Abstract
Many methods are available to gather data on wildlife population parameters, such as population abundance and density, yet few have been compared or validated. We compared the efficacy of three survey methods (live trapping, hair tubes and scats) for estimating abundance and population density of the European pine marten (Martes martes) in Galloway Forest, Scotland. We evaluated these methods by, firstly, comparing the accuracy of the population estimate derived from each method, and, secondly, comparing the financial cost of each method. Molecular analysis of samples from all three methods was used to determine sex and individual genotype. Population abundance estimates were derived from capture-recapture programme Capwire. The non-invasive methods (hair tubes and scats combined) detected 81% of known individuals, although hair tubes and scats performed poorly alone, detecting 48% and 52% of individuals, respectively. Live trapping was the individual method that detected the most individuals (77%). Hair tubes were the most expensive method, both in financial cost and personnel hours, whilst scat sampling was the cheapest method. There was a highly significant association between the sex of the animal and the total number of detections by method. The population abundance estimate from all methods combined was 32 (95% CI 31–35) and the population density estimate was 0.27 martens/km2. This study indicates that a combined sampling approach comprising hair tubes and scats maximises the number of detections and provides a viable alternative to invasive live trapping.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Determining the distribution and abundance of species is a fundamental issue in conservation biology (Riddle et al. 2003), and successful monitoring of these parameters is essential for effective management of wildlife populations (Gibbs et al. 1999). It can be problematic to collect accurate data on many wildlife species, particularly if they are elusive, cryptic, nocturnal, have large home ranges and occur at low population densities (Mills et al. 2000; Wilson and Delahay 2001; Riddle et al. 2003; Efford 2011).
Whilst a multitude of methods is available to monitor and gather data on wildlife populations, many have not been compared or validated with known population sizes or more rigorous methods of population estimation (Witmer 2005). Different methods can result in contrasting rates of detection, and the use of inappropriate sampling techniques might fail to detect a species when it is present (Diggins et al. 2016). Increasingly, studies are evaluating the efficacy of different methods for detecting and gathering population abundance and density data for wildlife populations (Edwards et al. 2000; Hackett et al. 2006; Long et al. 2007; Velli et al. 2015; Mumma et al. 2015; Diggins et al. 2016; Robinson et al. 2017; Riley et al. 2017).
The European pine marten (Martes martes) is a species of conservation and population management interest and is subject to national and international conservation legislation throughout much of its range (O’Mahony et al. 2017). In Britain, pine martens are of particular conservation interest because the population is recovering following a severe historical decline in which the species became confined to small parts of its former range (Langley and Yalden 1977; Croose et al. 2013, 2014). This recovery, and the potential role of pine martens in controlling populations of the invasive American grey squirrel (Sciurus carolinensis) (Sheehy and Lawton 2014; Sheehy et al. 2018), tempered with concern from some stakeholders of the impact on potentially vulnerable prey species such as the capercaillie (Tetrao urogallus) (Summers et al. 2009; Baines et al. 2016), has resulted in increased interest and need for the collection of accurate baseline abundance data and monitoring of population trends.
Methods used for estimating pine marten population abundance and density include non-invasive genetic sampling (Lynch et al. 2006; Mullins et al. 2010; Sheehy et al. 2013; O’Mahony et al. 2015; 2017; Kubasiewicz et al. 2016, 2017; Croose et al. 2016); camera trapping (Manzo et al. 2012); snow tracking (Kurki et al. 1998; Zalewski 1999); live trapping (Lynch et al. 2006; O’Mahony 2014); radio-telemetry (Balharry 1993; Bright and Smithson 1997); and territory mapping (Zalewski and Jędrzejewski 2006). Only one study to date has evaluated the efficacy of non-invasive hair sampling and live trapping for estimating pine marten population abundance (Lynch et al. 2006).
