Abstract
Dysphagia is a symptom suggestive of severe underlying pathology, although its causes include organic and non-organic disorders. The epidemiology of dysphagia is, however, poorly understood. We evaluated the prevalence of dysphagia in outpatients in Japan, measured the proportion ultimately found to have an organic cause, and recorded the nature of their symptoms and the underlying disorder. Of 5362 consecutive outpatients attending the Digestive Center at our hospital between June 1, 2010 and December 31, 2012, 186 patients (3.5 %) had dysphagia with a frequency score of ≥5 out of 6. The most common diagnosis was cancer (34 patients, 18.3 %), followed by gastroesophageal reflux disease (24 patients, 12.9 %). An esophageal motility disorder was diagnosed in 21 patients (11.3 %); the causes in the remaining 107 patients (57.5 %) were miscellaneous. Multivariable analysis identified the following predictors of cancer: age ≥ 54 years, weight loss, being a drinker of alcohol, and ≤2 gastrointestinal symptoms. Our findings can be used to inform the prioritization of referrals from primary care for investigation and treatment for patients with cancer for dysphagia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dysphagia is more common in the elderly [1, 2]. Turley and Cohen reported that 13.7 % of a cohort of individuals with a mean age of 82.4 years (range 58–97 years) reported dysphagia; of these, 20.6 % received early treatment and 42.9 % showed symptom improvement [3]. According to another study, the incidence of dysphagia in the elderly (65 years or older) ranges between 7 and 22 %, rising to 40–50 % in residents of care facilities [4]. Ekberg et al. have reported that dysphagia places a heavy psychosocial burden on the elderly, and substantially impairs their quality of life [5]. Furthermore, progression of dysphagia can lead to dehydration, malnutrition, pneumonia, and death [3, 5, 6]. As the aging of the population continues to accelerate, it will be increasingly important to recognize, investigate, and treat dysphagia promptly.
Previous studies have found a prevalence of cancer of 4–15 % in those referred with dysphagia [7–9]; excluding malignancy is the most important part of the assessment of patients with dysphagia. In contrast, it is well recognized that although dysphagia may be a symptom of malignancy, it may also be a consequence of a variety of other disorders [10]. Organic causes include inflammation and tumors, while functional causes include cerebrovascular disease, esophageal achalasia, and psychological disorders [6, 10–14]. Therefore, when encountering patients with dysphagia, it is not only important to determine that there is no underlying organic disease, but also necessary to identify the cause of the symptoms. Patients with malignant diseases usually require prompt treatment. However, a balance must be struck between the potential complications of any invasive investigation and its diagnostic utility. To do so, it is important to understand fully the clinical characteristics of patients with dysphagia [2], but at present there are insufficient epidemiologic data on patients with dysphagia [15, 16].
We aimed to identify the prevalence of cancer, gastroesophageal reflux disease (GERD), esophageal motility disorders, and miscellaneous causes in patients presenting to a tertiary care center with dysphagia. We also sought to establish patients’ demographic and clinical characteristics, and the nature and severity of their symptoms, so that we could identify factors that predict the presence of underlying malignant disease.
Patients and Methods
Between June 1, 2010 and December 31, 2012, 6069 patients (3196 men and 2873 women; mean age 58.7 ± 17.9 years) attended the Digestive Center of Kawasaki Medical School Hospital. All answered a self-administered questionnaire about their gastrointestinal (GI) symptoms [17–19], which we have previously validated [20], before undergoing medical examination. In addition to the outpatient physicians’ clinical assessment, blood tests, ultrasonography, and endoscopy were performed as necessary. From this cohort, patients with dysphagia were defined as those who had a score ≥ 5 for symptom frequency in response to the following question: “Does food get stuck in the throat/chest when you swallow?” If a patient answered the questionnaire more than once during the study period, the first was used in our analysis.
