Abstract
When a forest is fragmented, this increases the amount of forest edge relative to the interior. Edge effects can lead to loss of animal and plant species and decreased plant biomass near forest edges. We examined the influence of an anthropogenic forest edge comprising cattle pasture, coconut plantations, and human settlement on the mantled howler (Alouatta palliata), white-faced capuchin (Cebus capucinus), Central American spider monkey (Ateles geoffroyi), and plant populations at La Suerte Biological Research Station (LSBRS), Costa Rica. We predicted that there would be lower monkey encounter rate, mean tree species richness, and diameter at breast height (DBH) in forest edge versus interior, and that monkeys would show species-specific responses to edge based on diet, body size, and canopy height preferences. Specifically, we predicted that howler monkeys would show positive or neutral edge effects due to their flexible folivorous diet, large body size, and preference for high canopy, capuchins would show positive edge effects due to their diverse diet, small body size, and preference for low to middle canopy, and spider monkeys would show negative edge effects due their reliance on ripe fruit, large body size, and preference for high upper canopy. We conducted population and vegetation surveys along edge and interior transects at LSBRS. Contrary to predictions, total monkey encounter rate did not vary between the forest edge and forest interior. Furthermore, all three species showed neutral edge effects with no significant differences in encounter rate between forest edge and interior. Interior transects had significantly higher mean tree species richness than edge transects, and interior trees had greater DBH than edge trees, although this difference was not significant. These results suggest that forest edges negatively impact plant populations at La Suerte but that the monkeys are able to withstand these differences in vegetation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Forest fragmentation increases the amount of forest edge relative to interior. Forest edges represent boundaries, where one habitat type with its own distinct array of vegetation and inorganic characteristics grades into a habitat with diverse inorganic and organic attributes (Lovejoy et al. 1986). Forest edges occur naturally; for example, rivers naturally separate portions of otherwise continuous forests (Laurance 1991). However, human influence has dramatically altered natural landscapes, resulting in the large-scale creation of abrupt forest edges in habitats worldwide (Saunders et al. 1991). Such edges often occur at the margins of protected areas, where primary forest transitions sharply to farmland, road, or logged area (Stevens and Husband 1998; Lenz et al. 2014). Forest edges typically show differences from forest interior in soil type, moisture, sunlight, wind speed, and soil and air temperature, which lead to changes in both plant and animal species composition (Lovejoy et al. 1986; Harris 1988; Saunders et al. 1991; Mbora and Meikle 2004; Arroyo-Rodriguez and Mandujano 2006, 2009; Lehman et al. 2006a). Some ubiquitous and/or photophilic plant species may appear in greater concentration in edge zones (e.g., Pollock et al. 2017); however, overall edge effects can lead to the loss of animal and plant species, with both typically showing decreased biomass near forest edges (Estrada et al. 1999; Arroyo-Rodriguez and Mandujano 2006). A recent examination of global forest cover shows that 20% of the world’s forests lie within 100 m from an edge (Haddad et al. 2015), highlighting the urgency for research on the effects of edges on plant and animal communities.
Many studies have focused on quantifying abiotic factors and plant species composition at forest edges (e.g., Lovejoy et al. 1986; Harris 1988; Laurance 1991; Laurance and Yensen 1991; Saunders et al. 1991), while others have explored the impact of edge effects on mammals (e.g., small mammals, Stevens and Husband 1998; Gibson et al. 2013; large mammals, Brodie et al. 2015; carnivores, Balme et al. 2010), including primates (Malagasy strepsirrhines, Lehman et al. 2006a, b, c; Lehman 2007; McGoogan 2011; Burke and Lehman 2014; Ramsay et al. 2017; Kenyan catarrhines, Mbora and Meikle 2004). However, few studies have focused on the relationship between edge effects and platyrrhine primate species (but see: Peruvian platyrrhines, Kulp and Heymann 2015; de Vries 2017; Brazilian platyrrhines, Lenz et al. 2014). Anthropogenically caused deforestation is one of the key threats reducing primate populations worldwide (Estrada et al. 2017). Although some primates demonstrate resilience and adaptability to anthropogenic threat (McLennan et al. 2017), more than 50% of all primate taxa are currently at risk of extinction due to human-induced habitat modification (Mittermeier et al. 2012). South and Central America harbor the most primate species of the four regions where primates are found and more than 60% of these primate populations are in decline (Estrada et al. 2017). Gaining a better understanding of how edges affect platyrrhines is therefore especially important.
It is critical to investigate the relationship between anthropogenic edge effects and primate populations in tropical environments, where the ecological effects of habitat fragmentation have been understudied compared to temperate regions (Martin et al. 2012). In the Neotropics, sympatric platyrrhine species with different dietary and ecological niches co-exist in an increasingly fragmented tropical forest landscape. In Costa Rica, for example, deforestation has increased over the past few decades, largely for conversion to banana and pineapple plantations (Garber et al. 2010). As a result of agricultural development, forests have become divided into disconnected patches separated by matrix (e.g., cultivated areas). Some primate species found outside of Costa Rica, including black howler (Alouatta pigra) and golden-bellied capuchin monkeys (Sapajus xanthosternus), are known to be adaptable to human-altered landscapes and may persist in modified environments including agroforestry plantations (Canale et al. 2013; Zárate et al. 2014). However, adequate food resources are not always available to primates in deforested matrix environments. In most cases, habitat fragmentation negatively impacts wildlife, constituting a major cause of declining primate populations (Arroyo-Rodriguez and Dias 2010).