In 2014, we carried out a non-invasive genetic survey in Galloway Forest, southwest Scotland, to determine the distribution, population abundance and density of pine martens in a discrete area of the forest (Croose et al. 2016). We also investigated the effect of sample source (hair and scats) on population estimation. Previous studies using non-invasive sampling have used hair sampling alone to produce pine marten population estimates (Sheehy et al. 2013; O’Mahony et al. 2015; 2017). However, our 2014 study found that collection and genotyping of hair samples under-sampled the pine marten population (detecting only 33% of known individuals) compared with the collection and genotyping of scats (which detected 93% of individuals) (Croose et al. 2016). Consequently, we concluded that using a combined non-invasive sampling approach, comprising both hair and scat samples, was optimal to detect as many individuals within the population as possible to maximise the accuracy of population estimates.
Here, we describe a subsequent study carried out in 2017, where we conducted another survey of the pine marten population in the same area of Galloway Forest, comprising live trapping alongside non-invasive sampling. Our objective was to examine and compare the efficacy of three survey methods (live trapping, hair sampling and scats) for estimating pine marten abundance and population density. We evaluated these methods by, firstly, comparing the accuracy of the population estimate derived from each method, and, secondly, comparing the financial cost of each method. Finally, we considered how pine marten population abundance in the study area has changed between the previous study in 2014 and the current study.
Methods
Study area
The study was conducted in Galloway Forest, a predominantly commercial conifer plantation in southwest Scotland, UK (55° 02′ N, 4°16′ W). The study area covered a 100 km2 area known as the Fleet Basin, located at the south-eastern edge of Galloway Forest (as described in Croose et al. 2016). Dominant tree species are Sitka spruce (Picea sitchensis) (77%), Lodgepole pine (Pinus contorta) (11%) and other conifer species (12%), with minor coverage of broadleaves (6%). The dominant tree age structure is pole stage, mature and old forest stage crops with a smaller proportion at establishment or thicket stage (Forest Enterprise 2012). The study area has a mild maritime climate with average temperatures ranging from 1.3 °C in the winter to 19.5 °C in the summer (Met Office 2013) and an average annual rainfall of 1600 mm (Forest Enterprise 2012).
Sample collection
Live trapping
Live trapping of pine martens was undertaken during September 2017 under Scottish Natural Heritage licence. Live traps (Havahart Large 1-door collapsible traps, model 1089, Woodstream Europe Ltd., Fencing House, Oakham, Rutland, LE15 6RF, UK) were located off forest tracks and covered with dry hay and vegetation, such as moss. Trap sites were pre-baited for 2.5 weeks prior to trapping, using a hen’s egg and a mixture of honey, peanuts and raisins and a small amount of marten lure (Hawbakers Marten Lure, F&T Fur Harvester’s Trading Post, www.fntpost.com). A total of 40 trap sites were established at approximate 2 km spacing. The trapping was completed in two phases, whereby 20 traps were active in each phase and each trap was set for four nights and days. Traps were checked twice per day: once in the morning and once in the evening.
When a pine marten was captured, the trap was covered with a thick cloth and martens were calmly restrained at the rear of the trap using a purpose-built ‘comb’. A small hair sample for DNA analysis was removed with tweezers and a colour mark was applied to the marten (either the chest, flank or back) with stock marker spray (Carrs Billington Stock Marker Spray) in a distinctive pattern using a paintbrush, to identify individuals in any subsequent recaptures. Animals were released at the capture site immediately after sampling. Hair samples were taken on the first capture only and not on recaptures. If an animal was recaptured, it was identified by means of the colour markings applied at first capture.
Non-invasive sampling
Hair tubes
Hair samples were collected from a total of 99 hair tubes which were installed throughout the study area, at a density of one tube per 1 km2 Ordnance Survey grid square, with approximately 1 km spacing between each tube where practicable. The tubes were placed at the same locations as the previous survey in 2014, so that the sampling effort would be comparable across the two surveys. Due to active forestry operations, one site where a hair tube was located in 2014 was inaccessible, so only 99 hair tubes were installed, rather than the 100 installed during 2014.
