Abstract
Biodiversity is often highest at ecotones. However, edge effects vary among species and the spatial extent has rarely been quantified. Rodents form an important part of the food chain and thus are keystones of the ecosystem. We measured the species richness and abundance of rodents at ecotones between forests and three types of open agricultural biotopes (grasslands, rapeseed fields, and cereal fields) along perpendicular transects. The species richness and relative abundance of rodents were highest at the forest/grassland ecotone where the densities of the yellow-necked mouse (Apodemus flavicollis) and the striped field mouse (A. agrarius) were highest. The highest density of the forest-dwelling bank vole (Myodes glareolus) was recorded next to grasslands; however, the abundance of this species increased towards the forest interior. The positive edge effect of ecotones on species richness and total abundance did not exceed 10 m. Our results suggest that maintaining narrow grasslands at the margins of crop fields would strengthen rodent communities at ecotones as well as in adjacent forests.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Increasing demand for food and fuel production has prompted the replacement of diverse landscapes with uniform agricultural fields and earlier sustainable land use practices have shifted to the intensified management of monocultures (Robinson and Sutherland 2002; Díaz et al. 2006; Krauss et al. 2010). Due to agricultural intensification, several farmland plant, bird, and mammal populations have collapsed, resulting in a loss of functional diversity (Donald et al. 2001; Flynn et al. 2009; Cardinale et al. 2012; Dudley and Alexander 2017). Yet, it has been acknowledged that agriculture should not only target the highest yield in crop production but should also provide additional ecosystem services (Tilman et al. 2001; Robinson and Sutherland 2002; Garibaldi et al. 2017).
The highest species richness, diversity, or abundance is often found at ecotones, defined as transition areas between two ecological communities (Wiens 1976; Kark 2013). Ecotones connect different habitats and increase species richness and diversity in various taxonomic groups, such as plants (Luczaj and Sadowska 1997), insects (Magura et al. 2001; Sepp et al. 2004; Pe’er et al. 2011), birds (Paton 1994), and mammals (Lidicker 1999). This is referred to as a positive edge effect and its spatial extent may differ among taxa. For example, an edge effect may increase bird abundance and diversity up to 50 m from the exact edge (Paton 1994), whereas in several shrub and tree species, edge effects may be limited to a couple of meters (Luczaj and Sadowska 1997). Although opposite effects have been recorded (Hanski et al. 1996; Ross et al. 2005; Laiolo and Rolando 2005, Soga et al. 2013), the importance of ecotones and edge effects for wildlife in agricultural landscapes is widely acknowledged. However, these landscape elements are successively disappearing due to the ongoing intensification of agriculture and accompanying land consolidation (Benton et al. 2003; Dudley and Alexander 2017).
Small mammals are distributed in various habitats and are important for preserving biodiversity across landscapes (MacDonald and Barrett 1993; Kozakiewicz et al. 1999; Sullivan and Sullivan 2019). Although some taxonomic groups of small mammals (e.g., rodents) are often considered agricultural pests (Jacob et al. 2013), their ecological importance cannot be neglected, as they form an important part of the food chain (Andersson and Erlinge 1977; Sullivan and Sullivan 2019). Various small mammals forage on plants and thus help to spread plant seeds (Williams et al. 2001); they are also a staple food for various mammalian and avian predators (Korpimäki 1984; Goldyn et al. 2003; Elmeros 2006; Šàlek et al. 2010).
Habitat and plant community composition are important determinants of small mammal diversity and abundance (Hansson 1978; Väli and Tõnisalu 2021); however, human activity, particularly in forestry and agriculture, strongly influences habitats of small mammal communities (Panzacchi et al. 2010; Väli and Tõnisalu 2021). Habitat protection, especially in grasslands, is essential for small mammal communities (Bretagnolle et al. 2011; Benedek and Sîrbu 2018), and, vice versa, increasing areas of crop production are accompanied by declining vole populations (Butet and Leroux 2001).
In agricultural landscapes, small mammal species richness and abundance are highest at field margins (Ernoult et al. 2012; Fischer and Schröder 2014). Edge effects have also been detected in openings (Menzel et al. 1999) and at the border between clear-cuts and forest (Sekgororoane and Dilworth 1995) in forested landscapes. Hedgerows, in essence consisting of forest edge only, could even increase the abundance of some forest-associated species in farmland (Pollard and Relton 1970; Butet et al. 2006). On the other hand, edges have a negative impact on species preferring the habitat interior (Mills 1995; Stevens and Husband 1998; Haapakoski and Ylönen 2010; Panzacchi et al. 2010). Yet, the spatial extent of edge effects has rarely been studied quantitatively in small mammals (but see Williams and Marsh 1998; Mazzamuto et al. 2018), despite the substantial conservation implications. For instance, grassy margins supporting wildlife in agricultural landscapes influence crop yields and thus the effectiveness of such a conservation measure depends on the optimization of conditions at these margins. Finally, edge effects are not ubiquitous (Heske 1995; Kingston and Morris 2000); therefore, they should be analyzed during examinations of conservation value in each particular region, habitat type, and species complex.