Although platyrrhines are usually negatively affected by habitat fragmentation (Estrada and Coates-Estrada 1996; Estrada et al. 1999; Arroyo-Rodriguez and Dias 2010), we would expect anthropogenic forest edge environments to impact some Neotropical primate species more markedly than others based on differences in body size and dietary preferences (Lidicker 1999). The relationship between distance to the forest edge and level of preference for edge (tested as species density in forest edge vs. interior) has been investigated in primate taxa ranging from strepsirhines to platyrrhines to catarrhines, with varying results (e.g., Mbora and Meikle 2004; Lehman et al. 2006c; Lenz et al. 2014). Primates may respond to edge effects in ways that are (1) positive (i.e., increased density in forest edge compared to interior), (2) negative (i.e., decreased density in forest edge compared to interior), or (3) neutral (i.e., no difference in density between forest edge and interior) (Reis et al. 2004). Of the primate species that have been studied to date, positive edge effects have been found in species with diverse diets, possibly because of the greater number of potential food sources found at habitat intersections (Malagasy strepsirhines: rufous mouse lemur [Microcebus rufus], Milne-Edwards’ sifaka [Propithecus edwardsii], Lehman et al. 2006b, c; golden-brown mouse lemur [Microcebus ravelobensis]; Burke and Lehman 2014; platyrrhines: black-bearded sakis [Chiropotes chiropotes], golden-handed tamarins [Saguinus midas], Guianan brown capuchins [Cebus apella], Lenz et al. 2014). Negative edge effects have been found in large-bodied and/or highly frugivorous species, likely due to the absence in edges of the large, mature fruiting trees on which they rely (Lehman et al. 2006b; strepsirhines: gray mouse lemur [Microcebus murinus], Burke and Lehman 2014; greater dwarf lemur [Cheirogaleus major], Lehman et al. 2006c; rufous brown lemur [Eulemur rufus], Lehman 2007; Coquerel’s sifaka [Propithecus coquereli], McGoogan 2011; platyrrhines: Guianan spider monkey [Ateles paniscus], Lenz et al. 2014). Finally, positive or neutral edge effects have been found in folivores and folivore/frugivores, possibly due to their food trees containing more abundant young leaves and/or leaves having higher protein concentrations at forest edges (Chen et al. 1992; Ganzhorn 1995; strepsirhines: eastern woolly lemur [Avahi laniger], red-bellied lemur [Eulemur rubriventer], small-toothed sportive lemur [Lepilemur microdon], grey bamboo lemur [Hapalemur griseus], Lehman et al. 2006b, c, Lehman 2007; platyrrhines: red titi monkey [Callicebus cupreus], Kulp and Heymann 2015; Guianan red howler monkey [Alouatta macconelli], Lenz et al. 2014; catarrhines: Tana River red colobus [Procolobus rufomitratus], Mbora and Meikle 2004).
In the present study, we examine the relationship between anthropogenic edge effects and the density of wild mantled howler monkeys (Alouatta palliata), Central American spider monkeys (Ateles geoffroyi), and white-faced capuchin monkeys (Cebus capucinus) living in a fragmented tropical rainforest environment at the La Suerte Biological Research Station (LSBRS), Costa Rica. LSBRS is located in one of the top 25 global biodiversity hotspots (Myers et al. 2000), but in a region of Costa Rica that is increasingly deforested (Garber et al. 2010), making edge effects important to study. The way that all three of these sympatric monkey species interact with their forest habitat and respond to forest edges has important implications for conservation planning.
H1
Vegetation and primates show negative edge effects.
In this study, we predicted that there would be lower tree density, smaller mean tree diameter at breast height (DBH), less canopy cover, lower tree species richness, and lower monkey encounter rate overall in forest edge versus interior. These predictions are in line with past research suggesting that lower plant and animal biomass will be found at forest edges (Estrada et al. 1999; Arroyo-Rodriguez and Mandujano 2006).
H2
Primate species differ in response to edge effects.
We also expected to see species-level differences in monkey encounter rate at forest edge vs. interior at LSBRS, based on differences in body size, canopy height preferences, and dietary preferences between the three sympatric monkey species (Lidicker 1999). At LSBRS, protected rainforest sharply transitions to cattle pasture and coconut plantations (Molina 2015), meaning that minimal food is likely to be available to primates in the surrounding matrix. The mantled howler monkey, as a large-bodied folivore (Milton 1979), is expected to show neutral or positive edge effects, with higher encounter rate at forest edge and lower encounter rate in forest interior or equal encounter rates in both edge and interior. Due to their flexible diet, mantled howler monkeys have been able to persist in a wide variety of habitat types, ranging from undisturbed forest to habitats highly modified by humans (Estrada 2015; Garber and Kowalewski 2015). The greater availability of young, protein-rich leaves in edge habitat areas (Chen et al. 1992; Ganzhorn 1995) would promote howler monkey presence at habitat edge, but the lack of preferred large, mature trees, the lack of preferred high-canopy environments, and potential lack of preferred food species may minimize this advantage (Fleagle and Mittermeier 1980; Estrada 1984; Munoz et al. 2006). Previous research on other howler monkey species has shown positive edge effects (Lenz et al. 2014), suggesting that mantled howler monkeys may show the same trend.
The Central American spider monkey, a highly frugivorous and large-bodied primate (Ford and Davis 1992; Chapman et al. 1995), is expected to show negative edge effects, and should therefore have a lower encounter rate at forest edge and higher encounter rate in forest interior. Due to their large home and day range sizes (Klein and Klein 1977; Shimooka 2004, 2005), large body size (Glanz 1990), preference for the high upper canopy (Fleagle and Mittermeier 1980), and reliance on fruit from large, mature forest trees (van Roosmalen 1985), spider monkeys are one of the primates most vulnerable to habitat fragmentation (Peres and Dolman 2000; Defler et al. 2003; Stevenson et al. 2005; Boyle et al. 2009). Where edge effects have been tested in spider monkey species, they have been negative (Lenz et al. 2014), suggesting that Central American spider monkeys may show similar effects.