Hair tubes were constructed from 100 mm2 plastic cable trunking cut into 300 mm lengths and secured with a lid on the top. Three 40-mm diameter holes were drilled through the elevation of the tube facing the tree to allow the martens to grip the tree through the back of the tube whilst climbing into the tube. A further four 6-mm holes were drilled through the elevation of the tube facing the tree and bale string was tied through the holes to attach the tube to the tree. A clip-on cover was secured to the lid of the tube with a 100 mm length of duct tape to act as a hinge, allowing the front section of the tube to be detached for removing hair samples and replacing bait. The lid was also attached to the upper string passing through drilled holes to prevent detachment. Chicken wings were used as bait and secured inside the top of the tube by a hook comprising a 100 mm length of electrical cable armouring wire. An eye was formed at one end of the hook and it was threaded on to the upper length of tying string inside the tube with the wire end bent upwards. A small square patch of ‘mouse glue’ (Pest Control Supermarket, www.pestcontrolsupermarket.com) was stuck to a correspondingly sized square of self-adhesive backed Velcro and attached inside the tube 50 mm above the lower edge of the clip-on cover to catch the hair from an animal when it entered the tube. Hair tubes were placed within 30 m of forest tracks and were tied to a tree at a minimum height of 1.5 m above ground level. In areas of clearfell or recent timber harvesting where there were no trees on which to install hair tubes, tubes were installed on fencing posts driven into the ground.
Five sampling sessions were conducted during September and October 2017, with tubes checked every 7 days over a total of 36 days. During each session, any glue patches with hair samples were removed, fresh glue patches were fitted, fresh bait was placed in the tube and any remaining bait left on the ground below the tube. All hair samples were retained on the glue patches, stored individually in plastic tubes and frozen at − 4 °C within 12 h of collection. The same applied to hair samples taken from animals caught during live trapping.
Scat collection
Fresh pine marten scats (faeces) were collected during September and October 2017 in concurrence with the hair tube surveys. Scats were collected from forest tracks of varying lengths distributed throughout the study area, totalling 75.8 km. The distribution of scat survey effort was designed to maximise geographical coverage along forest tracks across the study area. Typically, one or two experienced surveyors searched the tracks extensively, covering both sides of the track, but not away from tracks, except when checking traps or hair tubes. Each track was surveyed once and tracks were not cleared prior to collection. Scats were also collected on an ad hoc basis whilst checking traps or hair tubes. Only fresh scats were collected for genotyping, but all scats observed were recorded as part of another study. All scats were frozen at − 4 °C within 12 h of collection.
Genetic analysis
The DNA extraction and analysis methods were as described in Croose et al. (2016). In this study, up to 10 microsatellite markers were used to identify individuals. These were Mel1; Gg7; Ma2; Mvi1341; Mer041 and Mvis075 (see Mullins et al. 2010) and Mar21; Mar64; Mar53 and Mar08 (Natali et al. 2010). All new individuals were genotyped with all 10 markers. The PI for 10 markers was 1.4 × 10−4 and PIsib was 1.1 × 10−2. Recaptures were identified using 6 markers (Mel1; Ma2; Mer041 and Mvis075; Mar64 and Mar08) and the PI was 5.1 × 10−3 and PIsib was 7.0 × 10−2.
Statistical analysis
Population estimation
Population abundance estimates for each individual method (live trapping, hair sampling and scat sampling) and all methods combined were derived from capture-recapture programme Capwire (Miller et al. 2005). Capwire has been shown to provide accurate population estimates for small populations with capture heterogeneity and takes account of multiple samples from an individual within the same sampling session (Miller et al. 2005). All captures, including recaptures, of individual pine martens, were grouped into a single sampling session scheme for analysis. The likelihood ratio test (LRT) was applied to choose the most suitable model: the Two-Innate Rates Model (TIRM), which allows for capturing heterogeneity and assigns a high or low capture probability to individuals, or the Even Capture Model (ECM), which assumes that each individual has an equal chance of being captured. The maximum population size was set to 100 and 95% confidence intervals were estimated using 1000 parametric bootstrap replicates.
