Abstract
Land-use change threatens a large number of tropical species (so-called ‘loser’ species), but a small subset of disturbance-adapted species may proliferate in human-modified landscapes (‘winner’ species). Identifying such loser and winner species is critically needed to improve conservation plans, but this task requires longitudinal studies that are extremely rare. We assessed this topic with small rodent assemblages in the Lacandona rainforest, a relatively new and highly dynamic agricultural frontier from southeastern Mexico. In particular, we measured the abundance of four rodent species in 12 forest sites during a 6 year period. We related changes in abundance to differences across time in landscape structure (i.e., percentage of forest cover, matrix contrast, number of forest patches, and forest edge density) surrounding each site. Total rodent abundance was almost two times higher in 2016 than in 2011, although abundances were generally low in all years. The abundance of Heteromys desmarestianus increased through time, mainly in forest sites with increasing matrix contrast. Oryzomys sp. also tended to increase in abundance, especially in sites with decreasing edge density. Sigmodon toltecus remained stable through time, but Peromyscus mexicanus tended to decrease in abundance, particularly in sites with decreasing edge density and increasing matrix contrast across time. Therefore, spatial variations in landscape structure lead to species-specific responses. If current deforestation rates persist, we predict a population decline of forest-specialist species (P. mexicanus), and an increase in generalist species (S. toltecus and Oryzomys sp.). Improving matrix quality is crucial for preventing the extinction of forest-specialist rodent species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tropical forests are being rapidly deforested and fragmented worldwide, mainly because of human activities such as agriculture, cattle ranching and selective logging (FAO 2016). As a consequence, the tropics are increasingly dominated by human-modified landscapes composed of patches of natural ecosystems embedded in matrices with different types of anthropogenic land covers (Melo et al. 2013). Depending on the types and proportions of each land cover in the landscape (landscape composition), and on their spatial distribution (landscape configuration), such emerging landscapes can have a highly heterogeneous structure (Fahrig et al. 2011). Yet, the relative influence of the different aspects of landscape structure on tropical species is still poorly understood, limiting our ability to develop efficient conservation strategies (Fahrig 2003; Fahrig et al. 2011).
Different species can show different responses to changes in landscape structure (Gorresen and Willig 2004; Vetter et al. 2011; Carrara et al. 2015; Arroyo-Rodríguez et al. 2016). Whereas many species can disappear in human-modified landscapes, other species tend to proliferate (‘loser’ and ‘winner’ species, respectively, sensu Tabarelli et al. 2012) or maintain stable populations. Defaunation—the human-driven decline of animal species—is a common process that negatively impacts biotic communities as well as ecosystem functioning in human-modified landscapes (Dirzo et al. 2014). Yet, some species, including some small rodents, can be highly resilient to the main drivers of defaunation, and even proliferate in these landscapes (Keesing and Young 2014; Galetti et al. 2015; Young et al. 2015; Rosin and Poulsen 2016). Proliferation of small rodents could be caused by the absence of predators or competitors, and/or by an increase in resource availability due to a higher productivity of forest edges (Dirzo et al. 2014; Mendes et al. 2015; Young et al. 2015). Yet, not all rodent species are equally resilient to disturbance (Trujano-Álvarez and Álvarez-Castañeda 2010; San-José et al. 2014; Howe and Davlantes 2017) and many species may be negatively impacted by habitat isolation in fragmented landscapes (Pardini et al. 2010; Banks-Leite et al. 2014).
Understanding the patterns and drivers of changes in rodent abundance in human-modified landscapes is urgently needed because these animals are involved in many biotic interactions (as prey, as competitors, as predators, as vectors), which are crucial for the maintenance and functioning of tropical forests (Andresen et al. 2018). For example, rodents play important roles in herbivory and seed dispersal (Dirzo et al. 2007; Wolff and Sherman 2007; Galetti et al. 2015), and hence, changes in population sizes could affect plant recruitment, potentially altering forest structure and diversity (Galetti and Dirzo 2013; Galetti et al. 2015; Rosin and Poulsen 2016).
Determining rodent population variations through time and the spatial determinants of such changes requires longitudinal studies, including several years and reproductive cycles. Although some studies include 2–3 years of rodent population analysis (e.g., Pardini et al. 2010; Lira et al. 2012; Banks-Leite et al. 2014; Young et al. 2015), longer studies are extremely rare (but see Isabirye-Basuta and Kasenene 1987; Fryxell et al. 1998; Gibson et al. 2013), thus limiting our understanding of population and community dynamics in human-modified landscapes (Fahrig and Merriam 1994; del Castillo 2015). Longitudinal studies could help us understand the relative importance of human-induced proliferation or decline of rodents, and identify the landscape spatial attributes that might have an impact on these fluctuations. Here, we present the first longitudinal assessment of small rodent assemblages in the Lacandona rainforest—a species-rich region (Instituto Nacional de Ecología 2000) that harbors the highest diversity of mammals in Mexico (Medellín 1994). Covering about 1.3 million ha (Instituto Nacional de Ecología 2000), this region has suffered very high rates of forest loss, fragmentation and degradation in the last three decades due to the advance of the agricultural frontier (Carabias et al. 2015).