Finally, the white-faced capuchin monkey, as a small-bodied frugivore with a diverse diet (Ford and Davis 1992; Rose 1994), is expected to show positive edge effects, and should therefore have a higher encounter rate at forest edge and lower encounter rate in forest interior. Due to their extreme dietary adaptability, capuchins have been known to increase in number in some forest fragments, even as other primate species decrease in number and species richness (Cunha et al. 2006). Capuchins also show preference for the middle to lower forest canopy (Fleagle and Mittermeier 1980), suggesting that the potentially lower tree heights in forest edge zones would not negatively impact their foraging behavior. Because other capuchin species have shown positive edge effects (Lenz et al. 2014), we predict that the white-faced capuchin will demonstrate the same preference.
Methods
Study species
Mantled howler monkeys are large-bodied primates with adult weight ranging from 3.1 to 9.8 kg (Ford and Davis 1992). They live in groups of 1–40 individuals (di Fiore et al. 2011), and usually live in groups of greater than ten (Ryan et al. 2008). Howler monkeys including the mantled howler are traditionally known as the only folivorous New World monkeys, subsisting largely on leaves, but they include substantial amounts of fruit and flowers in their diets depending on resource availability (Glander 1982; Asensio et al. 2007; di Fiore et al. 2011). Howler monkeys are known to prefer young leaves, which are more protein-rich and lower in undesirable secondary compounds such as tannins (Milton 1979; Glander 1982; Estrada 1984).
Central American spider monkeys are similarly large-bodied primates with adult weight ranging from 6 to 9.4 kg (Ford and Davis 1992). They live in fission–fusion groups over large ranges (Klein and Klein 1977; Shimooka 2005). As preferential frugivores, their diet may comprise entirely fruit, or be supplemented with flowers and/or leaves (Chapman et al. 1995).
White-faced capuchins are small-bodied primates with adult weight ranging from 2.6 to 3.9 kg (Ford and Davis 1992). They live in groups of 9–20 individuals (Perry 1997; Rose 1997). Capuchins are mostly frugivorous, but have a wide-ranging and varied diet supplemented by insects, young leaves, flowers, eggs, and small vertebrates (Freese 1983; Rose 1994, 1997; Cunha et al. 2006).
Study site and monkey population
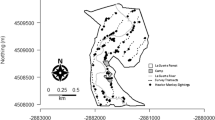
We conducted this study at the La Suerte Biological Research Station (LSBRS) in northeastern Costa Rica (10°26′N, 83°46′W). LSBRS is a tropical lowland rainforest totaling approximately 3 km2 of primary forest, secondary forest, and regenerating pastures (Pruetz and Leasor 2002; Garber et al. 2010). The main forested area where we conduct research comprises two connected forest patches (“Large Forest” = 0.935 km2 and “Small Forest” = 0.35 km2) as well as a partially cleared area for “camp” (0.071 km2) (Molina 2015). A reforestation project began in 2005 to build a corridor to connect the two fragments (Garber et al. 2010). LSBRS represents one of increasingly few forested areas in a region of Costa Rica that has been largely deforested since the 1970s, primarily due to cattle ranching and large-scale banana and pineapple production by major corporations (Garber et al. 2010; Molina 2015). Although primates are not hunted or otherwise directly threatened by humans at LSBRS or surrounding area to our knowledge, abrupt forest edges exist between many areas of LSBRS and the neighboring properties, with barbed wire fences marking the sharp transitions between protected rainforest, cattle pasture, and coconut plantations (Molina 2015). Coconut trees are not a food source for these monkey populations to our knowledge, with no feeding observed since our research in this forest began in 2009 (Schreier, unpublished data). The distinct forest edges at LSBRS, with minimal primate food resources outside forest boundaries, make LSBRS an ideal site at which to investigate the relationship between anthropogenic edge effects and the density of the three monkey species present.
Although relatively little systematic research has been conducted at the site with respect to primate population structure (Pruetz and Leasor 2002), past survey estimates suggest that the Small Forest contains two (Pruetz and Leasor 2002) or three (Garber et al. 2010) groups of mantled howler monkeys and one group of white-faced capuchin monkeys (Garber et al. 2010; Pruetz and Leasor 2002), while the Large Forest contains 6–8 (Garber et al. 2010) or 7–8 (Pruetz and Leasor 2002) groups of mantled howler monkeys and one (Garber et al. 2010) to three (Pruetz and Leasor 2002) groups of white-faced capuchin monkeys. One group of Central American spider monkeys ranges throughout the Large Forest (Pruetz and Leasor 2002; Garber et al. 2010).
Vegetation survey
We collected the data reported here on vegetation and population structure from May–August 2015 (vegetation and population surveys), May–July 2016 (population surveys), and May–June 2017 (vegetation surveys). These time periods all comprise the wet season at LSBRS, and therefore seasonal differences are not a confounding factor in our analyses. The primary vegetation data we recorded (i.e., tree species richness, DBH, and tree density) are not expected to vary considerably over a couple of years, and therefore can effectively be compared to monkey population data collected during the same months of 2015 and 2016.