The questionnaire items included date of completion, sex, date of birth, age, height, weight, occupation, the disease for which the patient was currently being treated in the outpatient setting, history of drug allergy, history of pregnancy, alcohol and coffee intake, and smoking history. In this study, patients were classified as ‘never’, ‘ex’-smokers, or ‘current’ smokers. ‘Ever’ smokers included current smokers, and ex-smokers who were not smoking at the time of the study. Smoking intensity was defined as the self-reported average number of cigarettes smoked per day and smoking duration (years). We derived the number of pack-years of cigarette exposure by calculating (daily number of cigarettes/20) × (smoking years). For alcohol intake, we asked participants whether they currently drank alcohol, or were life-long non-drinkers. Alcohol intake was based on the usual daily intake of sake (Japanese rice wine), shochu (Japanese spirits), beer, and wine among current drinkers. The daily amount of alcohol consumption was assessed in terms of grams of alcohol; for example, one unit of Japanese sake contains approximately 22 g alcohol. Weight loss was defined as positive if the patient self-reported weight loss or if weight loss was detected during history taking. The questions on GI symptoms specifically included: “Do you experience heartburn?” “Does your stomach sometimes get bloated?” “Do you sometimes have a heavy feeling in the stomach after meals?” “Do you sometimes subconsciously rub your chest with your hand?” “Do you sometimes feel sick after meals?” “Do you experience heartburn after meals?” “Do you have an unusual (e.g., burning) sensation in your throat?” “Do you sometimes feel early satiety?” “Does food get stuck in the throat/chest when you swallow?” “Do you sometimes feel bitter liquid (gastric acid) coming up into your throat?” “Do you burp a lot?” “Do you experience heartburn when you bend over?” “Do you sometimes suffer from stomach ache?” “Do you suffer from constipation?” and “Do you suffer from diarrhea?” (Table 1). As with dysphagia, other GI symptoms with a score ≥ 5 for symptom frequency were considered to be clinically significant and therefore to represent a positive answer.
We initially classified the causes of dysphagia into four categories: cancer, GERD, esophageal motility disorders, and miscellaneous causes. We compared the demographic and clinical characteristics of these four groups, and then sought to establish whether there were any factors predictive of a final diagnosis of malignancy.
Our institutional ethics committee approved the study (reference 1912), and authorization for the use of medical records for research purposes was given before they were accessed.
Statistical Analysis
Data are expressed as the mean ± standard deviation, median (minimum, maximum), or proportions (%). The four groups were compared using one-way ANOVA, the Kruskal–Wallis test, or Chi-squared test as appropriate. Multiple comparisons of continuous variables of each group were made by the Scheffé’s F test. These analyses were performed using the SPSS statistical package release 17.0 (SPSS Inc., Chicago, IL, USA). Fisher’s exact test (exact significance probability (2-tailed)) was performed using the SPSS 22.0. Cutoffs for age and the number of GI symptoms other than dysphagia with a severity score ≥ 5 were obtained from analysis of receiver operating characteristic (ROC) curves performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a modified version of R commander designed to add statistical functions frequently used in biostatistics (R Foundation for Statistical Computing, Vienna, Austria) [21]. Odds ratios (ORs) of the risk factors for cancers were calculated using univariate and multivariate logistic regression analyses. p values <0.05 were regarded as being statistically significant.
Results
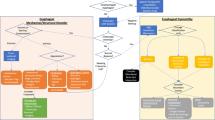
Of 6069 patients invited to participate in this study, 5362 answered the questionnaire (response rate 88.4 %, Fig. 1). The data of all those who answered a questionnaire were included in the analysis.
Classification According to Cause of Dysphagia
In total, 208 patients (3.9 %) had a dysphagia score of ≥5 out of 6. Of these, 11 who had undergone gastric surgery, one who had undergone esophageal surgery, one who had undergone head and neck surgery, and nine with unidentified causes were excluded from the analysis. The data of the remaining 186 (3.5 %, 92 men and 94 women, mean age 58.2 ± 17.1 years) were analyzed. This cohort of these 186 patients was divided into four groups according to the cause of dysphagia. There were 34 patients with cancer (Cancer group), 24 with GERD (GERD group), 21 with esophageal motility disorders (EMD group), and 107 with dysphagia due to miscellaneous causes (Miscellaneous causes group). In the GERD group, there were nine patients with erosive esophagitis and 15 with non-erosive reflux disease. The Miscellaneous causes group included eight patients with organic diseases where endoscopy detected organic abnormalities such as an ulcer of the epiglottis caused by Behçet’s disease; no organic abnormalities could be detected in the remaining 99 patients in the Miscellaneous causes group (Fig. 1).