Previous studies in the region show that among the common terrestrial small rodent species, Heteromys desmarestianus (Desmarest’s Spiny Pocket Mouse) and Peromyscus mexicanus (Mexican Deer Mouse) are mainly found in conserved forests, while Sigmodon toltecus (Toltec Cotton Rat) is found in disturbed sites, and Oryzomys sp. (Rice Rat) in both types of vegetation (Medellín and Equihua 1998; Reid 2009). Yet, the local and landscape determinants of rodent abundances are largely unknown. To our knowledge, there is only one study that assesses the effects of landscape structure on the rodent community, but it uses a temporally static landscape approach to suggest species-specific responses to landscape structure (San-José et al. 2014). Yet, as the landscape structure in this region is not static but rather changes over time, longitudinal assessments are needed to accurately identify which rodent species may be proliferating in this region (i.e., ‘winners’), which species may be declining in abundance (i.e., ‘losers’), and what landscape structural attributes can predict these patterns.
Here, we evaluated (1) changes in landscape structure in the Lacandona region between 2010 and 2016; (2) temporal changes (i.e. 2011, 2012, 2014 and 2016) in the abundance of four terrestrial rodent species within 12 forest sites; and (3) the landscape structure variables as predictors of changes in rodent abundance through time. We measured four landscape structure variables, two compositional (forest cover and matrix contrast) and two configurational variables (fragmentation degree and forest edge density). We expected species-specific responses to changes in landscape structure, with populations of forest-specialist species (i.e., H. desmarestianus and Peromyscus mexicanus) declining in more disturbed landscapes (i.e., more deforested, fragmented, and/or with higher forest edge density and matrix contrast). Yet, following previous studies in the region (Medellín and Equihua 1998; San-José et al. 2014), we predicted an increase in the abundance of more generalist, disturbance-adapted species (i.e., S. toltecus and Oryzomys sp.) through time, especially in more disturbed landscapes.
Methods
Study area
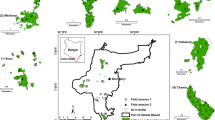
The Lacandona rainforest region is situated in the north-eastern portion of the Mexican State of Chiapas State (16°05′N, 90°25′W; Fig. 1). With a warm and humid climate (average monthly temperatures ranging from 24 to 26 °C, and mean annual precipitation ranging from 1500 to 3500 mm; Instituto Nacional de Ecología 2000), it was originally covered by mature tropical rainforest. This region is considered of highest conservation priority because of its exceptional biodiversity (Arriaga et al. 2000). Within this region, the Montes Azules Biosphere Reserve (MABR) encompasses an area of 331,200 ha of continuous rainforest (Instituto Nacional de Ecología 2000). South of the MABR, across the Lacantún River, the Marqués de Comillas Region (MCR) is comprised of 203,999 ha of fragmented forests embedded in a matrix dominated by agricultural lands and human settlements (Fig. 1). All the area is highly threatened by several human-related pressures, such as illegal flora and fauna extraction and land-use change (Carabias et al. 2015). The study was conducted inside the MABR (three continuous forest sites), as well as in forest patches outside the reserve, in the MCR (nine forest patches, ranging from 3 to 92 ha; Fig. 1). All sites were located at similar altitudes (0–200 m a.s.l.), and continuous forest sites were located at least 1 km from the nearest border of the Lacantún River (see further details in San-José et al. 2014).
Location of the study sites in the Lacandona rainforest, Mexico. Points represent the center of each site, where rodent surveys were carried out. From the geographic center of each site we estimated the structure of seven different-sized landscapes (see example in the top left side of the map). Circles surrounding each point represent the largest buffer (1400 m radius)
Sampling design
We sampled small (< 1 kg) terrestrial rodents within each site once a year, from April to September (including periods of both the dry and rainy seasons) in 2011, 2012, 2014 and 2016. In each year, we sampled the 12 sites following a random order to avoid potential confounding effects of temporal variations in resource availability and environmental conditions. In each site, we placed 120 Sherman traps in a grid of 90 × 110 m2 (distance between traps = 10 m). We located the grid in the center of each forest patch (avoiding tree-fall gaps). In continuous forest sites, the grid was located more than 1 km away from the nearest forest edge (i.e., the Lacantún river, Fig. 1). We baited each trap with a mixture of oats, sunflower seeds, and vanilla. In each site, we captured rodents for eight consecutive nights (960 trap-nights per site, 11,520 per field session, totaling 46,080 trap-nights). Animals were marked with gentian violet to account for recaptures within each year. This non-invasive short-term marking technique allows the identification of marked individuals for several months, and has no adverse physiological effects on mammals (Silvy et al. 2005). We used the number of individuals captured as a proxy of abundance within each site. Ten species of small-sized rodents are reported for the area (Medellín and Equihua 1998); however, this study is only focused on the most common terrestrial species. We excluded from the analyses arboreal species (Nyctomys sumichrasti, Ototylomys phyllotis and Tylomys nudicaudatus). Oryzomys (= Handleyomys) species, were treated at the genus level because of inconsistent taxonomy in the region, and the non-invasive nature of this study (San-José et al. 2014).