In this study, we tested for the effect of habitat edges on rodent populations at the margins of an agricultural landscape at the community and species levels. We compared relative abundances of rodent species and their total abundance at ecotones between a forest and different types of agricultural land (i.e., grasslands, rapeseed, and cereal fields) to detect the most suitable ecotone for rodents. We hypothesized that (1) the species richness and abundance of rodents are higher in ecotones than in open land and the forest interior and (2) grasslands support more rodent species and have a higher total abundance compared with rapeseed and cereal fields, which support mainly granivorous species. We quantified the edge effect spatially to improve the development of conservation strategies for small mammal populations in agricultural landscapes.
Methods
Study species
Our study focuses on four species, the bank vole (Myodes glareolus Schreber, 1780), yellow-necked mouse (Apodemus flavicollis Melchior, 1834), striped field mouse (Apodemus agrarius Pallas, 1771), and field vole (Microtus agrestis Linnaeus, 1761). All four species are mainly herbivorous and granivorous with small proportions of invertebrates in their diet. The bank vole prefers forested habitats of different type and age; the yellow-necked mouse opportunistically occupies various types of forests and agricultural landscapes; the striped field mouse prefers grassy margins and heterogeneous farmland); the field vole thrives in open habitats (especially grasslands) with medium-height and tall vegetation (MacDonald and Barrett 1993). All species are abundant and widespread in the study area.
Study area
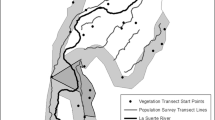
The study was conducted in 2014 and in 2018 in Estonia, northern Europe, in three areas located near Tartu (58°27′ N 26°32′ E), Viljandi (58°19′ N 25°44′ E), and Põlva (58°04′ N 26°59′ E in 2014 and 57°59′ N 27°01′ E in 2018), 50–100 km from each other (Fig. 1A). In each of the three areas, ecotones between a forest (mostly middle-aged mixed forests) and three types of open agricultural land (grassland, rapeseed oil field, and cereal field; Fig. 1B) were selected, for a total of nine plots annually. In the study area, ecotones are rather abrupt transitions from forest to crop field. Estonian managed forests are kept in rather natural conditions and the forest interior does not differ drastically from edges (e.g., undergrowth is not removed in managed forests), other than lower foliage and the occurrence of bushes on edges. Grasslands included permanent grasslands (Viljandi) and 1- or 2-year grasslands, formed on a rotational basis (Tartu, Viljandi). Owing to the lack of similar types of grasslands in all study areas, the two types of grasslands were pooled for analyses. Among cereal fields, wheat was included once (Tartu) and barley twice (Viljandi and Põlva) in 2014, and barley was included once (Viljandi) and wheat twice (Tartu and Põlva) in 2018, according to the availability of crop types. Trapping was conducted only at unharvested crop fields.
Design of the study. A Location of the three study areas in Estonia (location in Europe is shown in the upper left corner). B Each study area contained lines of trap groups in three different ecotone types. C Each type of ecotone contained three replicates of lines of trap groups. D Each trap group consisted of five Sherman box traps
Trapping of rodents
In each study plot, three replicate trapping transects were established. Each transect included seven trap groups, 10 m apart from each other, starting in the forest 30 m from the edge and ending in open land 30 m from the edge (Fig. 1C). In each trap group, five traps were placed in a circle with diameter up to 2 m; traps were located at least 0.5 m apart from each other (Fig. 1D). On the edge, the diameter was usually smaller to ensure that traps were located exactly on the edge. Sherman large box traps (7.6 × 8.9 × 22.9 cm) baited with black bread, which was warm-flavored by sunflower oil, were used. Only a few traps were disarmed due to activity of large animals (Table 1).
Trapping was conducted from 25 July to 18 August in 2014 and from 2 to 27 August in 2018, i.e., in late summer when the rodent abundance reaches its annual peak (van Apeldoorn et al. 1992; Heroldovà et al. 2007; Väli and Tõnisalu 2021), and the temperatures were sufficient to prevent the avoidance of metal traps. To avoid the effects of harvesting, rodents were only trapped for two consecutive nights in each plot. Traps were set up in the evening and checked the next morning. The number of trapped animals in each 5-piece trap-group did not exceed three; hence, our trapping did not suffer from oversaturation and our estimates on species relative density and richness are accurate despite the short trapping sessions. Captured rodents were identified to the species level and marked by cutting a small piece of body hair to detect re-trapped individuals. As the number of recaptures was low (13.6% of all captures) and our aim was to estimate relative abundance (not absolute abundance), recaptures were excluded from further analysis.