Clinical Characteristics of Each Group
The demographic and clinical characteristics of each of the four groups are shown in Table 2. There were significant differences in the mean age, the proportion of men, the number of symptoms other than dysphagia, pack-years of smoking exposure, the proportion of current drinkers, and the proportion of patients with weight loss between the groups.
Multiple comparisons showed that the Cancer group was significantly older than the GERD group (p < 0.01). The proportion of men was also significantly higher in the Cancer group than in both GERD group (p = 0.04) and the Miscellaneous causes group (p < 0.01), and it was also significantly higher in the EMD group than that in the Miscellaneous causes group (p < 0.01). The number of symptoms other than dysphagia was significantly less common in the Cancer group than the Miscellaneous causes group (p < 0.01). Although there was no significant differences in the proportion of ‘ever’ smokers between the groups, smoking exposure measured by pack-years was significantly higher in the Cancer group than the GERD group (p = 0.03) and the Miscellaneous causes group (p < 0.01). There was a significantly higher proportion of current alcohol drinkers in the Cancer group than the GERD group (p = 0.03) and the Miscellaneous causes group (p < 0.01). Weight loss was also detected significantly more often in the Cancer group and EMD group than the Miscellaneous causes group (p < 0.01 and p = 0.02, respectively).
Gastrointestinal Symptoms of Each Group
There was a significant difference in the number of symptoms other than dysphagia reported with a score of ≥5 among the 14 on the questionnaire among the four groups (Table 2). Symptoms other than dysphagia were less common in the Cancer group than the other three groups.
There were significant differences in patients’ responses to the questionnaire items “Does your stomach sometimes get bloated?” “Do you sometimes have a heavy feeling in the stomach after meals?” “Do you have an unusual (e.g., burning) sensation in your throat?” “Do you sometimes suffer from stomach ache?” and “Do you suffer from diarrhea?” (Table 3). Further comparison of patients with cancer with those with others found that the proportion giving positive answers to the following questions was significantly lower in the Cancer group: “Do you experience heartburn?” (p = 0.01); “Does your stomach sometimes get bloated?” (p < 0.01); “Do you sometimes have a heavy feeling in the stomach after meals?” (p < 0.01); “Do you sometimes suffer from stomach ache?” (p < 0.01); and “Do you suffer from diarrhea?” (p = 0.02).
Risk Factors for Cancer
Analysis of ROC curves revealed that the optimal cutoff for the number of GI symptoms in patients with dysphagia to predict the presence of cancer was two (sensitivity, 0.59; specificity, 0.68; positive predictive value (PPV), 0.29; negative predictive value (NPV), 0.88), with an area under the curve (AUC) of 0.66 (95 % confidence interval [CI] 0.57–0.76) (Fig. 2). The optimal age cutoff was 54.0 years (sensitivity 0.97; specificity, 0.43; PPV, 0.28; NPV, 0.99), AUC 0.66, 95 % CI 0.58–0.74).
Univariate analysis identified the following as predictors for underlying cancers in patients with dysphagia: age ≥ 54 years (OR 25.3; 95 % CI 3.4–190.0); male sex (OR 5.2; 95 % CI 2.1–12.6); being a drinker of alcohol (OR 3.9; 95 % CI 1.8–8.4); two or fewer GI symptoms (OR 3.1; 95 % CI 1.4–6.6); weight loss (OR 3.0; 95 % CI 1.3–7.0); and cigarette smoking (OR 2.5; 95 % CI 1.2–5.4). Multivariate analysis revealed that the following factors were significantly associated with cancers in dysphagic patients: age ≥ 54 years (OR 26.4, 95 % CI 3.2–218.5; p < 0.01); weight loss (OR 6.0, 95 % CI 1.8–20.0; p < 0.01); alcohol consumption (OR 3.8, 95 % CI 1.4–10.6; p = 0.01); and two or fewer GI symptoms (OR 3.3, 95 % CI 1.2–9.2; p = 0.02). In our cohort, all patients with underlying cancer had at least one of these “warning” signs.