Landscape metrics
We used a site-landscape approach (sensu Fahrig 2013), that is, response variables were recorded in the 12 forest sites, and landscape variables were measured in the surrounding landscape containing each site. We considered all sites as independent samples because they were separated from each other by at least 1.5 km (continuous forest sites were separated by at least 2.5 km), and small-sized rodents are known to have a very low vagility and are not likely to migrate between forest sites (McNab 1963; Maza et al. 1973). Independence also increased by measuring landscape predictors in non-overlapping landscape buffers (Eigenbrod et al. 2011). Note that the few landscapes that showed some spatial overlap were separated by large rivers, thus contributing to the independence between sites (Fig. 1). We used high resolution (10 m) satellite images from 2010 (SPOT 5) and 2016 (Sentinel 2A)—both sensors with similar radiometric characteristics (Hagolle et al. 2015)—to classify six different land-cover types using Spring 3.3 (Camara et al. 1996): old-growth forests, secondary forests, cattle pastures, annual crops (e.g., chili, corn), arboreal crops (e.g., rubber and palm plantations), and human settlements (e.g., houses, roads). Overall, classification accuracy was 80.0% in 2010 and 80.3% in 2016.
We then used Conefor software extensions (Saura and Torné 2009) to measure landscape metrics in 2010 and 2016. In particular, we measured two metrics of landscape composition (percentage of forest cover and matrix contrast), and two metrics of landscape configuration (number of forest patches and forest edge density). Forest cover included only old-growth forests, and matrix contrast (sensu Campbell et al. 2011) was the percentage of the matrix composed of open areas: cattle pastures, annual crops, and human settlements. Forest edge density was measured as the length of all old-growth forest edges divided by the total area of the landscape (m/ha; McGarigal et al. 2012). We selected these landscape predictors because: (1) rodents’ abundance in the region seems to be related to landscape forest cover and matrix contrast (San-José et al. 2014); (2) forest fragmentation can shape population movements, migration dynamics and diversity patterns (Malcolm 1995; Bennett and Saunders 2010); and (3) edge density may affect resource availability and predation/competition interactions (Mendes et al. 2015).
Since the response of species to landscape structure can be scale-dependent (San-José et al. 2014; Smith et al. 2011; Galán-Acedo et al. 2018), we assessed each landscape metric within seven different-sized radii (200- to 1400-m radius, at 200-m intervals) from the center of each forest site to assess the scale of landscape effect (see below). The 1400-m radius was the largest radius until a minimum overlap between two buffers started to appear, thus avoiding dependence (i.e., pseudoreplication) problems in our analyses (Eigenbrod et al. 2011). To include continuous forest sites in regression models, we considered these sites as having 100% forest cover, 0% matrix contrast, no fragmentation (number of forest patches = 1), no edges (edge density = 0) and no isolation (mean inter-patch distance = 0).
Data analyses
We first used repeated measures models to test for differences between the first and last sampling year (∆ = 2016 value − 2010 value) in the value of each landscape metric at the largest scale (1400 m radius). We then used a similar statistical procedure to test for differences in the abundance of each rodent species among the four survey years (2011, 2012, 2014, and 2016). To identify the temporal changes in rodent abundance we summed up the data of all traps from each site and year and tested for variations (i.e., 4 survey years) in the abundance of each species through time within each site using Spearman correlations (i.e., correlation between abundance and time). The correlation coefficient of each species within each site was used as a measure of the average tendency of change in abundance, as it indicates whether a given rodent population is increasing (rs > 0), decreasing (rs < 0) or shows no trend (rs ~ 0) through time in a given site. We then assessed the landscape drivers of these temporal changes in rodent abundance with generalized linear models. In particular, we related the obtained correlation coefficients in each site with the differences (∆) between years (2016–2010) in each landscape metric. A positive ∆ value indicates that a given metric is increasing through time in a given landscape, i.e., it shows a higher value in 2016 than in 2010, whereas a negative value indicates the opposite.
As we did not know a priori the landscape size that best predicts rodent responses to landscape patterns, we assessed the so-called “scale of effect” of each landscape metric following Jackson and Fahrig (2015). In brief, we used Spearman correlations to assess the association between each landscape metric (∆ values) and each response variable (changes in abundance) at each spatial scale (i.e., different-sized radii) to identify the scale that yields the strongest response-predictor relationship (i.e., the highest correlation coefficient; Table A1 in supplementary material). We then used a multimodel averaging approach (sensu Burnham and Anderson 2002) with generalized linear models to assess the effect of each landscape predictor (measured at the optimal scale; Table A1) on correlation coefficients with the ‘glmulti’ package (Calcagno and de Mazancourt 2010) for R version 3.3.3. To avoid multicollinearity problems in multiple models, we estimated the variance inflation factor (VIF) of each predictor using the ‘car’ package for R 3.3.3 (Fox and Weisberg 2011). VIF values for the number of forest patches (VIF = 13.0) and forest cover (VIF = 8.4) indicated collinearity between these two predictors (see Neter et al. 1996). Therefore, we excluded the former predictor from the analysis. For each response variable we constructed 8 models, which represent all combinations of three explanatory variables and the null model (which includes only the intercept). For each model, we calculated the Akaike’s Information Criterion corrected for small samples (AICc). We ranked the models from the best (i.e., lowest AICc) to the worst (i.e., highest AICc). We then calculated the sum of Akaike weights (∑wi) of each landscape predictor, i.e., the sum of Akaike weights of the models in which each variable appeared. This metric represents the probability that each variable is within the true best model, and it was used to weight model-averaged parameter estimates (Burnham and Anderson 2002). To be conservative, we considered that the variation (Δ) in a given landscape attribute through time was an important explanatory variable for a given change in rodent abundance if the following three criteria were met: (1) it showed a high sum of Akaike weights; (2) the model-averaged unconditional variance was lower than the model-averaged parameter estimate (Burnham and Anderson 2002); and (3) the complete model showed a high percentage of explained deviance (i.e., high goodness-of-fit; Crawley 2007).