Data analysis
Data analysis was conducted hierarchically. We first compared abundances and species richness in three main habitat types (forest, open land, edge). Second, we assessed differences in abundances and species richness in different crop types (data from edges and forests excluded) and edge effects in relation to various crop types, indicated as significances of interactions between habitat factors (crop type vs general habitat type (open land and edge or forest and edge; third habitat type excluded in both cases)). Third, we quantified edge effect by comparing species richness and abundances at various distances from the edge. Here, we separately analyzed effects of distance from edge into open land and forest interiors, which enabled us to test for significance of various distances (groups) from the edge (intercept in models). Again, we excluded forest data when quantifying edge effect in open land and, vice versa, we excluded opn land data when quantifying edge effect in forest.
We used generalized linear models (GLMs) with Poisson error distribution and logarithmic link function, because the ratio between the residual deviance and degrees of freedom indicated no over-dispersion of the data for the Poisson distribution; a test of overdispersion using the dispersiontest function in the R package AER v. 1.2–9 (Kleiber and Zeileis 2008) indicated only slight overdispersion (< 1.15). The number of species (0–2; relative species richness) or trapped individuals (0–3; relative abundance) in each trap group per trap night were used as dependent variables and biotope (forest, edge, or open land), crop type (grassland, rapeseed, or cereal) or distance from the edge (0, 10 m, 20 m, or 30 m) as categorical descriptive factors. Year (2014 and 2018) and study area (Põlva, Tartu, Viljandi) were included as blocking variables. Due to low number of levels, these variables were treated as fixed (not random) factors. The significance of models was evaluated using likelihood-ratio tests with chi-square approximation by comparing models with and without predictors. Tukey post hoc tests were conducted using the R package multcomp (Hothorn et al. 2008) to detect significant differences between specific levels. R 4.0.0 (R Core Team 2020) in RStudio 1.2.5042 (R Studio Team 2016) was used for data analysis.
Results
Most of the 241 rodents, trapped during 3775 trap nights, were bank voles (53.5%) and yellow-necked mice (37.8%), while striped field mice (6.6%) and field voles (2.2%) were trapped only occasionally (Table 1).
The general habitat type significantly influenced the species richness and relative abundance of the three species (pooled). The abundance was significantly higher at edges than in open habitats (GLM post hoc Tukey contrasts for species richness: z = 3.92, Padj < 0.001 and abundance: z = 4.74, Padj < 0.001) as well as in forest than in open land (species richness: z = 6.46, Padj < 0.001, abundance: z = 7.51, Padj < 0.001). No difference between the forest and edge was found (species richness: z = 1.40, Padj = 0.33, abundance: z = 1.54, Padj = 0.26). In all models evaluating differences between general habitat types, year had only marginal effect (z = 0.27–0.34, Padj = 0.035–0.081), and only two study areas (Tartu and Põlva) differed from each other significantly (z = 0.93–1.31, Padj < 0.001).
Among open habitats, rodent abundance was highest in rape fields (GLM: χ2 = 15.26, df = 2, P < 0.001, Tukey contrasts with the other two crop types: z = 2.93, Padj = 0.009), being similarly low in other two crop types. However, the positive effect of edge was strongest next to grasslands (Fig. 2A), indicated by a significant interaction of habitat types (open/edge) in grasslands (in GLM of species richness: z = 3.08; P = 0.002 and abundance: z = 2.96; P = 0.003). However, the crop type did not affect the edge effect in forests (interactions in GLM of species richness: z < 0.9; P > 0.33 and abundance: z < 1.1; P > 0.28). Compared with edges, a significantly lower abundance was recorded at all distances into open habitat interior (Table 2), indicating that the extent of the edge effect was < 10 m. In forests, a higher abundance was found only at 30 m from the edge (Table 3).
Relative abundances (loess-smooths of mean number of individuals trapped in a 5-trap group) of A all rodents, B bank voles and C yellow-necked mice at various distances from the ecotone in grasslands green solid line), cereal (blue-dotted line) and rapeseed (purple-dashed line). Shading indicates 95% confidence intervals
The relative abundance of the bank vole was significantly influenced by general habitat types. This species preferred forests over edges (Tukey contrasts: z = 4.07, Padj < 0.001) and open habitats (z = 6.59, Padj < 0.001), but no difference was found between edges and open habitats (z = 1.07, Padj = 0.51). We did not analyze the edge effect towards open habitat in a statistical framework owing to the low number of bank voles. However, we detected a gradual increase in abundance towards the forest interior; the abundance was significantly higher already at 10 m from the edge (Table 3). A different pattern was found only next to grasslands, where the density was equally high at all distances from the edge (Fig. 2B).