Discussion
We found that 3.5 % of patients presenting to the Digestive Center of a university hospital in Japan had dysphagia, and that 18.3 % of these had a cancer. We also found that those ultimately diagnosed with cancer reported significantly fewer GI symptoms at the time of initial history taking.
Being an alcohol drinker and cigarette smoking are well-recognized risk factors for esophageal and head and neck cancers [22, 23]. We found that the number of GI symptoms other than dysphagia reported by patients with dysphagia and who were ultimately found to have cancer was significantly fewer than the number of those who had other diagnoses. Few studies have examined the coexistence of GI symptoms other than dysphagia in patients with dysphagia, and only one study has reported the prevalence of lower GI symptoms in addition to upper GI symptoms [24]. Ours is the first study to have investigated the number of GI symptoms reported by patients with dysphagia with and without an ultimate diagnosis of cancer.
Eslick and Talley reported a prevalence of dysphagia of 16 % in the general population [16]. In addition, Cho et al. reported that 3 % of men and 3 % of women are aware of dysphagia at least once a week [24]. Our cohort included not only patients who presented to the hospital with the chief complaint of dysphagia, but also those who presented with other GI symptoms and answered the questionnaire. Of these patients, the questionnaires of those who scored ≥5 in response to the question “Does food get stuck in the throat/chest when you swallow?” were included in our analysis. Although the population of patients in our study was different from that of previous studies, the prevalence of dysphagia (3.5 %) was not greatly different.
Van Zanten et al. investigated patients with dyspepsia who reported a symptom severity of 4 or more on a 7-point Likert scale [25]. Manabe et al. [20] conducted a similar investigation in which scores of ≥4 were defined as the “presence of symptoms” in Japanese patients with dyspepsia. We elected to use scores ≥5 (often or always) to define the presence of symptoms and select patients for our analysis, as we thought that the presence of mild dysphagia (score 4) would not be sufficiently objective.
Cho et al. classified dysphagia into frequent (more than once a week), occasional (once a week or less), or absent [24]. Although dysphagia was associated with organic disease in 43 % of the patients with frequent dysphagia, the underlying cause could not be identified in 57 % [24]. They also reported that GERD was the most common underlying disease in patients with frequent dysphagia, and that GERD was significantly more common in patients with frequent dysphagia than in those with occasional dysphagia or no dysphagia [24]. Eslick et al. also reported an association between GERD and dysphagia [16]. Our finding that GERD was the second most frequent underlying cause of dysphagia is consistent with these studies [16, 24].
Patients with organic causes for dysphagia, such as those with cancer, erosive esophagitis in the GERD group, and those with organic abnormalities detected by endoscopic examination in the Miscellaneous causes group comprised 27.4 % of our cohort, which is a smaller proportion than that reported by Cho et al. [24]. The incidence of organic diseases is influenced by the definition used, and our definition fitted with our routine clinical practice. In our cohort, malignancy accounted for 66.7 % of all organic disease associated with dysphagia, with esophageal cancer (including recurrent esophageal cancer) being the most common (accounting for 73.5 %).
Early diagnosis of cancer in primary care leads to better cancer treatment outcomes and improves survival [26]. In general, dysphagia, hematuria, hemoptysis, and rectal bleeding are considered to be alarm symptoms [26, 27], although Cho et al. reported that only one of their patients complaining of frequent dysphagia had cancer [24]. While dysphagia is considered to be an important alarm symptom of esophageal cancer, many patients with dysphagia do not have malignant disease [28–30]. In our cohort, 18.3 % of patients complaining of dysphagia had malignant disease, suggesting that dysphagia should indeed be considered an alarm symptom.