Results
Landscape structure showed important changes through time in each forest site (Fig. A1). For example, while some landscapes lost up to 10% of forest cover between 2010 and 2016, others actually showed an increase in forest cover of up to 40%. Thus, when averaged across all landscapes, we did not find significant differences in landscape metrics between the 2 years (Table 1). Yet, mean matrix contrast tended to increase over time, mostly due to the expansion of annual crops, such as corn.
We recorded four species of small terrestrial rodents: Heteromys desmarestianus, Sigmodon toltecus, Peromyscus mexicanus and Oryzomys sp. in all years. In total, we collected 76 individuals in 2011, 82 in 2012, 49 in 2014 and 153 in 2016. The abundance of three species (H. desmarestianus, Oryzomys sp., and P. mexicanus) showed a significant temporal trend, but populations of S. toltecus remained stable through time (Table 2, Fig. A2 in supplementary material). In particular, the abundance of H. desmarestianus and Oryzomys sp. tended to increase through time, whereas the abundance of P. mexicanus tended to decrease.
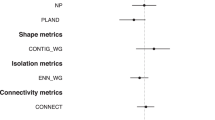
Considering the scale of effect of each landscape variable on each response (Table A1), we found strong associations between temporal changes in landscape structure and changes in rodent abundance (36–50% of explained deviance), with each rodent species showing different responses to changes in each landscape metric (Fig. 2; Table 3). In particular, the abundance of Oryzomys sp. increased mainly in sites with decreasing forest edge density (Fig. 2; Table 3; Table A2 in supplementary material). Yet, the abundance of H. desmarestianus increased in sites surrounded by increasing matrix contrast. The abundance of S. toltecus mainly decreased in landscapes suffering a decrease in matrix contrast. Finally, the abundance of P. mexicanus increased in landscapes exposed to increasing matrix contrast and decreasing edge density (Tables 3 and A2, Fig. 2).
Effect of temporal (2016–2010) differences in landscape structure on temporal trends in rodent abundance in the Lacandona rainforest, Mexico. We show the sum of Akaike weights (Σwi) of each landscape predictor (bars). The sign (±) of parameter estimates are indicated with different colors: grey bars for positive responses, and black bars for negative responses (see averaged parameters and associated unconditional variances in Table 3). We also indicate values of pseudo-R2, i.e., the percentage of explained deviance by complete models. Each landscape metric was measured at the optimal scale (see Table A1 in supplementary material)
Discussion
To our knowledge, the present study is the first in assessing the patterns and potential landscape drivers of rodent populations using a longitudinal approach, covering several sampling years and reproductive cycles. With such an approach, we were able to demonstrate that the Lacandona rainforest—a relatively new agricultural frontier in southeastern Mexico (Meli et al. 2015)—is suffering a rapid process of land-use change that promotes high spatial variations in landscape structure. Such variations in landscape structure lead to species-specific changes in the abundance of small rodents. Although total rodent abundance was two times higher in 2016 than in 2011, the mean number of individuals in all sites was very low, probably because the region has not suffered a significant defaunation process (Garmendia et al. 2013), allowing a top–down control of rodent populations by natural predators (Dirzo et al. 2014; Bovendorp et al. 2018). However, as discussed below, if current annual deforestation rates continue in the region (− 2.1% between 1990 and 2010; Courtier et al. 2012) we can expect significant changes in the abundance of small rodents in the near future, including the population increase of some species (winner species) and population decline (loser species) of others.
Our findings support previous studies on the contrasting responses of different rodent species to habitat disturbance (San-José et al. 2014). Whereas the abundance of H. desmarestianus and Oryzomys sp. increased through time in most sites, the abundance of P. mexicanus tended to decrease and populations of S. toltecus remained relatively stable through time. These population trends were strongly related to temporal changes in landscape structure in each site. For example, two out of four species (H. desmarestianus and P. mexicanus) increased their populations in forest sites that had experienced an increase in matrix contrast through time (i.e., higher percentage of open area in the matrix). As both species are forest specialists that mainly live in old-growth and secondary forests (Ceballos and Oliva 2005; Reid 2009), these patterns do not mean that they are benefited by increasing matrix contrast, but that individuals in landscapes with higher matrix contrast could be ‘forced’ to take refuge in the remaining forest patches, concentrating there. This ecological process has been demonstrated in other forest-specialist species, such as Australian tropical non-volant mammals (Laurance 1991) and howler monkeys (Arroyo-Rodríguez and Dias 2010). If this process is verified in future studies, these two species could be good candidates of ‘loser’ species (sensu Tabarelli et al. 2012), as it is well-known that forest-dependent species that avoid using the matrix are more prone to extinction in fragmented rainforests (Laurance 1991, 1994; Gascon et al. 1999; Vetter et al. 2011; Carrara et al. 2015). This is not only related to resource scarcity in forest patches, but also to lower connectivity in landscapes with higher matrix contrast, which may promote population isolation and a limited gene flow (Bennett and Saunders 2010; Mech and Hallett 2001).