The abundance of the yellow-necked mouse also differed significantly among the three main habitat types: the species was found in edges (Tukey contrasts: z = 3.62, Padj < 0.001) and forests (z = 3.59, P adj < 0.001) more often than in open habitats. No difference between forests and edges was detected (z = 0.71, P adj = 0.76). The abundance was significantly higher at edges than at any distance towards the interior of open land; no edge effect was found towards the forest interior (Tables 2 and 3). The greatest difference between edges and open habitats was recorded in grasslands; however, the difference was only subtle in cereal fields and absent in rape fields (Fig. 2C).
The relative abundance of the striped field mouse was higher at edges than in forests (Tukey contrasts: z = 3.0, P = 0.007) and open habitats (z = 2.6, P = 0.025), with no difference between the latter two habitat types (z = 1.66, P = 0.21). In open habitats, the abundance of the striped field mouse was lowest at 10 m from the edge; however, the small sample size limited the quantitative analysis of the edge effect (Table 2).
Discussion
We detected most rodent individuals in forests, with a similar abundance at ecotones and significantly fewer individuals in open land. Despite the low number of detected species, we observed the same pattern in an analysis of species richness. Our methodological approach might not effectively capture the full species richness and abundance. An additional trap type (e.g., pitfall) and longer trapping period would increase the numbers of trapped individuals and species. However, given that our aim was not to estimate total species richness and abundances but to study habitat associations (by comparing relative abundances in different habitats or parts of habitats), we believe that our data were sufficient. Additionally, larger study would enable also more detailed analysis of different vegetation types at edges and in various habitats. Therefore, we limit our conclusions only to three agricultural land use types and do not analyze effects of vegetation in forests.
Relatively high species richness or abundance at ecotones is a rather universal pattern (Wiens 1976). For instance, there is a strong edge effect among plants in forest–grassland borders (e.g., shrubs, trees, and bryophytes; Luczaj and Sadowska 1997). Larger ecotone areas between agricultural landscapes and forests could increase the abundance and diversity of bumblebees (Sepp et al. 2004; Sõber et al. 2020) and wide edge habitats next to arable fields can support a high abundance of butterflies (Pe’er et al. 2011). However, exceptions to these patterns occur too (Kark 2013) and various edges may have interacting effects (Porensky and Young 2013). In the current study, species-specific patterns revealed whether the high abundance and diversity of rodents reflected a positive edge effect per se or just the proximity to forest habitats.
The most abundant species in ecotones was the yellow-necked mouse, but the striped field mouse also preferred edges. Both species are frequently recorded at edges of crop fields and on cultivated landscapes (Kozakiewicz et al. 1999; Marsh et al. 2001). We detected the highest abundance of yellow-necked mouse at the border between forest and grassland. Similarly, a positive edge effect on rodents has been found in delayed hay fields in prairies (Pasitschniak-Arts and Messier 1998). However, grasslands themselves harbored relatively low densities of yellow-necked mice among open habitats. This is surprising in the context of the critical role of grasslands in shaping the distribution and abundance of various rodents and thus the food web in general (Bretagnolle et al. 2011). A low abundance in grasslands in our study might be explained by the high share of intensively managed hayfields (mowed 2–3 times a year), which is a poor habitat for small mammals (Garratt et al. 2012; Fischer and Schröder 2014), and the low proportion of permanent unfertilized meadows (mowed only once a year), a favorable habitat for rodents. Nevertheless, our results support the view that there is a positive effect of grasslands and indicate that adjacent forests (ecotone) support the utilization of these areas by rodents.
In the current study, the edge effect on yellow-necked mouse was only subtle at edges of cereal fields and the species was detected at all distances in rape fields. This is in line with the results of previous studies showing the importance of rapeseed for the closely related Ural field mouse Apodemus uralensis Pallas, 1811 during the reproductive period (Heroldovà et al. 2004). In addition to large quantities of food for granivorous rodents, such as Apodemus species, rapeseed fields provide cover against avian predators (Panek and Hušek 2014). Additionally, cereal crops, such as barley and wheat, provide favorable conditions for voles for reproduction and foraging until harvest; several mouse species may stay in cereal fields until plowing, but such fields eventually become unsuitable (Heroldovà et al. 2007). Finally, although Apodemus species use open habitats for foraging, the forest provides more opportunities for hiding and should be nearby (Kozakiewicz et al. 1999); hence, edges may just be a pathway between forests and open land, as suggested by the marginal edge effect found in the current study.