We conducted vegetation surveys along the forest edge (within 100 m of the forest edge) and interior transects (more than 100 m from forest edge). Each transect was 50 m × 5 m; we aimed to distribute them evenly throughout the Large and Small Forests and camp. Overall, we conducted 17 edge and 12 interior transects. We conducted ten edge and nine interior transects in the Large Forest (one transect per 0.049 km2), five edge and three interior transects in the small forest (one transect per 0.044 km2), and two transects around camp (all of camp is edge; one transect per 0.035 km2; Fig. 1). Along the entire transect and within 2.5 m of either side of the transect line, we recorded all trees with circumferences at breast height > 10 cm and identified tree species when possible. From these tree abundance and circumference data, we calculated tree density and mean DBH for each transect. We also determined tree species richness for transects located in the Large Forest. Calculating tree species richness in the Small Forest was not possible due to time constraints and the very high number of trees and tree species there. We also estimated tree cover using a point-sampling method. At each 1-m interval, we estimated tree cover by looking straight above and assigning a score of 1–4 (1 = 0–25% coverage, 2 = 26–25% coverage, 3 = 51–75% coverage, and 4 = 76–100% coverage). Because our data were not normally distributed (Shapiro–Wilk test of normality), we analyzed our vegetation data via non-parametric statistical tests. We compared mean tree species richness, mean DBH, and mean tree density across edge and interior vegetation transects using Mann–Whitney U tests. We used SPSS version 23 for all vegetation analyses and significance was set at p < 0.05.
Population survey
Following Pruetz and Leasor (2002), we conducted a systematic primate population survey using 11 line transects that were spaced approximately 150 m apart (Fig. 1). Transect lengths ranged from 0.26 to 1.29 km. One transect was walked ten times and abandoned after 2015 due to safety concerns. The other ten transects were walked 19–34 times each during the study period, with variation in number of times walked due to transect accessibility (e.g., some transects were inaccessible for several days after heavy rains due to flooding; some individual transect surveys were started, and then abandoned when heavy rains began). We walked a total of 156.7 km in transect length. In order to reduce destruction to the habitat, we used existing trails as the basis for our transects as much as possible, with minimal off-trail area included. We independently walked the transects at a speed of approximately 1.5 km/h (Peres 1999), stopping every 100 m for 2 min to conduct a detailed search for monkey species (Pruetz and Leasor 2002). Upon encountering any monkey species, we recorded the time and location (trail marker and GPS point using a Garmin GPSMAP 62 s Handheld GPS Navigator). Each of these records represents a single monkey group (Pruetz and Leasor 2002). We conducted line transects throughout daylight hours, but primarily during the morning (06:00–10:00) and afternoon (14:00–18:00) to correspond with periods of peak monkey activity (Peres 1999). We rotated transects to minimize observer bias (Peres 1999) and alternated daily between sampling odd-numbered and even-numbered transects to avoid re-sampling the same monkey groups on the same day.
To compare group encounter rates (with group encounter rate being a proxy for monkey density) between the edge and interior, we fit four generalized linear mixed models (GLMM): one for each of the three monkey species and one combining all monkey sightings together. In each model, we assumed the number of encounters on each transect walk followed a Poisson distribution whose log mean depended on forest location (edge or interior) as a fixed effect and transect as a random effect. Each model also used a constant offset term to account for differing effort on transects of different length. We fit models with the lme4 package (Bates et al. 2015) in R version 3.3.2 (R Core Team 2016). We assessed differences in encounter rate between forest edge and interior by examining overlap of 95% confidence intervals.
Results
Vegetation survey
As predicted, mean tree species richness was significantly higher in the interior (mean = 6.1 trees, SD = 2.1) than the edge (mean = 3.8 trees; SD = 1.8; U = 22.5, p = 0.038). Mean tree DBH in interior transects (34.1 cm, SD = 29.6) was higher than mean DBH in the edge (22.4 cm, SD = 11.6), but this difference was not statistically significant (U = 75.0, p = 0.245). Mean tree cover was also higher in the interior than the edge; 92.0% of 1-m intervals in the interior had between 51 and 100% cover compared with 73.6% of 1-m intervals in the edge. Contrary to predictions, there was no difference in mean tree density between the edge (0.11/mm2, SD = 0.10) and the interior (0.08/m2, SD = 0.07; U = 92.5, p = 0.679).
Population survey
Contrary to our predictions, total monkey encounter rate did not vary between the forest edge (1.17 groups/km, CI: 0.85, 1.62) and forest interior (1.15 groups/km, CI: 0.85, 1.57; p = 0.933; Figs. 2, 3). We did, however, find species-level differences in monkey encounter rate at forest edge vs. interior, but not consistently in the predicted directions. As predicted, howler monkeys showed neutral edge effects. Howler encounter rate was slightly higher in the interior (0.86 groups/km, CI: 0.62, 1.19) than the edge (0.69 groups/km, CI: 0.47–1.01), but this difference was not significant (p = 0.35; Fig. 2). Contrary to predictions, capuchin and spider monkeys also displayed neutral edge effects, with no significant differences between encounter rates in the edge vs. interior. Capuchin encounter rate in the edge was 0.50 groups/km (CI: 0.32, 0.80) and in the interior was 0.37 groups/km (CI: 0.22, 0.62; p = 0.33). Spider monkey encounter rate in the edge (0.29, CI: 0.16, 0.51) was almost identical to that in the interior (0.29, CI: 0.18, 0.50; p = 0.93).
Discussion
Our hypothesis (H1) that there would be lower tree species richness, smaller tree DBH and less canopy cover in the forest edge vs. interior was partially supported, in that interior environments showed higher tree species richness and mean canopy cover. However, there was no difference in monkey encounter rate between the edge and interior. Thus, there were some negative edge effects for vegetation but not for the overall primate community at La Suerte.
