dig Dis Sci 49(3):353–360
Vicentine FP, Herbella FA, Allaix ME, Silva LC, Patti MG (2014) High-resolution manometry classifications for idiopathic achalasia in patients with Chagas’ disease esophagopathy. J Gastrointest Surg 18(2):221–224
Ribolsi M, Balestrieri R, Holloway H, Emerenziani S, Cicala M (2016) Intra-bolus pressure and esophagogastric gradient, assessed with high-resolution manometry, are associated with acid exposure and proximal migration of refluxate. Dis Esophagus 29:1020–1026
Pandolfino JE, El-Serag HB, Zhang Q, Shah N, Ghosh SK, Kahrilas PJ (2006) Obesity: a challenge to esophagogastric junction integrity. Gastroenterology 130:639–649
Pstras L, Thomaseth K, Waniewski J, Balzani I, Bellavere F (2016) The Valsalva manoeuvre: physiology and clinical examples. Acta Physiol (Oxf) 217(2):103–119. https://doi.org/10.1111/apha.12639(Epub 2016)
Gorodner MV, Galvani C, Fisichella PM, Patti MG (2004) Preoperative lower esophageal sphincter pressure has little influence on the outcome of laparoscopic Heller myotomy for achalasia. Surg Endosc 18(5):774–778
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Anefalos, A., Herbella, F.A.M. & Patti, M.G. Upper Esophageal Sphincter Motility and Thoracic Pressure are Determinants of Pressurized Waves in Achalasia Subtypes According to the Chicago Classification. World J Surg 44, 1932–1938 (2020). https://doi.org/10.1007/s00268-020-05396-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-020-05396-3