On the other hand, edges have a negative impact on species preferring the habitat interior (Mills 1995; Stevens and Husband 1998; Haapakoski and Ylönen 2010; Panzacchi et al. 2010). Forest edges are associated with a high mortality due to a higher occurrence of predators and lower vegetation cover (Ferguson 2004; Orrock et al. 2004; Mirski and Väli 2021); differences may also arise from differences in forest productivity (Mills 1995). The high total abundance of rodents in forests, especially next to grasslands, was mainly driven by the bank vole, previously identified as typical forest-dwelling species (van Apeldoorn et al. 1992; Kozakiewicz et al. 1999; Marsh et al. 2001; Alejūnas and Stirkė 2010; Balestrieri et al. 2015; Benedek and Sîrbu 2018). Moreover, we found a negative edge effect on the abundance of the bank vole, as its numbers increased towards the forest interior. Interestingly, a lower abundance of bank vole in the forest interior, compared with the edge, was detected in Italy (Mazzamuto et al. 2018). Different patterns may be explained by the different scales, since traps were set at 50 and 100 m from the edge in the Italian study (Mazzamuto et al. 2018), whereas we studied edge effects up to 30 m towards the forest interior. Alternatively, these differences may be explained by the spatial variability of responses. To summarize, the preferential use of edges by the rodent community may be explained by a combination of factors. For example, it may be explained by the avoidance of open land (bank vole), an inevitable consequence of movement between foraging and roosting habitats (striped field mouse), or a combination of various responses to edges and different crop types (yellow-necked mouse).
Maintaining natural grasslands at edges of arable fields is a well-known method to preserve biodiversity in agricultural landscapes; however, this reduces the income of farmers (Ausden 2007). The abundance and species richness of rodents were significantly lower at all distances from the edge, indicating that a positive edge effect of ecotones did not exceed 10 m. This suggests that “green margins” surrounding arable fields may be rather narrow for supporting small mammals and thus affordable to farmers interested in supporting biodiversity in and adjacent to their crops; in other ecosystems where the habitat change is less dramatic than at edges between forest and farmland, the extent of edge effect may be different. Furthermore, the lower abundance on grasslands and the positive effect of grasslands towards the forest interior suggest that grassy margins may effectively reduce crop damage by granivorous rodents (Jacob et al. 2013), which are expected to stay in ecotones with sufficient food resources and opportunities for hiding. In conclusion, our results show that edges between forests and farmland harbor a viable community of rodents, including species with contrasting habitat preferences.
Data availability
All data generated or analyzed during this study will be included in this published article and its supplementary information files.
References
Alejūnas P, Stirkė V (2010) Small mammals in northern Lithuania: species diversity and abundance. Ekologija 56:110–115. https://doi.org/10.2478/v10055-010-0016-6
Andersson M, Erlinge S (1977) Influence of predation on rodent populations. Oikos 29:591–597
Ausden M (2007) Habitat management for conservation: a handbook of techniques. Oxford University Press, Oxford. https://doi.org/10.1093/acprof:oso/9780198568728.001.0001
Balestrieri A, Remonti L, Morotti L, Saino N, Prigioni C, Guidali F (2015) Multilevel habitat preferences of Apodemus sylvaticus and Clethrionomys glareolus in an intensively cultivated agricultural landscape. Ethol Ecol Evol 29:38–53. https://doi.org/10.1080/03949370.2015.1077893
Benedek AM, Sîrbu I (2018) Responses of small mammal communities to environment and agriculture in a rural mosaic landscape. Mamm Biol 90:55–65. https://doi.org/10.1016/j.mambio.2018.02.008
Benton TG, Vickery JA, Wilson JD (2003) Farmland biodiversity: is habitat heterogeneity the key? Trends Ecol Evol 18:182–188. https://doi.org/10.1016/S0169-5347(03)00011-9
Bretagnolle V, Gauffre B, Meiss H, Badenhausser I (2011) The role of grassland areas within arable cropping systems for the conservation of biodiversity at the regional level. In Lemaire G, Hodgson JA, Chabbi A (Eds), Grassland productivity and ecosystem services pp 251–260. Wallingford: CABI. https://doi.org/10.1079/9781845938093.0251