Density estimates
Population density estimates were derived by dividing the population abundance estimates by the effective trapping area (Otis et al. 1978). The effective trapping area was calculated as per the previous study in 2014 (Croose et al. 2016). Briefly, a convex hull around the study area was delineated by the locations of the outmost hair tubes and scat transects using MapInfo Professional (v12.0). A buffer strip was included to account for ‘edge effects’ caused by the movement of animals in and out of the study area (Otis et al. 1978; Royle et al. 2013). The buffer strip was derived by calculating the mean maximum distance moved by pine martens to provide an estimator of home range diameter (mean = 2.42 km), then the width of half mean maximum distance moved (1021 m) was applied to the convex hull to create the effective trapping area (Royle et al. 2013). Significant areas of open ground and lochs were excluded from the calculation of the effective trapping area.
Other
Chi-squared analysis was used to test if the number of detections (‘captures’) per detection method (‘trap type’) was independent of animal sex.
Data availability
The datasets generated and analysed during this study are available from the corresponding author on reasonable request.
Results
Sample collection and DNA analyses
A total of 160 trap days of live trapping were completed, resulting in a total of 48 captures of pine martens during 320 trap checks. DNA analysis of hair samples taken of captured animals identified 24 individuals: 17 males and seven females (Table 1). Genotyping success rate of these hair samples was 100%. The number of times an individual marten was captured ranged from 1 to 4. On one occasion, a recaptured animal could not be identified with certainty from the colour markings applied by stock spray on the first capture, so this recapture was excluded from further analysis.
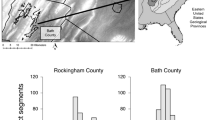
In total, 157 hair samples were collected from the hair tubes. Of these, 57% were sex-typed, resulting in 61 male detections and 28 female detections. 44% of the samples were successfully genotyped, detecting 15 individuals (10 males and 5 females) (Table 1). The cumulative proportion of hair tubes that collected hair samples increased over the sampling period, from 6% in the first sampling session to 64% in the fifth sampling session. The number of new (previously undetected) genotypes detected in the hair tubes increased through the sampling sessions, with no new genotypes detected in the fifth and final sampling session (Fig. 1). The number of times an individual marten was detected in a hair tube ranged from 1 to 12.
In total, 103 scats were collected. Of these, 80% were sex-typed, resulting in 38 male detections and 44 female detections. 32% of the samples were successfully genotyped, detecting 16 individuals (6 males and 10 females) (Table 1). The number of times an individual marten was detected via scats ranged from 1 to 4.
When all methods are combined, the number of times an individual marten was detected ranged from 1 to 18 (mean = 4.55) (Fig. 2).
Population estimation
The minimum population size derived from genotyping samples from all three methods combined was 31 individuals, comprising 19 males and 12 females. The minimum population size detected from each individual method was 24 individuals from live trapping, 15 from hair tubes and 16 from scats (Table 2).
In Capwire, the TIRM model was selected for estimates derived from the following sample groups: hair tubes; scats; hairs and scats combined; all methods combined; the ECM model was selected for estimates from live trapping only. The population abundance estimates derived from Capwire were 30 (95% CI 24–37) for live trapping; 15 (95% CI 15–16) for hair tubes; 26 (95% CI 16–37) for scats; 27 (95% CI 25–31) for hair tubes and scats combined; and 32 (95% CI 31–35) for all methods combined (Table 2).
There was considerable variation in the average number of observations per individual for each method, with hair tubes yielding the highest average number of observations per individual (4.6) and live trapping yielding the lowest average number of observations per individual (2.0) (Table 2).
The population density estimate for pine martens in the Fleet Basin was 0.27 martens/km2 for all methods combined (and ranged from 0.13 to 0.25 martens/km2 for individual methods alone) (Table 2).