The abundance of three species (Oryzomys sp., H. desmarestianus and P. mexicanus) tended to increase in forest sites located in landscapes with decreasing forest edge density. This is consistent with Pardini (2004), who found a negative response of small terrestrial mammals to increasing forest edges in the Brazilian Atlantic forest. Pfeifer et al. (2017) also show that many forest-interior specialist species, including amphibians, reptiles, birds, and mammals, respond negatively to forest edges, reaching peak abundances only at sites farther than 200–400 m from sharp high-contrast forest edges. Such negative response can be related to both abiotic and biotic changes along forest edges. For example, forest temperature can increase at forest edges, especially in fragmented rainforests (Arroyo-Rodríguez et al. 2017), potentially making basic activity patterns, such as feeding and traveling, more energetically costly (Tuff et al. 2016). Also, predation pressures may be higher at forest edges, because these habitats are exposed to higher light incidence than forest interiors, making preys more easily located by predators at edges than interiors (e.g., Barbaro et al. 2013, and references therein). Whatever the proximate cause of these negative edge effects, these patterns support the hypothesis that H. desmarestianus and P. mexicanus may represent ‘loser’ species.
Besides forest-dependence and specialization, other species’ ecological attributes and life history traits can help to explain the observed rodent responses to landscape structure. For example, extinction risk in human-modified landscapes has also been inversely related to reproductive rate, and directly associated with life span extension (Laurance 1991; Terborgh et al. 2001; Vetter et al. 2011). In this sense, both potentially ‘loser’ species (i.e., H. desmarestianus and P. mexicanus) have relatively lower reproductive rates and longer life expectancies, whereas the potentially ‘winner’ species (Oryzomys sp. and S. toltecus) have higher reproductive rates and lower life expectancies (Ceballos and Oliva 2005; Reid 2009). Additionally, H. desmarestianus feeds mainly on seeds and fruits, which could further increase its vulnerability to disturbance, compared with the other three species (all omnivores), as frugivory has been related with a higher sensitivity to habitat disturbance in fragmented landscapes (Laurance 1991).
Species traits can also influence the scale of landscape effect, i.e., the landscape size that best predicts rodent responses to landscape structure (Jackson and Fahrig 2015). In particular, the scale of effect is expected to be higher in species with greater vagility, as they are expected to interact with its environment across larger spatial scales (Miguet et al. 2016). Here, the scale of effect varied from 200 to 1400 m radius, with Oryzomys sp. showing the lowest scale of effect, followed by H. desmarestianus, S. toltecus and P. mexicanus (Table A1). Following Miguet et al. (2016), this finding suggests that Oryzomys sp. may have lower vagility, which is reasonably expected for a generalist species that is able to find resources in both conserved and disturbed forests (Medellín and Equihua 1998). In other words, Oryzomys sp. probably does not need to move much to find enough resources, at least compared to P. mexicanus, which is a forest-specialist species, and probably needs to move more frequently or larger distances to find adequate resources in the landscape. Unfortunately, we found no information on the ranging behavior or dispersal distances of these species, and thus, this represents an interesting avenue for future research.
Conclusions and conservation implications
The Lacandona rainforest is suffering a rapid process of land-use change, which is spatially variable (Fig. A1). Such spatial variations in landscape changes, however, did not result in a generalized increase in rodent abundance (i.e., ‘rodentization’, sensu Mendes 2014), but rather lead to species-specific responses to landscape structure. This can be related to the relatively short period of deforestation in the region (< 50 years ago), the relatively high amount of forest cover (~ 40%), and a highly heterogeneous matrix that includes different land covers, many of which can be used by small rodents as temporal or permanent habitat (Medellín 1994; Medellín and Equihua 1998). Whatever the cause of this pattern, it represents ‘good news’, as it suggests that the biotic interactions and ecological processes in which rodents are involved (e.g., seed dispersal, seed predation) have not suffered great alteration in the region. Yet, we cannot reject the hypothesis that populations of some rodent species will increase in the future, as the low values of final model-averaged parameter estimates (β estimates) suggest that the impact of landscape spatial changes on rodent population dynamics act at very low rates. In other words, it seems that the impact of landscape spatial changes on rodent communities will only be fully appreciated at the long-term.
In particular, if current land-use changes are maintained in the region, we can expect a population decline of forest specialist species (P. mexicanus and H. desmarestianus), and an increase in habitat generalist species (S. toltecus and Oryzomys sp.). H. desmarestianus showed an increase in abundance (not a decrease, as expected), but such increase seems to be ‘forced’ by increasing matrix contrast in the local landscape (see above). Regarding S. toltecus, their populations remained without significant changes, but given the tolerance of this species to human-induced disturbances (Medellín and Equihua 1998), they would probably benefit from future landscape disturbance.