Our study had several limitations. First, it is generally thought that patients with dysphagia report difficulty in swallowing when the cause lies within the oropharynx, but report the sensation that the food gets stuck when the cause lies within the esophagus [10]. Our questionnaire did not differentiate between these different sensations of dysphagia, so dysphagia arising from a disorder of the oropharynx or the esophagus could not be investigated separately. Oropharyngeal dysphagia is associated with other oropharyngeal symptoms, such as coughing during meals and regurgitation of food or liquid into the nose. Furthermore, dysphagia occurring within 1 s of swallowing is also thought to arise from a disorder of the oropharynx, while dysphagia arising from disorders of the esophagus is associated with symptoms localized to the lower sternum, epigastrium and abdomen, and chest pain and odynophagia [2]. While oropharyngeal dysphagia associated with late regurgitation of undigested food may be caused by a large Zenker’s diverticulum, it has been proposed that this symptom may also suggest distal esophageal stasis caused by peptic stricture, malignancy, or achalasia [2]. Further study will be needed to identify whether it is possible to distinguish clearly between these causes based on symptoms alone. Second, some patients with dysphagia are referred to the otorhinolaryngology department [31], and others with central nervous system disorders such as stroke and parkinsonism may be under the care of a neurologist [2, 4, 6]. By collaborating with other departments in our hospital, we could have recruited a larger cohort. Patients may also present to the hospital by other means, such as to the gerontology or emergency departments, and others may be treated in primary care alone without referral. As our study was conducted in a university hospital, the patient population studied may be different from the primary care patient population. It will be necessary to conduct large-scale multi-center studies in primary, secondary, and tertiary settings to confirm our findings. Third, we did not enquire about the duration of gastrointestinal symptoms, including dysphagia. It is reported that the duration of symptoms is generally longer in those with non-organic disorders [32]. Finally, ours was a relatively small cohort, and therefore it is difficult to generalize our findings outside Japan.
In conclusion, we found that age ≥ 54 years, weight loss, being a drinker of alcohol, and two or fewer GI symptoms predicted an underlying diagnosis of cancer in patients with dysphagia. The presence of these risk factors could inform urgent referral from primary care to a specialist center for prompt diagnosis and treatment. Because all patients in this study with cancer had at least one of these warning signs, our findings can be used to inform urgent referrals from primary care for investigation and treatment for patients with dysphagia.
References
Khan A, Carmona R, Traube M. Dysphagia in the elderly. Clin Geriatr Med. 2014;30(1):43–53.
Lind CD. Dysphagia: evaluation and treatment. Gastroenterol Clin North Am. 2003;32(2):553–75.
Turley R, Cohen S. Impact of voice and swallowing problems in the elderly. Otolaryngol Head Neck Surg. 2009;140(1):33–6.
Easterling CS, Robbins E. Dementia and dysphagia. Geriatr Nurs. 2008;29(4):275–85.
Ekberg O, Hamdy S, Woisard V, Wuttge-Hannig A, Ortega P. Social and psychological burden of dysphagia: its impact on diagnosis and treatment. Dysphagia. 2002;17(2):139–46.
Lieu PK, Chong MS, Seshadri R. The impact of swallowing disorders in the elderly. Ann Acad Med Singapore. 2001;30(2):148–54.
Murray IA, Palmer J, Waters C, Dalton HR. Predictive value of symptoms and demographics in diagnosing malignancy or peptic stricture. World J Gastroenterol. 2012;18(32):4357–62.
Kapoor N, Bassi A, Sturgess R, Bodger K. Predictive value of alarm features in a rapid access upper gastrointestinal cancer service. Gut. 2005;54(1):40–5.
Spahos T, Hindmarsh A, Cameron E, et al. Endoscopy waiting times and impact of the two week wait scheme on diagnosis and outcome of upper gastrointestinal cancer. Postgrad Med J. 2005;81(961):728–30.
Kruger D. Assessing esophageal dysphagia. JAAPA. 2014;27(5):23–30.
Regan J, Sowman R, Walsh I. Prevalence of Dysphagia in acute and community mental health settings. Dysphagia. 2006;21(2):95–101.
Bazemore PH, Tonkonogy J, Ananth R. Dysphagia in psychiatric patients: clinical and videofluoroscopic study. Dysphagia. 1991;6(1):2–5.
Bhattacharyya N. The prevalence of dysphagia among adults in the United States. Otolaryngol Head Neck Surg. 2014;151(5):765–9.
Cook IJ. Oropharyngeal dysphagia. Gastroenterol Clin North Am. 2009;38(3):411–31.
Roy N, Stemple J, Merrill RM, Thomas L. Dysphagia in the elderly: preliminary evidence of prevalence, risk factors, and socioemotional effects. Ann Otol Rhinol Laryngol. 2007;116(11):858–65.