Efficacy of survey methods
The highest population estimate produced by Capwire (32 (95% CI 31–35)) was derived from all three methods combined. The method which detected the most individual pine martens was live trapping, detecting 77% of known individuals within the population (n = 24) (Table 1) and producing the highest and most accurate population estimate (30 (95% CI 24–37)) from Capwire (Table 2). Hair tubes detected fewer than half of known individuals within the population (48%) and yielded the lowest and least accurate population estimate (n = 15). Scat sampling detected just over half of known individuals (52%) and yielded a more accurate population estimate than hair tubes (n = 26). However, hair tubes and scat sampling combined detected 81% of known individuals (n = 25), a more accurate minimum population estimate than live trapping alone.
There were individual variations in the pattern of detections by the three sampling methods: only five individuals (16% of all individuals detected) were detected by all three methods; 45% of individuals (n = 14) were detected by two methods, and 39% of individuals (n = 12) were detected by only one method (Fig. 2). Interestingly, six individuals were live-trapped on 13 occasions but were never detected via hair tubes or scat samples, three individuals were detected in hair tubes but never in live traps or scat samples and three individuals were detected only by scat sampling (Fig. 2).
There was a highly significant association between the sex of the animal and the total number of detections by method (χ2 = 9.7965, df = 2, p = 0.00746). Female detections were over-represented in scat sampling, whereas male detections were over-represented in live trapping and hair tubes. However, the results of the test for an association between sex and the number of individuals detected per method was not statistically significant (χ2 = 4.8264, df = 2, p = 0.08953).
Financial cost
Hair tubes were the most expensive method (accounting for the most personnel hours, highest vehicle mileage and highest genotyping costs), detected the fewest individuals and produced the lowest and least accurate population estimate (Table 3). The relatively high cost is due to sampling from hair tubes taking place over 5 weeks (longer than the live trapping and scat collection exercises) and producing the highest number of samples for genotyping. Conversely, live trapping was completed in only 8 days, and, because hair samples were taken from captured animals during the first capture only, there were many fewer samples for genotyping. Scat sampling was the least costly method in terms of personnel hours, mileage and genotyping costs (Table 3).
Discussion
Comparison of efficacy of methods
This is the first study to compare the efficacy of live trapping, hair tubes and scat sampling in providing population abundance and density estimates for pine martens.
In this study, live trapping was the single method that detected the most individual pine martens (77% of known individuals). However, it did yield the lowest average number of observations per individual, compared with hair tube and scat sampling, and resulted in a bias towards male detections. Whilst hair tube and scat sampling performed fairly poorly alone in detecting individuals, both of these methods combined detected 81% of known individuals, producing a more accurate minimum population estimate than live trapping alone. In particular, hair tubes yielded the highest average number of observations per individual marten compared with the other methods (Table 2). Due to the higher number of detections per individual and the wider geographic spread of hair tubes and scat transects, non-invasive sampling can provide a better indication of the distribution of martens in the study area and an individual marten’s home range, which can be useful in the absence of fine-scale spatial data from radio-tracking. The results from the hair tubes support our previous study in 2014, which showed that using hair tubes alone under-samples the population by failing to detect all individuals and is not an accurate method to use in isolation (Croose et al. 2016). The population estimate derived from Capwire (n = 32) was very close to the minimum population estimate derived from genotyping (n = 31), suggesting that, in combination, the three survey methods successfully sampled most of the population.
The effectiveness of live trapping and non-invasive sampling was very similar (detecting 77% and 81% of known individuals, respectively). However, the significant association between the sex of the animal and the total number of detections by method indicates a bias, which should be considered for future survey design. Live trapping and hair tubes resulted in a bias towards male detections, whereas scats resulted in a bias towards female detections. A combined sampling approach using hair tubes and scats takes account of the sex bias. Nevertheless, the sex bias arising from methods may vary by study area, as a previous study elsewhere in Scotland found higher detection probabilities for female than male martens in hair samples (Sheehy et al. 2018). Personality or behavioural traits may also influence detectability of individuals and studies have shown that bold, active, exploratory individuals are most detected (Merrick and Koprowski 2017). Thus, live trapping and hair tubes may under-sample shy, less exploratory individuals, whereas these individuals may be detected via scat surveys, as this does not require the animal to undertake any potentially risky behaviour, such as entering a trap or hair tube. Moreover, the spatial distribution of hair tubes and trap sites and pine martens’ use of these sites, along with the status of individuals (particularly whether they are resident or dispersing), may influence detectability (Belbachir et al. 2015). This may explain the noticeable variations in detections of individual pine martens by different methods in our study (Fig. 2).