With respect to hypothesis (H2), our predictions about individual species’ responses to the edge were supported for one species, but not for the others. As predicted, mantled howler monkey encounter rate did not vary between the forest edge and interior, therefore showing neutral edge effects. This finding contrasts with results from mantled howler monkeys in Costa Rica’s Osa Peninsula, which were observed more frequently in the forest edge compared to the interior (Skrinyer 2016) as well as the Guianan red howler monkey (Lenz et al. 2014), which was found to prefer edge environments. Our results thus bely the idea that howler monkeys, due to their flexible diet, are so resilient to anthropogenic habitat modification that they favor edge environments (Garber et al. 2006; McKinney et al. 2015; Marsh et al. 2016). However, it may be that mantled howler monkeys are able to thrive in some types of edge habitat while not gaining any particular advantage from others. In the Osa Peninsula, edge habitats surveyed by Skrinyer (2016) included natural edge (e.g., two transects—beach and river) as well as agricultural anthropogenic edge (two transects), with only one edge transect bordering a habitat with minimal vegetation (road edge along fences). Similarly, Lenz et al. (2014) surveyed two lightly logged primary forest transects as edge habitat, which likely had more transitional matrix and thus more primate food sources than the forest edge at LSBRS. At LSBRS, forest edges show a sharp transition to road and/or cattle grazing land with minimal food sources available in the surrounding matrix, possibly driving the neutral edge response of howler monkeys in this environment.
Recent research suggests that howler monkeys are in fact vulnerable to the effects of habitat fragmentation (Arroyo-Rodriguez and Dias 2010). Their diet is species-selective and grazing occurs exclusively on trees, with only 27 species from 15 families fed upon in one Mexican rainforest setting (Estrada 1984). Mantled howler monkeys have been reported to preferentially use trees with larger DBH than the average DBH in forest fragments (Munoz et al. 2006), which may explain why we found no significant difference in howler monkey encounter rate in edge vs. interior at LSBRS. Howler monkeys may be drawn to the forest interior by their preference for the large, mature forest trees more commonly found there at LSBRS. Although tree height in forest edge vs. interior was not assessed in this study, previous research on the red howler monkey (Alouatta seniculus) has shown that howler monkeys prefer high canopy environments, which may also be more likely to be found in forest interior (Fleagle and Mittermeier 1980). Preliminary research on feeding behavior at LSBRS showed mantled howler monkeys feeding on trees from their most preferred plant family, Moraceae (Estrada 1984), at both edge and interior locations (Schlaht, unpublished data). Similarly, past research on mantled howler monkey diet at LSBRS found that monkey groups from both Large and Small forests were able to obtain adequate nutrition from all forest areas surveyed (Occhibove et al. 2015). Preferred howler monkey feeding trees are therefore present throughout the LSBRS site, which may explain the lack of preference for edge over interior at LSBRS.
In contrast to predictions, Central American spider monkeys and white-faced capuchin monkeys at LSBRS also displayed no difference in encounter rate between forest edge and interior, and therefore showed neutral edge effects. These findings contrast with results from the Guianan spider monkey and brown capuchin monkey (Lenz et al. 2014), which were found to prefer interior and edge environments, respectively. However, the Central American spider monkey was observed to use forest edge and interior equally in the Osa Peninsula, Costa Rica, which was attributed to the abundance of Attalea butryacea palm fruit in the edge (Skrinyer 2016). Our neutral edge results for these monkey species also correspond with our finding that there is no difference in tree density between the edge and interior at LSBRS, perhaps suggesting that tree density is more important than tree size or canopy height for these two species. Additionally, Central American spider monkeys may not show preference for edge or interior at LSBRS due to preferred foods, such as native lipid-rich fruiting trees like lemon guava (Psidium guajava) (Skrinyer 2016; Schlaht, unpublished data), being found throughout edge and interior regions. It is also possible that our neutral edge results are due to differences in spider monkey detection accuracy in edge and interior, with the smaller trees on the forest edge making it more likely that we would detect typically high-canopy spider monkeys if present. Further research is needed to investigate the full range of factors that may be contributing to the neutral edge response in the Central American spider monkey at LSBRS, including how fruiting tree species are distributed.
The white-faced capuchin monkey similarly did not show preference for forest edge or interior at LSBRS, and may not have done so due to the presence of diverse food sources throughout the site. As frugivores with highly varied diets (Freese 1983; Rose 1994, 1997; Cunha et al. 2006), capuchins may have been able to find a range of appropriate food sources throughout both edge and interior environments. Initial results regarding capuchin tree use at LSBRS show that although they rested in both edge and interior, they fed predominantly from interior trees (Schlaht, unpublished data). Their neutral edge response may therefore be attributed to an abundance of food sources in the interior. When the capuchins did feed on the edge, they did so from trees with larger DBH than those they fed from in the interior (Schlaht, unpublished data). The small body size of capuchins and their preference for low to middle forest canopy may have allowed them to move freely through both small and large trees in all forest zones, allowing them to target feeding trees throughout the forest. Further research is needed on food resource abundance and tree use at LSBRS to clarify the factors that may be influencing this neutral edge response in the white-faced capuchin monkey.
Overall, our results do not support Lidicker’s (1999) findings that animal species respond to forest fragmentation in different ways that may relate to their body size and dietary preferences. Our primate population surveys were conducted from June–August, a time of year encompassing the start of the wet season in northeastern Costa Rica. It is possible that population surveys conducted at other times of the year may yield different results for the edge and interior responses of each species. However, Chapman (1988) studied variation in ranging and foraging patterns in mantled howler monkeys, Central American spider monkeys, and white-faced capuchin monkeys during wet and dry seasons in Santa Rosa National Park, Costa Rica, and found no significant relationship between seasonality and feeding behavior. All monkey species were selective of food items and showed variation in range usage, but did not show seasonal differences (Chapman 1988). Seasonal differences, then, would not necessarily be expected to influence primate ranging patterns or primate preferences for edge vs. interior in our study. It is also important to note that our neutral edge preference results for all primate species may have been impacted by our sampling methods, with 11 short transects sampled multiple times to generate encounter rates. If our sampling methods had consisted of a larger number of longer transects sampled fewer times, our encounter rates may have been different. However, given the small size of forest fragments at LSBRS and limited area to walk the transects, this was unavoidable.