Eslick GD, Talley NJ. Dysphagia: epidemiology, risk factors and impact on quality of life–a population-based study. Aliment Pharmacol Ther. 2008;27(10):971–9.
Kusano M, Shimoyama Y, Sugimoto S, et al. Development and evaluation of FSSG: frequency scale for the symptoms of GERD. J Gastroenterol. 2004;39(9):888–91.
Svedlund J, Sjödin I, Dotevall G. GSRS—a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig Dis Sci. 1988;33(2):129–34.
Carlsson R, Dent J, Bolling-Sternevald E, et al. The usefulness of a structured questionnaire in the assessment of symptomatic gastroesophageal reflux disease. Scand J Gastroenterol. 1998;33(10):1023–9.
Manabe N, Haruma K, Hata J, et al. Clinical characteristics of Japanese dyspeptic patients: is the Rome III classification applicable? Scand J Gastroenterol. 2010;45(5):567–72.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–8.
Tanabe H, Yokota K, Shibata N, Satoh T, Watari J, Kohgo Y. Alcohol consumption as a major risk factor in the development of early esophageal cancer in patients with head and neck cancer. Intern Med. 2001;40(8):692–6.
Grønbaek M, Becker U, Johansen D, Tønnesen H, Jensen G, Sørensen TI. Population based cohort study of the association between alcohol intake and cancer of the upper digestive tract. BMJ. 1998;317(7162):844–7.
Cho SY, Choung RS, Saito YA, et al. Prevalence and risk factors for dysphagia: a USA community study. Neurogastroenterol Motil. 2015;27(2):212–9.
Veldhuyzen van Zanten SJ, Chiba N, Armstrong D, et al. A randomized trial comparing omeprazole, ranitidine, cisapride, or placebo in helicobacter pylori negative, primary care patients with dyspepsia: the CADET-HN Study. Am J Gastroenterol. 2005;100(7):1477–88.
Dregan A, Møller H, Charlton J, Gulliford MC. Are alarm symptoms predictive of cancer survival? Population-based cohort study. Br J Gen Pract. 2013;63(617):e807–12.
Stapley S, Peters TJ, Neal RD, Rose PW, Walter FM, Hamilton W. The risk of oesophago-gastric cancer in symptomatic patients in primary care: a large case-control study using electronic records. Br J Cancer. 2013;108(1):25–31.
Andrews JM, Fraser RJ, Heddle R, Hebbard G, Checklin H. Is esophageal dysphagia in the extreme elderly (> or = 80 years) different to dysphagia younger adults? A clinical motility service audit. Dis Esophagus. 2008;21(7):656–9.
Vakil N, Moayyedi P, Fennerty MB, Talley NJ. Limited value of alarm features in the diagnosis of upper gastrointestinal malignancy: systematic review and meta-analysis. Gastroenterology. 2006;131(2):390–401 Quiz 659–60.
Vakil NB, Traxler B, Levine D. Dysphagia in patients with erosive esophagitis: prevalence, severity, and response to proton pump inhibitor treatment. Clin Gastroenterol Hepatol. 2004;2(8):665–8.
Lindgren S, Janzon L. Prevalence of swallowing complaints and clinical findings among 50-79-year-old men and women in an urban population. Dysphagia. 1991;6(4):187–92.
Simrén M, Svedlund J, Posserud I, Björnsson ES, Abrahamsson H. Health-related quality of life in patients attending a gastroenterology outpatient clinic: functional disorders versus organic diseases. Clin Gastroenterol Hepatol. 2006;4(2):187–95.
Acknowledgments
We are grateful to Dr. Satoshi Morita, PhD, of the Department of Biomedical Statistics and Bioinformatics, Kyoto University Graduate School of Medicine, Japan, for his helpful advice and guidance on statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsukamoto, M., Manabe, N., Kamada, T. et al. Number of Gastrointestinal Symptoms is a Useful Means of Identifying Patients with Cancer for Dysphagia. Dysphagia 31, 547–554 (2016). https://doi.org/10.1007/s00455-016-9712-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00455-016-9712-z