Non-invasive sampling is advantageous over live trapping, because trapping has the potential for discomfort, distress, physical injury, loss of fitness or even incidental mortality of captured animals (Putman 1995). Furthermore, pine martens are a protected species in Britain, and, as such, a licence from the appropriate Statutory Nature Conservation Organisation is required to capture pine martens, whereas a licence is not required for non-invasive methods. Live trapping can be better justified if other samples (e.g. blood) are required that cannot be collected non-invasively, or if physical capture is necessary for condition assessment, PIT-tagging or radio-collaring.
Nevertheless, there are drawbacks to non-invasive methods, notably the degradation of DNA in samples and associated low genotyping success. DNA amplification success rates are affected by the length of time that samples are in the field, temperature and dew point (Murphy et al. 2007). The lower genotyping success rate for non-invasive samples (44% for hairs and 32% for scats in this study) means that it is almost certain that more individuals would have been sampled by non-invasive methods, but the DNA was of insufficient quality to amplify to genotype. Scat samples have a lower genotyping success rate than hairs, which has led to them being discounted from analysis in some previous studies (Mullins et al. 2010; Kubasiewicz et al. 2017). Conversely, with live trapping, the hair samples taken from animals during capture were fresh and consisted of more hairs, and thus resulted in a 100% genotyping success rate. Any methodological improvements that lead to increased genotyping success rates will improve the value and accuracy of non-invasive sampling methods in the future.
The relatively small proportion of samples from the hair tubes genotyped was partly attributable to the glue patches inside the tubes failing to collect adequate quantities of hairs on several occasions due to an accumulation of flies stuck to the patches, thus reducing their adhesive ability. The number of individual detections and resulting population estimates from hair tubes could be improved by increasing the number of hair follicles in each reaction, as previous research indicates that including more follicles (up to 13) reduces PCR failure rates (Kubasiewicz et al. 2016). In this study, five hair tube sampling sessions were completed (one more than during the 2014 study). Interestingly, whilst the number of individuals detected by hair tubes increased cumulatively through the sampling sessions, no new genotypes were identified during the fifth and final sampling session; however, the genotyping success rate for hair samples collected during this final session was poor (24% of samples genotyped).
When considering financial cost, the cost of non-invasive sampling was much higher than live trapping, with hair tubes being the most expensive individual method and scat sampling the cheapest individual method. The relatively high cost incurred by personnel hours for the hair tubes could be reduced by having only four sampling sessions, as no new genotypes were identified during the fifth and final sampling session in this study.
Whilst hair tubes and scat samples have proved successful methods in our studies in southwest Scotland and also studies in Ireland (Lynch et al. 2006; Mullins et al. 2010), it is worth noting that the efficacy of different survey methods may vary by locality. In a study in Italy, Bartolommei et al. (2013) found both scat surveys and hair tubes to be ineffective at detecting pine martens, and camera trapping was the only effective method to estimate pine marten population density. In other studies in Scotland and Poland, hair tubes have been unsuccessful at collecting pine marten hair samples at sites where pine martens were known to be present and marten scats were detected (Kubasiewicz et al. 2017; Power 2015). In Lynch et al.’s (2006) study in Ireland, scats, hair trapping and live trapping were decreasingly effective at detecting the presence of pine martens, although population abundance was not estimated in this study.