Our findings indicate that edge effects do not yet appear to negatively affect the monkey population at LSBRS. These neutral edge responses provide hope that primates and other wildlife populations may be able to withstand severe habitat fragmentation. As forests throughout Costa Rica and other tropical regions become increasingly fragmented, it is more and more important to understand the species-specific responses of primates to deforestation. The La Suerte region is experiencing additional anthropogenic pressure each year due to an increase in the number and size of banana and pineapple plantations, which eventually cannot fail to put additional stress on the monkey population. Given the ongoing deforestation of the wider region, it is critical to continue to monitor the monkeys over time to assess their continuing response to fragmentation.
References
Arroyo-Rodriguez V, Dias P (2010) Effects of habitat fragmentation and disturbance on howler monkeys: a review. Am J Primatol 72:1–16
Arroyo-Rodriguez V, Mandujano S (2006) Forest fragmentation modifies habitat quality for Alouatta palliata. Int J Primatol 27:1079–1096
Arroyo-Rodriguez V, Mandujano S (2009) Conceptualization and measurement of habitat fragmentation from the primates’ perspective. Int J Primatol 30:497–514
Asensio N, Cristobal-Azkarate J, Dias P, Vea J, Rodriguez-Luna E (2007) Foraging habits of Alouatta palliata mexicana in three forest fragments. Folia Primatol 78:141–153
Balme G, Slotow R, Hunter L (2010) Edge effects and the impact of non-protected areas in carnivore conservation: leopards in the Phinda-Mkhuze Complex, South Africa. Anim Conserv 13:315–323
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Boyle S, Lourenco W, Da Silva L (2009) Travel and spatial patterns change when Chiropotes satanas chiropotes inhabit forest fragments. Int J Primatol 30:515–531
Brodie J, Giordano A, Ambu L (2015) Differential responses of large mammals to logging and edge effects. Mammal Biol 80:7–13
Burke R, Lehman S (2014) Edge effects on morphometrics and body mass in two sympatric species of mouse lemurs in Madagascar. Folia Primatol 85:277–291
Canale G, Kierulff M, Chivers D (2013) A critically endangered capuchin monkey (Sapajus xanthosternos) living in a highly fragmented hotspot. In: Marsh L, Chapman C (eds) Primates in fragments. Springer, New York, pp 299–311
Chapman C (1988) Patterns of foraging and range use by three species of Neotropical primates. Primates 29:177–194
Chapman C, Wrangham R, Chapman L (1995) Ecological constraints on group size: an analysis of spider monkey and chimpanzee subgroups. Behav Ecol Sociobiol 36:59–70
Chen J, Franklin J, Spies T (1992) Vegetation response to edge environments in old-growth Douglas fir forests. Ecol Appl 2:387–396
Cunha A, Vieira M, Grelle C (2006) Preliminary observations on habitat, support use and diet in two non-native primates in an urban Atlantic forest fragment: the capuchin monkey (Cebus sp.) and the common marmoset (Callithrix jacchus) in the Tijuca forest, Rio de Janeiro. Urban Ecosyst 9:351–359
de Vries M (2017) How “edgy” are tamarins? A preliminary investigation of spatial variation in the behaviour of two sympatric callitrichids. Department of Anthropology, University of Toronto, Toronto
Defler T, Rodriguez J, Hernandez-Camacho J (2003) Conservation priorities for Colombian primates. Primate Conserv 19:10–18
di Fiore A, Link A, Campbell C (2011) The atelines: behavioral and sociological diversity in a New World monkey radiation. In: Campbell C, Fuentes A, MacKinnon K, Panger M, Bearder S (eds) Primates in perspective. Oxford University Press, New York, pp 155–188
Estrada A (1984) Resource use by howler monkeys in the rain forest of Los Tuxtlas, Veracruz, Mexico. Int J Primatol 5:105–131
Estrada A (2015) Conservation of Alouatta: social and economic drivers of habitat loss, information vacuum, and mitigating population declines. In: Kowalewski M, Garber P, Cortes-Ortiz L, Urbani B, Youlatos D (eds) Howler monkeys, developments in primatology: progress and prospects. Springer, New York, pp 383–409
Estrada A, Coates-Estrada R (1996) Tropical rain forest fragmentation and wild populations of primates at Los Tuxtlas, Mexico. Int J Primatol 17:759–783
Estrada A, Anzures A, Coates-Estrada R (1999) Tropical rain forest fragmentation, howler monkeys (Alouatta palliata), and dung beetles at Los Tuxtlas, Mexico. Am J Primatol 48:253–262
Estrada A, Garber PA, Rylands AB, Roos C, Fernandez-Duque E, Di Fiore A, Li B (2017) Impending extinction crisis of the world’s primates: why primates matter? Sci Adv 3:e1600946
Fleagle J, Mittermeier R (1980) Locomotor behavior, body size, and comparative ecology of seven Surinam monkeys. Am J Phys Anth 52:301–314
Ford S, Davis L (1992) Systematics and body size: implications for feeding adaptations in New World monkeys. Am J Phys Anth 88:415–468
Freese C (1983) Cebus capucinus. In: Jazen D (ed) Costa Rican natural history. Cambridge University Press, Cambridge, pp 458–460
Ganzhorn J (1995) Low-level forest disturbance effects on primary production, leaf chemistry, and lemur populations. Ecology 76:2084–2096
Garber P, Kowalewski M (2015) New challenges in the study of howler monkey behavioral ecology and conservation: where we are and where we need to go? In: Kowalewski M, Garber P, Cortes-Ortiz L, Urbani B, Youlatos D (eds) Howler monkeys, developments in primatology: progress and prospects. Springer, New York, pp 413–428