Butet A, Leroux ABA (2001) Effects of agriculture development on vole dynamics and conservation of Montagu’s harrier in western French wetlands. Biol Cons 100:289–295. https://doi.org/10.1016/S0006-3207(01)00033-7
Butet A, Paillat G, Delettre Y (2006) Seasonal changes in small mammal assemblages from field boundaries in an agricultural landscape of western France. Agr Ecosyst Environ 113:364–369. https://doi.org/10.1016/j.agee.2005.10.008
Cardinale BJ, Duffy JE, Gonzalez A et al (2012) Biodiversity loss and its impact on humanity. Nature 486:59–67. https://doi.org/10.1038/nature11148
Díaz S, Fargione J, Chapin FS III, Tilman D (2006) Biodiversity loss threatens human well-being. PLoS Biol 4:1300–1305. https://doi.org/10.1371/journal.pbio.0040277
Donald PF, Green RE, Heath MF (2001) Agricultural intensification and the collapse of Europe’s farmland bird populations. Proceedings of the Royal Society of London B 268:25–29. https://doi.org/10.1098/rspb.2000.1325
Dudley N, Alexander S (2017) Agriculture and biodiversity: a review. Biodiversity 18:45–49. https://doi.org/10.1080/14888386.2017.1351892
Elmeros M (2006) Food habits of stoats Mustela erminea and weasels Mustela nivalis in Denmark. Acta Theriol 51:179–186. https://doi.org/10.1007/BF03192669
Ernoult A, Vialette A, Butet A, Michel N, Rantier Y, Jambon O, Burel F (2012) Grassy strips in their landscape context, their role as new habitat for biodiversity. Agr Ecosyst Environ 166:15–27. https://doi.org/10.1016/j.agee.2012.07.004
Ferguson SH (2004) Influence of edge on predator–prey distribution and abundance. Acta Oecologica 25:111–117. https://doi.org/10.1016/j.actao.2003.12.001
Fischer C, Schröder B (2014) Predicting spatial and temporal habitat use of rodents in a highly intensive agricultural area. Agr Ecosyst Environ 189:145–153. https://doi.org/10.1016/j.agee.2014.03.039
Flynn DFB, Gogol-Prokurat M, Nogeire T et al (2008) Loss of functional diversity under land use intensification across multiple taxa. Ecol Lett 12:22–33. https://doi.org/10.1111/j.1461-0248.2008.01255.x
Garibaldi LA, Gemmill-Herren B, D’Annolfo R, Graeub BE, Cunningham SA, Breeze TD (2017) Farming approaches for greater biodiversity, livelihoods, and food security. Trends Ecol Evol 32:68–80. https://doi.org/10.1016/j.tree.2016.10.001
Garratt CM, Minderman J, Whittingham MJ (2012) Should we stay or should we go now? What happens to small mammals when grass is mown, and the implications for birds of prey. Ann Zool Fenn 49:113–123. https://doi.org/10.5735/086.049.0111
Goldyn B, Hromada M, Surmacki A, Tryjanowski P (2003) Habitat use and diet of the red fox Vulpes vulpes in an agricultural landscape in Poland. Eur J Wildl Res 49:191–200. https://doi.org/10.1007/BF02189737
Haapakoski M, Ylönen H (2010) Effects of fragmented breeding habitat and resource distribution on behavior and survival of the bank vole (Myodes glareolus). Popul Ecol 52:427–435. https://doi.org/10.1007/s10144-010-0193-x
Hanski IK, Fenske TJ, Niemi GJ (1996) Lack of edge effect in nesting success of breeding birds in managed forest landscapes. Auk 113:578–585
Hansson L (1978) Small mammal abundance in relation to environmental variables in three Swedish forest phases. Studia Forestalia Suecica 147:5–40
Heroldovà M, Bryja J, Zejda J, Tkadlec E (2007) Structure and diversity of small mammal communities in agriculture landscape. Agr Ecosyst Environ 120:206–210. https://doi.org/10.1016/j.agee.2006.09.007
Heroldovà M, Zejda J, Zapletal M, Obdržàlkovà D, Jànovà E, Bryja J, Tkadlec E (2004) Importance of winter rape for small rodents. Plant Soil Environ 50:175–181. https://doi.org/10.17221/4079-PSE
Heske EJ (1995) Mammalian abundances on forest-farm edges versus forest interiors in Southern Illinois: is there an edge effect? J Mammal 76:562–568. https://doi.org/10.2307/1382364
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biom J 50:346–363. https://doi.org/10.1002/bimj.200810425
Jacob J, Manson P, Barfknecht R, Fredricks T (2013) Common vole (Microtus arvalis) ecology and management: implications for risk assessment of plant protection products. Pest Manag Sci 70:869–878. https://doi.org/10.1002/ps.3695
Kark S (2013) Ecotones and ecological gradients. In: R. Leemans (Eds.), Ecological Systems pp. 147–160. Springer, New York. https://doi.org/10.1007/978-1-4614-5755-8_9
Kingston SR, Morris DW (2000) Voles looking for an edge: habitat selection across forest ecotones. Can J Zool 78:2174–2183. https://doi.org/10.1139/cjz-78-12-2174
Kleiber C, Zeileis A (2008) Applied econometrics with R. Springer-Verlag, New York. ISBN 978–0–387–77316–2. https://CRAN.R-project.org/package=AER