Based on the empirical evidence available to date for this biodiversity hotspot (Medellín 1994; Medellín and Equihua 1998; San-José et al. 2014), preventing the expansion of open areas (e.g., cattle pastures and annual crops) in the region seems to be crucial for increasing landscape connectivity and resource availability for forest-dwelling rodent species, such as P. mexicanus and H. desmarestianus. Our findings also support the importance of preventing potential negative edge effects (Pardini 2004; Pfeifer et al. 2017), for example, by increasing forest cover (and thus, the amount of core areas) in the landscape. Although the percentage of forest cover showed a weaker effect than other landscape metrics, it showed a positive effect on both forest-specialist species, thus highlighting the importance of stopping forest loss and increasing forest cover through passive or active restoration.
Data availability
Data used in this study are archived at Figshare (https://doi.org/10.6084/m9.figshare.6171623).
References
Andresen E, Arroyo-Rodríguez V, Escobar F (2018) Tropical biodiversity: the roles of biotic interactions in its origin, maintenance, function and conservation. In: Dáttilo W, Rico-Gray V (eds) Ecological networks in the tropics. Springer, New York, pp 1–13
Arriaga L, Espinoza J, Aguilar C et al (2000) Regiones terrestres prioritarias de México. CONABIO, Mexico City
Arroyo-Rodríguez V, Dias PAD (2010) Effects of habitat fragmentation and disturbance on howler monkeys: a review. Am J Primatol 72:1–16
Arroyo-Rodríguez V, Rojas C, Saldaña-Vázquez RA et al (2016) Landscape composition is more important than landscape configuration for phyllostomid bat assemblages in a fragmented biodiversity hotspot. Biol Conserv 198:84–92
Arroyo-Rodríguez V, Saldaña-Vázquez RA, Fahrig L, Santos BA (2017) Does forest fragmentation cause an increase in forest temperature? Ecol Res 32:81–88
Banks-Leite C, Pardini R, Tambosi LR et al (2014) Using ecological thresholds to evaluate the costs and benefits of set-asides in a biodiversity hotspot. Science 345:1041–1045
Barbaro L, Giffard B, Charbonnier Y et al (2013) Bird functional diversity enhances insectivory at forest edges: a transcontinental experiment. Divers Distrib 20:149–159
Bennett AF, Saunders DA (2010) Habitat fragmentation and landscape change. In: Sodhi NS, Ehrlich PR (eds) Conservation biology for all. Oxford University Press, Oxford, pp 88–106
Bovendorp RS, Brum FT, McCleery RA et al (2018) Defaunation and fragmentation erode small mammal diversity dimensions in tropical forests. Ecography. https://doi.org/10.1111/ecog.03504
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Springer, New York
Calcagno V, de Mazancourt C (2010) Glmulti: an R package for easy automated model selection with (generalized) linear models. J Stat Soft 34:1–29
Camara G, Souza RCM, Freitas UM et al (1996) SPRING: integrating remote sensing and GIS by object-oriented data modelling. Comp Graph 20:395–403
Campbell RE, Harding JS, Ewers RM et al (2011) Production land use alters edge response functions in remnant forest invertebrate communities. Ecol Appl 21:3147–3161
Carabias J, de la Maza J, Cadena R (2015) Conservación y desarrollo sustentable en la Selva Lacandona. 25 años de actividades y experiencias. Natura y Ecosistemas Mexicanos, Mexico City
Carrara E, Arroyo-Rodríguez V, Vega-Rivera JH et al (2015) Impact of landscape composition and configuration on forest specialist and generalist bird species in the fragmented Lacandona rainforest, Mexico. Biol Conserv 184:117–126
Ceballos G, Oliva G (2005) Los mamíferos silvestres de México. CONABIO & Fondo de Cultura Económica, Mexico City
Courtier S, Núñez JM, Kolb M (2012) Measuring tropical deforestation with error margins: a method for REDD monitoring in south-eastern Mexico. In: Sudarshana P (ed) Tropical forests. InTech, Shanghai, pp 269–296
Crawley MJ (2007) The R book. Wiley, London
del Castillo RF (2015) A conceptual framework to describe the ecology of fragmented landscapes and implications for conservation and management. Ecol Appl 25:1447–1455
Dirzo R, Mendoza E, Ortiz P (2007) Size-related differential seed predation in a heavily defaunated Neotropical rainforest. Biotropica 39:355–362
Dirzo R, Young HS, Galetti M et al (2014) Defaunation in the Anthropocene. Science 345:401–406
Eigenbrod F, Hecnar SJ, Fahrig L (2011) Sub-optimal study design has major impacts on landscape-scale inference. Biol Conserv 144:298–305
Fahrig L (2003) Effects of habitat fragmentation on biodiversity. Annu Rev Ecol Evol Syst 34:487–515
Fahrig L (2013) Rethinking patch size and isolation effects: the habitat amount hypothesis. J Biogeogr 40:1649–1663
Fahrig L, Merriam G (1994) Conservation of fragmented populations. Conserv Biol 8:50–59
Fahrig L, Baudry J, Brotons L et al (2011) Functional landscape heterogeneity and animal biodiversity in agricultural landscapes. Ecol Lett 14:101–112