Garber P, Estrada A, Pavelka M (2006) New perspectives in the study of Mesoamerican primates: concluding comments and conservation priorities. In: Estrada A, Garber P, Pavelka M, Luecke L (eds) New perspectives in the study of Mesoamerican primates: distribution, ecology, behavior and conservation. Kluwer Academic/Plenum Publishers, New York, pp 563–584
Garber P, Molina A, Molina R (2010) Putting the community back in community ecology and education: the role of field schools and private reserves in the ethical training of primatologists. Am J Primatol 72:785–793
Gibson L, Lynam A, Bradshaw C, He F, Bickford D, Woodruff D, Laurance W (2013) Near-complete extinction of native small mammal fauna 25 years after forest fragmentation. Science 341:1508–1510
Glander K (1982) The impact of plant secondary compounds on primate feeding behavior. Yearbook Phys Anth 15:1–18
Glanz W (1990) Neotropical mammal densities: how unusual is the community on Barro Colorado Island, Panama? In: Gentry A (ed) Four Neotropical rainforests. Yale University Press, New Haven, pp 287–313
Haddad NM, Brudwig LA, Clobert J, Davies KF, Gonzalez A, Holt RD, Townshend JR (2015) Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci Adv 1:e1500052
Harris L (1988) Edge effects and conservation of biotic diversity. Conserv Biol 2:330–332
Klein L, Klein D (1977) Feeding behaviour of the Colombian spider monkey. In: Clutton-Brock T (ed) Primate ecology: studies of the feeding and ranging behavior in lemurs, monkeys and apes. Academic Press, London, pp 153–181
Kulp J, Heymann E (2015) Ranging, activity budget, and diet composition of red titi monkeys (Callicebus cupreus) in primary forest and forest edge. Primates 56:273–278
Laurance W (1991) Edge effects in tropical forest fragments: application of a model for the design of nature reserves. Biol Conserv 57:205–219
Laurance W, Yensen E (1991) Predicting the impacts of edge in fragmented habitats. Biol Conserv 55:77–92
Lehman S (2007) Spatial variations in Eulemur fulvus rufus and Lepilemur mustelinus densities in Madagascar. Folia Primatol 78:46–55
Lehman S, Rajaonson A, Day S (2006a) Edge effects on the density of Chierogaleus major. Int J Primatol 27:1569–1588
Lehman S, Rajaonson A, Day S (2006b) Edge effects and their influence on lemur density and distribution in southeast Madagascar. Am J Phys Anthropol 129:232–241
Lehman S, Rajaonson A, Day S (2006c) Lemur responses to edge effects in the Vohibola III classified forest, Madagascar. Am J Primatol 68:293–299
Lenz B, Jack K, Spironello W (2014) Edge effects in the primate community of the biological dynamics of Forest Fragments Project, Amazonas, Brazil. Am J Phys Anth 155:436–446
Lidicker W (1999) Responses of mammals to habitat edges: an overview. Landscape Ecol 14:333–343
Lovejoy T, Bierregaard R, Rylands A, Malcolm J, Quintela C, Harper L, Brown K, Powell A, Powell G, Schubart H, Hays M (1986) Edge and other effects of isolation on Amazon forest fragments. In: Soule M (ed) Conservation biology: the science of scarcity and diversity. Sunderland/Sinauer, New York, pp 257–285
Marsh C, Link A, King-Bailey G, Donati G (2016) Effects of fragment and vegetation structure on the population abundance of Ateles hybridus, Alouatta seniculus and Cebus albifrons in Magdalena Valley, Colombia. Folia Primatol 87:17–30
Martin L, Blossey B, Ellis E (2012) Mapping where ecologists work: biases in the global distribution of terrestrial ecological observations. Front Ecol Environ 10:195–201
Mbora D, Meikle D (2004) Forest fragmentation and the distribution, abundance and conservation of the Tana River red colobus (Procolobus rufomitratus). Biol Conserv 118:67–77
McGoogan K (2011) Edge effects on the behaviour and ecology of Propithecus coquereli in northwest Madagascar. Department of Anthropology, University of Toronto, Toronto
McKinney T, Westin J, Serio-Silva J (2015) Anthropogenic habitat modification, tourist interactions and crop-raiding in howler monkeys. In: Kowalewski M, Garber P, Cortes-Ortiz L, Urbani B, Youlatos D (eds) Howler monkeys, developments in primatology: progress and prospects. Springer, New York, pp 281–311
McLennan M, Spagnoletti N, Hockings K (2017) The implications of primate behavioral flexibility for sustainable human-primate coexistence in anthropogenic habitats. Int J Primatol 38:105–121
Milton K (1979) Factors influencing leaf choice by howler monkeys: a test of some hypotheses of food selection by generalist herbivores. Am Nat 114:362–378
Mittermeier R, Schwitzer C, Rylands A, Taylor L, Chiozza F, Williamson E, Wallis J (2012) Primates in peril: the world’s 25 most endangered primates 2012–2014. IUCN/SSC Primate Specialist Group (PSG), International Primatological Society (IPS), Conservation International (CI), Bristol Conservation and Science Foundation, Bristol, pp 1–40
Molina M (2015) A brief history of the Molina family, and the birth of the Maderas Rainforest Conservancy at the La Suerte and Ometepe Field Stations—a narrative. In: Huettman F (ed) Central American biodiversity: conservation, ecology and a sustainable future. Springer Science + Business Media, New York, pp 199–214