Korpimäki E (1984) Population dynamics of birds of prey in relation to fluctuations in small mammal populations in western Finland. Ann Zool Fenn 21:287–293
Kozakiewicz M, Gortat T, Kozakiewicz A, Barkowska M (1999) Effects of habitat fragmenta-tion on four rodent species in a Polish farm landscape. Landscape Ecol 14:391–400. https://doi.org/10.1023/A:1008070610187
Krauss J, Bommarco R, Guardiola M et al (2010) Habitat fragmentation causes immediate and time-delayed biodiversity loss at different trophic levels. Ecol Lett 13:597–605. https://doi.org/10.1111/j.1461-0248.2010.01457.x
Laiolo P, Rolando A (2005) Forest bird diversity and ski-runs: a case of negative edge effect. Anim Conserv 8:9–16
Lidicker WZ (1999) Responses of mammals to habitat edges: an overview. Landscape Ecol 14:333–343. https://doi.org/10.1023/A:1008056817939
Luczaj L, Sadowska B (1997) Edge effect in different groups of organisms: vascular plant, bryophyte and fungi species richness across a forest-grassland border. Folia Geobotanica Et Phytotaxonomica 32:343–353. https://doi.org/10.1007/BF02821940
MacDonald DW, Barrett P (1993) Mammals of Britain and Europe. Harper-Collins, London
Magura T, Tóthmérész B, Molnár T (2001) Forest edge and diversity: carabids along forest-grassland transects. Biodivers Conserv 10:287–300. https://doi.org/10.1023/A:1008967230493
Marsh ACW, Poulton S, Harris S (2001) The yellow-necked mouse Apodemus flavicollis in Britain: status and analysis of factors affecting distribution. Mammal Rev 31:203–227. https://doi.org/10.1111/j.1365-2907.2001.00089.x
Mazzamuto MV, Wauters LA, Preatoni D, Martinoli A (2018) Behavioural and population responses of ground-dwelling rodents to forest edges. Hystrix, the Italian Journal of Mammalogy 29:211–215. https://doi.org/10.4404/hystrix-00119-2018
Menzel MA, Ford WM, Laerm J, Krishon D (1999) Forest to wildlife opening: habitat gradi-ent analysis among small mammals in the southern Appalachians. For Ecol Manage 114:227–232. https://doi.org/10.1016/S0378-1127(98)00353-3
Mills LS (1995) Edge effects and isolation: red-backed voles on forest remnants. Conserv Biol 9:395–403. https://doi.org/10.1046/j.1523-1739.1995.9020395.x
Mirski P, Väli Ü (2021) Movements of birds of prey reveal the importance of tree lines, small woods and forest edges in agricultural landscapes. Landscape Ecol 36:1409–1421. https://doi.org/10.1007/s10980-021-01223-9
Orrock JL, Danielson BJ, Brinkerhoff RJ (2004) Rodent foraging is affected by indirect, but not by direct, cues of predation risk. Behav Ecol 15:433–437. https://doi.org/10.1093/beheco/arh031
Panek M, Hušek J (2014) The effect of oilseed rape occurence on main prey abundance and breeding success of the Common Buzzard (Buteo buteo). Bird Study 61:457–464. https://doi.org/10.1080/00063657.2014.969192
Panzacchi M, Linnell JDC, Melis C, Odden M, Odden J, Gorini L, Andersen R (2010) Effect of land-use on small mammal abundance and diversity in a forest-farmland mosaic landscape in south-eastern Norway. For Ecol Manage 259:1536–1545. https://doi.org/10.1016/j.foreco.2010.01.030
Porensky LM, Young TP (2013) Edge‐effect interactions in fragmented and patchy landscapes. Conserv Biol 27:509–519
Pasitschniak-Arts M, Messier F (1998) Effects of edges and habitats on small mammals in a prairie ecosystem. Can J Zool 76:2020–2025. https://doi.org/10.1139/z98-144
Paton PWC (1994) The effect of edge on avian nest success: how strong is the evidence? Conserv Biol 8:17–26
Pe’er G, van Maanen C, Turbé A, Matsinos YG, Kark S (2011) Butterfly diversity at the eco-tone between agricultural and semi-natural habitats across a climatic gradient. Divers Distrib 17:1186–1197. https://doi.org/10.1111/j.1472-4642.2011.00795.x
Pollard E, Relton J (1970) Hedges V A study of small mammals in hedges and cultivated fields. J Appl Ecol 7:549–557. https://doi.org/10.2307/2401977
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org (last accessed 01.10.2020)
Robinson RA, Sutherland WJ (2002) Post-war changes in arable farming and biodiversity in Great Britain. J Appl Ecol 39:157–176. https://doi.org/10.1046/j.1365-2664.2002.00695.x
Ross JA, Matter SF, Roland J (2005) Edge avoidance and movement of the butterfly Parnassius smintheus in matrix and non-matrix habitat. Landscape Ecol 20:127–135. https://doi.org/10.1007/s10980-004-1010-8
R Studio Team (2016) RStudio: integrated development for R. RStudio Inc., Boston, MA. http://www.rstudio.com/ (last Accessed 10 Jan 2018).