FAO (2016) State of the World’s Forests 2016. Forests and agriculture: land-use challenges and opportunities. FAO, Rome
Fox J, Weisberg S (2011) An R companion to applied regression, 2nd edn. SAGE publications Inc, Thousand Oaks
Fryxell JM, Falls JB, Falls EA, Brooks RJ (1998) Long-term dynamics of small-mammal populations in Ontario. Ecology 79:213–225
Galán-Acedo C, Arroyo-Rodríguez V, Estrada A et al (2018) Drivers of the spatial scale that best predict primate responses to landscape structure. Ecography 41:1–11
Galetti M, Dirzo R (2013) Ecological and evolutionary consequences of living in a defaunated world. Biol Conserv 163:1–6
Galetti M, Bovendorp R, Guevara R (2015) Defaunation of large mammals leads to an increase in seed predation in the Atlantic forests. Global Ecol Conserv 3:824–830
Garmendia A, Arroyo-Rodríguez V, Estrada A et al (2013) Landscape and patch attributes impacting medium- and large-sized terrestrial mammals in a fragmented rain forest. J Trop Ecol 29:331–344
Gascon C, Lovejoy TE, Bierregaard ROJ et al (1999) Matrix habitat and species richness in tropical forest remnants. Biol Conserv 91:223–229
Gibson L, Lynam AJ, Bradshaw CJA et al (2013) Near-complete extinction of native small mammal fauna 25 years after forest fragmentation. Science 341:1508–1510
Gorresen M, Willig MR (2004) Landscape responses of bats to habitat fragmentation in Atlantic forest of Paraguay. J Mamm 85:688–697
Hagolle O, Sylvander S, Huc M et al (2015) SPOT-4 (Take 5): simulation of Sentinel-2 time series on 45 large sites. Remote Sens 7:12242–12264
Howe HF, Davlantes J (2017) Waxing and waning of a cotton rat (Sigmodon toltecus) monoculture in early tropical restoration. Trop Conserv Sci 10:1–11
Instituto Nacional de Ecología (2000) Programa de Manejo Reserva de la Biósfera Montes Azules. Secretaría de Medio Ambiente y Recursos Naturales, Mexico City
Isabirye-Basuta G, Kasenene JM (1987) Small rodent populations in selectively felled and mature tracts of Kibale Forest, Uganda. Biotropica 19:260–266
Jackson HB, Fahrig L (2015) Are ecologists conducting research at the optimal scale? Global Ecol Biogeogr 24:52–63
Keesing F, Young TP (2014) Cascading consequences of the loss of large mammals in an African Savanna. Bioscience 64:487–495
Laurance WF (1991) Ecological correlates of extinction proneness in Australian tropical rain forest mammals. Conserv Biol 5:79–89
Laurance WF (1994) Rainforest fragmentation and the structure of small mammal communities in tropical Queensland. Biol Conserv 69:23–32
Lira PK, Ewers RM, Banks-Leite C, Pardini R, Metzger JP (2012) Evaluating the legacy of landscape history: extinction debt and species credit in bird and small mammal assemblages in the Brazilian Atlantic Forest. J Appl Ecol 49:1325–1333
Malcolm JR (1995) Forest structure and the abundance and diversity of Neotropical small mammals. In: Lowman MD, Nadkarni NM (eds) Forest canopies. Academic Press, San Diego, pp 179–197
Maza BG, French NR, Aschwanden AP (1973) Home range dynamics in a population of heteromyid rodents. J Mamm 54:405–425
McGarigal K, Cushman SA, Ene E (2012) FRAGSTATS v4: spatial pattern analysis program for categorical and continuous maps. http://www.umass.edu/landeco/research/fragstats/fragstats.html. Accessed 07 November 2018
McNab BK (1963) Bioenergetics and the determination of home range size. Am Nat 97:133–140
Mech SG, Hallett JG (2001) Evaluating the effectiveness of corridors: a genetic approach. Conserv Biol 15:467–474
Medellín R (1994) Mammal diversity and conservation in the Selva Lacandona, Chiapas, Mexico. Conserv Biol 8:780–799
Medellín R, Equihua W (1998) Mammal species richness and habitat use in rainforest and abandoned agricultural fields in Chiapas, Mexico. J Appl Ecol 35:13–23
Meli P, Hernández-Cárdenas G, Carabias J et al (2015) La deforestación de los ecosistemas naturales en Marqués de Comillas. In: Carabias J, de la Maza J, Cadena R (eds) Conservación y desarrollo sustentable de la Selva Lacandona. Natura y Ecosistemas Mexicanos A.C, Mexico City, pp 247–259
Melo F, Arroyo-Rodríguez V, Fahrig L et al (2013) On the hope for biodiversity-friendly tropical landscapes. Trends Ecol Evol 28:462–468
Mendes C (2014) Patch size, shape and edge distance influences seed predation in a keystone palm in tropical rainforests. MS Thesis, Universidade Estadual Paulista, Instituto de Biociencias de Rio Claro, Rio Claro
Mendes C, Ribeiro MC, Galetti M (2015) Patch size, shape and edge distance influence seed predation on a palm species in the Atlantic forest. Ecography 38:1–11
Miguet P, Jackson HB, Jackson ND, Martin AE, Fahrig L (2016) What determines the spatial extent of spatial landscape effects on species? Landsc Ecol 31:1177–1194
Neter J, Kutner MH, Nachtshein CJ et al (1996) Applied linear statistical models, 4th edn. McGraw-Hill/Irwin, New York
Pardini R (2004) Effects of forest fragmentation on small mammals in an Atlantic Forest landscape. Biodivers Conserv 13:2567–2586