Munoz D, Estrada A, Naranjo E, Ochoa S (2006) Foraging ecology of howler monkeys in a cacao (Theobroma cacao) plantation in Comalcalco, Mexico. Am J Primatol 68:127–142
Myers N, Mittermeier R, Mittermeier C, Fonseca G, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858
Occhibove F, Ferro C, Campera M, Liponi G, Borgognini-Tarli S, Ganzhorn J, Donati G (2015) Living in islands of forests: nutritional ecology of the howler monkey (Alouatta palliata) at La Suerte Biological Field Station, North-eastern Costa Rica. In: Huettman F (ed) Central American biodiversity: conservation, ecology and a sustainable future. Springer Science + Business Media, New York, pp 525–538
Peres C (1999) General guidelines for standardizing line-transect surveys of tropical forest primates. Neotrop Primates 7:11–15
Peres C, Dolman P (2000) Density compensation in Neotropical primate communities: evidence from 56 hunted and non-hunted Amazonian forests of varying productivity. Oecologia 122:175–189
Perry S (1997) Male-female social relationships in wild white-faced capuchins (Cebus capucinus). Behaviour 134:477–510
Pollock S, Nielsen S, St. Clair C (2017) A railway increases the abundance and accelerates the phenology of bear-attracting plants in a forested, mountain park. Ecosphere 8:e01985
Pruetz J, Leasor H (2002) Densities of primate species in forest fragments at La Suerte Biological Field Station, Costa Rica. Neotrop Primates 10:4–9
R Core Team (2016) R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. Available from: https://www.R-project.org
Ramsay M, Razafindrakoto A, Lehman S (2017) The effects of a national highway on the endangered golden-brown mouse lemur Microcebus ravelobensis in Ankarafantsika National Park, Madagascar. Oryx. https://doi.org/10.1017/S0030605317001284
Reis L, Fletcher R, Battin J, Sisk T (2004) Ecological responses to habitat edges: mechanisms, models, and variability explained. Annu Rev Ecol Syst 35:491–522
Rose L (1994) Sex differences in diet and foraging behaviour in white-faced capuchins (Cebus capucinus). Int J Primatol 15:95–114
Rose L (1997) Vertebrate predation and food-sharing in Cebus and Pan. Int J Primatol 18:727–765
Ryan S, Starks P, Milton K, Getz W (2008) Intersexual conflict and group size in Alouatta palliata: a 23-year evaluation. Int J Primatol 29:405–420
Saunders D, Hobbs R, Margules C (1991) Biological consequences of ecosystem fragmentation: a review. Conserv Biol 5:18–32
Shimooka Y (2004) Seasonal variation in association patterns of wild spider monkeys (Ateles belzebuth belzebuth) at La Macarena, Colombia. Am J Primatol 52:13–29
Shimooka Y (2005) Sexual differences in ranging of Ateles belzebuth belzebuth at La Macarena, Colombia. Int J Primatol 26:385–406
Skrinyer AJ (2016) Living on the edge: an assessment of habitat disturbance and primate use on the Osa Peninsula, Costa Rica. MA Thesis: Kent State University
Stevens S, Husband T (1998) The influence of edge on small mammals: evidence from Brazilian Atlantic forest fragments. Biol Conserv 85:1–8
Stevenson P, Link A, Ramirez B (2005) Frugivory and seed fate in Bursera inversa at Tinigua National Park, Colombia: implications for primate conservation. Biotropica 37:431–438
van Roosmalen M (1985) Habitat preferences, diet, feeding strategy and social organization of the black spider monkey (Ateles paniscus paniscus Linnaeus 1758) in Surinam. Acta Amazonica 15:1–238
Zárate D, Andresen E, Estrada A, Serio-Silva J (2014) Black howler monkey (Alouatta pigra) activity, foraging and seed dispersal patterns in shaded cocoa plantations versus rainforest in southern Mexico. Am J Primatol 76:890–899
Acknowledgements
We are grateful to Renee Molina and the Maderas Rainforest Conservancy for their support and facilitation of our research at the La Suerte Biological Research Station, Costa Rica. We thank Renate Schlaht for sharing feeding tree data, Palomo Aguilar, Marie-dominique Franco, Arlene Ruddy, and Caleb Garzanelli for help mapping and clearing transects, Michael Ennis for population survey data collection, Nicholas Hug for GIS assistance, and Ryan Janzen for technological consultation. We also thank Madison Azzara, Troy Levinson, Tim Cooney, Tianna Wagner, Macy Cox, Natalie Lopez-Esquibel, Katie Pepperl, and Renate Schlaht for help conducting vegetation surveys. Finally, we thank the Primates associate editor and reviewers Kimberley Hockings and Giuseppe Donati for their helpful comments which have improved this paper. Our research complies with the ethical standards in the treatment of animals corresponding with the guidelines laid down by the Primate Society of Japan, NIH (US), and EC. This research protocol was approved by the Regis University Animal Care Committee and was conducted with the permission of the Costa Rican government, the Maderas Rainforest Conservancy, and the Molina family. This research was supported by University Research and Scholarship Council (URSC) Faculty and Student Research and Scholarship Grants (Regis University) and the Maderas Rainforest Conservancy.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Bolt, L.M., Schreier, A.L., Voss, K.A. et al. The influence of anthropogenic edge effects on primate populations and their habitat in a fragmented rainforest in Costa Rica. Primates 59, 301–311 (2018). https://doi.org/10.1007/s10329-018-0652-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10329-018-0652-0