Population abundance and density
The pine marten population in the Fleet Basin as determined in this study (n = 32) has apparently almost doubled since the previous study in 2014 (n = 18; Croose et al. 2016). This was contrary to our expectations that the population may have declined due to extensive timber harvesting and a consequent reduction in mature woodland habitat between the study years: between September 2014 and September 2017, 632.54 ha of land was felled (approximately 7% of the forested part of the study area). Whilst the mechanisms of the population increase are unknown, we suggest some hypotheses.
Firstly, the availability of den sites for martens in the Fleet Basin has substantially increased since the 2014 population estimate. Pine martens require sheltered, elevated den sites for resting and breeding, and a scarcity of suitable arboreal sites may be a critical constraint for marten populations (Brainerd et al. 1995). In autumn 2014, 50 artificial den boxes were installed in the Fleet Basin at a density of one box per 2 km2 (JB, JM & GV, unpublished data). This increase in den site availability in a commercial forestry plantation where natural cavities are scarce may help to reduce predation risk and energetic costs, particularly for breeding females.
Secondly, predator populations may be influenced by the abundance and population cycles of prey species, and in the Netherlands, pine marten litter size corresponds with abundance of wood mice (Apodemus sylvaticus) (Kleef and Wijsman 2015). Both 2014 and 2017 appeared to be peak years for field voles (Microtus agrestis) in Galloway Forest, a key prey item for martens (JB, unpublished data), so in this case, differences in vole abundance are unlikely to have been a driver of the population increase.
Finally, the apparent increase in martens may be due in part to some individuals within the population being under-sampled or failing to be detected in the 2014 study. It is possible that if live trapping had been conducted during the 2014 study, more individuals would have been detected. However, if only considering individuals detected in 2017 through hair tubes and scats, and discounting those detected only by live trapping, to allow a more direct comparison with the sampling effort during 2014, there is still a clear increase from 15 individuals detected in 2014 to 25 individuals in 2017. This suggests that there has been a real population increase over the 3 years between the two studies, and it is not merely attributable to differences in sampling effort.
Three individuals that were detected in the 2014 study were also detected in the 2017 study: two males and one female. The rest of the animals identified in 2017 had not previously been detected.
The population density estimate for the Fleet Basin produced by this study (0.27 martens/km2, an increase from 0.15/km2 in the previous study in 2014; Croose et al. 2016) is within the middle range of population densities reported in other studies in Scotland, which have ranged from < 0.10 martens/km2 (Bright and Smithson 1997; Kubasiewicz et al. 2017) to 0.58 martens/km2 (Halliwell 1997). This is predominantly lower than densities reported elsewhere in Europe, which typically range from 0.01–1.75 martens/km2 (Zalewski and Jędrzejewski 2006), but have been reported to be as high as 4.42 martens/km2 in the midlands of Ireland (Sheehy et al. 2013).
Recommendations and conclusion
The data from this study indicate that, of the three methods used, the most effective for estimating pine marten populations is a combined sampling approach comprising hair tubes and scat sampling. This approach maximises the number of detections, accounts for the sex bias resulting from detection methods, avoids the invasive nature of live trapping and does not require a protected species licence. Although not used in this study, camera traps and the random encounter model have proved to be an effective method for estimating pine marten population density elsewhere (Manzo et al. 2012). Therefore, it would be worthwhile comparing the efficacy of camera traps with non-invasive methods and live trapping in future studies. Nevertheless, the necessity of some studies to collect genetic material, such as hairs and scats, will dictate that camera trapping alone is not a suitable method. As the pine marten population in Britain continues to recover and expand its range, combined non-invasive sampling methods should be employed to monitor the population and inform conservation and management efforts.