Šàlek M, Kreisinger J, Sedlàcek F, Albrecht T (2010) Do prey densities determine preferences of mammalian predators for habitat edges in an agricultural landscape? Landsc Urban Plan 98:86–91. https://doi.org/10.1016/j.landurbplan.2010.07.013
Sekgororoane GB, Dilworth TG (1995) Relative abundance, richness, and diversity of small mammals at induced forest edges. Can J Zool 73:1432–1437. https://doi.org/10.1139/z95-168
Sepp K, Mikk M, Mänd M, Truu J (2004) Bumblebee communities as an indicator for landscape monitoring in the agri-environmental programme. Landsc Urban Plan 67:173–183. https://doi.org/10.1016/S0169-2046(03)00037-9
Soga M, Kanno N, Yamaura Y, Koike S (2013) Patch size determines the strength of edge effects on carabid beetle assemblages in urban remnant forests. J Insect Conserv 17:421–428
Stevens SM, Husband TP (1998) The influence of edge on small mammals: evidence from Brazilian Atlantic forest fragments. Biol Cons 85:1–8. https://doi.org/10.1016/S0006-3207(98)00003-2
Sullivan TP, Sullivan DS (2019) Similarity in occupancy of different-sized forest patches by small mammals on clearcuts: conservation implications for red-backed voles and small mustelids. Mammal Research 65:255–266. https://doi.org/10.1007/s13364-019-00467-w
Sõber V, Leps M, Kaasik A, Mänd M, Teder T (2020) Forest proximity supports bumblebee species richness and abundance in hemi-boreal agricultural landscape. Agr Ecosyst Environ 298:106961. https://doi.org/10.1016/j.agee.2020.106961
Tilman D, Fargione J, Wolff B et al (2001) Forecasting agriculturally driven global environmental change. Science 292:281–284. https://doi.org/10.1126/science.1057544
Väli Ü, Tõnisalu G (2021) Community- and species-level habitat associations of small mammals in a hemiboreal forest–farmland landscape. Ann Zool Fenn 58:1–11. https://doi.org/10.5735/086.058.0101
van Apeldoorn RC, Oostenbrink WT, van Winden A, van der Zee FF (1992) Effects of habitat fragmentation on the bank vole Clethrionomys glareolus in an agricultural landscape. Oikos 65:265–274
Wiens J (1976) Population responses to patchy environments. Annu Rev Ecol Evol Syst 7:81–120. https://doi.org/10.1146/annurev.es.07.110176.000501
Williams PA, Karl BJ, Bannister P, William GL (2001) Small mammals as potential seed dispersers in New Zealand. Austral Ecol 25:523–532. https://doi.org/10.1046/j.1442-9993.2000.01078.x
Williams SE, Marsh H (1998) Changes in small mammal assemblage structure across a rain forest/open forest ecotone. J Trop Ecol 14:18–198. https://doi.org/10.1017/S0266467498000157
Acknowledgements
Ulvi Selgis, Kylle Kiristaja and Marilin Mõtlep assisted us during fieldwork. We would also like to thank Tiit Maran and Jaanus Remm for useful recommendations for establishing the methodology for trapping small mammals.
Funding
The study was supported by the NGO Eagle Club and by institutional research funding IUT21-1 at the Estonian Ministry of Education and Research.
Author information
Authors and Affiliations
Contributions
Conceptualization, GT and ÜV; methodology, GT and ÜV; formal analysis, ÜV; investigation GT; data curation, GT and ÜV; writing — original draft preparation, GT and ÜV; writing — review & editing, GT and ÜV; visualization, GT and ÜV; project administration, ÜV; funding acquisition, ÜV.
Corresponding author
Ethics declarations
Ethics approval
The work was conducted in accordance with relevant national legislation. Animals were live-trapped and released, no experiment was conducted and no approval by an ethics committee was required.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tõnisalu, G., Väli, Ü. Edge effect in rodent populations at the border between agricultural landscapes and forests. Eur J Wildl Res 68, 34 (2022). https://doi.org/10.1007/s10344-022-01580-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10344-022-01580-z