Pardini R, Bueno AA, Gardner TA et al (2010) Beyond the fragmentation threshold hypothesis: regime shifts in biodiversity across fragmented landscapes. PLoS ONE 5:e13666
Pfeifer M, Lefebvre V, Peres CA et al (2017) Creation of forest edges has a global impact on forest vertebrates. Nature 551:187–191
Reid FA (2009) A field guide to the mammals of Central America & Southeast Mexico. Oxford University Press, New York
Rosin C, Poulsen JR (2016) Hunting-induced defaunation drives increased seed predation and decreased seedling establishment of commercially important tree species in an Afrotropical forest. Forest Ecol Manage 382:206–213
San-José M, Arroyo-Rodríguez V, Sánchez-Cordero V (2014) Association between small rodents and forest patch and landscape structure in the fragmented Lacandona rainforest, Mexico. Trop Conserv Sci 7:403–422
Saura S, Torné J (2009) Conefor Sensinode 2.2: a software package for quantifying the importance of habitat patches for landscape connectivity. Environ Mod Soft 24:135–139
Silvy NJ, López RR, Peterson MJ (2005) Wildlife marking techniques. In: Braun EE (ed) Research and management techniques for wildlife and habitats. The Wildlife Society Inc., Bethesda, pp 339–376
Smith AC, Fahrig L, Francis CM (2011) Landscape size affects the relative importance of habitat amount, habitat fragmentation, and matrix quality on forest birds. Ecography 34:103–113
Tabarelli M, Peres CA, Melo FPL (2012) The ‘few winners and many losers’ paradigm revisited: emerging prospects for tropical forest biodiversity. Biol Conserv 155:136–140
Terborgh J, López L, Nuñez P et al (2001) Ecological meltdown in predator-free forest fragments. Science 294:1923–1926
Trujano-Álvarez AL, Álvarez-Castañeda ST (2010) Peromyscus mexicanus (Rodentia: Cricetidae). Mamm Species 42:111–118
Tuff KT, Tuff T, Davies KF (2016) A framework for integrating thermal biology into fragmentation research. Ecol Lett 19:361–374
Vetter D, Hansbauer MM, Végvári Z et al (2011) Predictors of forest fragmentation sensitivity in Neotropical vertebrates: a quantitative review. Ecography 34:1–8
Wolff PW, Sherman PW (2007) Rodent societies: an ecological & evolutionary perspective. The University of Chicago Press, Chicago
Young HS, McCauley DJ, Dirzo R et al (2015) Context-dependent effects of large-wildlife declines on small-mammal communities in central Kenya. Ecol Appl 25:348–360
Acknowledgements
We thank financial support provided by PAPIIT-DGAPA, UNAM (Grant IN-204215), CONACyT (Project 2015-253946), and Rufford Small Grants (No. 22049-1). N.P.A.P. obtained a graduate scholarship from CONACyT. This paper constitutes a partial fulfillment of the PhD program of the Posgrado en Ciencias Biológicas, Universidad Nacional Autónoma de México (UNAM). V.A.-R. thanks PASPA-DGAPA-UNAM for funding his sabbatical stay at the Geomatics and Landscape Ecology Laboratory, Carleton University. The Instituto de Investigaciones en Ecosistemas y Sustentabilidad, UNAM, provided logistical support. We thank Carlos Palomares Magaña (Escuela Nacional de Estudios Superiores Morelia, UNAM) for his technical support in GIS. H. Ferreira, A. Valencia and A. López also provided technical support. Three anonymous reviewers provided valuable insights on the manuscript. Livia León Paniagua (Faculty of Sciences, UNAM) and IDEA WILD provided some Sherman traps. We thank the landowners from the Marqués de Comillas region (Ixcán, Loma Bonita, Chajul, Pirú, Reforma, Galacia, Flor de Marqués), for allowing us to collect data on their properties, as well as the Montes Azules Biosphere Reserve, Natura y Ecosistemas Mexicanos A.C., and the National Commission of Natural Protected Areas (CONANP). A special acknowledgement to Audón Jamangapé and his family, as this study would not have been possible without their assistance in the field.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no other conflict of interest.
Ethical approval
This research adhered to national and international guidelines for the treatment of research animals, and was conducted in accordance with the legal requirements of the National Autonomous University of Mexico (UNAM), and the country of Mexico. We assured the welfare of all rodents captured in the study. We were granted access to the study sites by local communities, landowners, and the Montes Azules Biosphere Reserve, part of the National Commission of Natural Protected Areas of Mexico (CONANP).
Additional information
Communicated by Akihiro Nakamura.
This article belongs to the Topical Collection: Forest and plantation biodiversity.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Arce-Peña, N.P., Arroyo-Rodríguez, V., San-José, M. et al. Landscape predictors of rodent dynamics in fragmented rainforests. Biodivers Conserv 28, 655–669 (2019). https://doi.org/10.1007/s10531-018-1682-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-018-1682-z