References
Baines D, Aebischer NJ, Macleod A (2016) Increased mammalian predators and climate ch1ange predict declines in breeding success and density of Capercaillie tetrao urogallus, an old stand specialist, in fragmented Scottish forests. Biodivers Conserv 25(11):2171–2186
Balharry D (1993) Factors affecting the distribution and population density of pine martens (Martes martes) in Scotland. Ph.D. dissertation. University of Aberdeen
Bartolommei P, Mazon E, Cozzolino R (2013) Evaluation of three indirect methods for surveying European pine marten in a forested area of central Italy. Hystrix Italian J Mammal 23(2):91–94
Belbachir F, Pettorelli N, Wacher T, Belbachir-Bazi A, Durant SM (2015) Monitoring rarity: the critically endangered Saharan cheetah as a flagship species for a threatened ecosystem. PLoS One 10(1):e0115136. https://doi.org/10.1371/journal.pone.0115136
Brainerd SM, Helldin J-O, Lindstrӧm ER, Rolstad E, Storch I (1995) Pine marten (Martes martes) selection of resting and denning sites in Scandinavian managed forests. Ann Zool Fenn 32:151–157
Bright PW, Smithson TJ (1997) Species Recovery Programme for the pine marten in England: 1995-96. English Nature Research Reports No 240
Croose E, Birks JDS, Schofield HW (2013) Expansion zone survey of pine marten (Martes martes) distribution in Scotland. Scottish Natural Heritage Commissioned Report No. 520
Croose E, Birks JDS, Schofield HW, O’Reilly C (2014) Distribution of the pine marten (Martes martes) in southern Scotland in 2013. Scottish Natural Heritage Commissioned Report No. 740
Croose E, Birks JDS, O’Reilly C, Turner P, Martin J, MacLeod ET (2016) Sample diversity adds value to non-invasive genetic assessment of a pine marten (Martes martes) population in Galloway Forest, southwest Scotland. Mammal Res 61:131–139
Diggins CA, Gilley M, Kelly CA, Ford M (2016) Comparison of survey techniques on detection of northern flying squirrels. Wildl Soc Bull 40(4):654–662
Edwards GP, de Preu ND, Shakeshaft BJ, Crealy IV (2000) An evaluation of two methods of assessing feral cat and dingo abundance in central Australia. Wildl Res 27:143–149
Efford MG (2011) Estimation of population density by spatially explicit capture– recapture analysis of data from area searches. Ecology. 92(12):2202–2207
Forest Enterprise (2012) Galloway Forest Fleet Basin Forest Design Plan 2012–2022. Dumfries and Galloway: Forest Enterprise
Gibbs JP, Snell HL, Causton CE (1999) Effective monitoring for adaptive wildlife management: lessons from the Galapagos Islands. J Wildl Manag 63:1055–1065
Hackett HM, Lesmeister DB, Desanty-Combes J, Montague WG, Millspaugh JJ, Gompper ME (2006) Detection rates of eastern spotted skunks (Spilogale Putorius) in Missouri and Arkansas using live capture and non-invasive techniques. Am Midl Nat 158(1):123–131
Halliwell E (1997) The ecology of red squirrels in Scotland in relation to pine marten predation. Ph.D. dissertation. University of Aberdeen
Kleef HL, Wijsman HJW (2015) Mast, mice and pine marten (Martes martes): the pine marten’s reproductive response to wood mouse (Apodemus sylvaticus) fluctuations in the Netherlands. Lutra. 58(1):23–33
Kubasiewicz LM, Minderman J, Woodall LC, Quine CP, Coope R, Park KJ (2016) Fur and faeces: an experimental assessment of non-invasive DNA sampling for the European pine marten. Mammal Res 61:299–307
Kubasiewicz LM, Quine CP, Summers RW, Coope R, Cottrell JE, A’Hara SW, Park KJ (2017) Non-invasive genotyping and spatial mark-recapture methods to estimate European pine marten density in forested landscapes. Hystrix Ital J Mammal 28(2):265–271
Kurki A, Nikula A, Helle P, Lindon H (1998) Abundances of red fox and pine marten in relation to the composition of boreal forest landscapes. J Anim Ecol 67:874–886
Langley PJW, Yalden DW (1977) The decline of the rarer carnivores in Great Britain during the nineteenth century. Mammal Rev 7:95–116
Long RA, Donovan TM, Mackay P, Zielinski WJ, Buzas JS (2007) Comparing scat detection dogs, cameras and hair snares for surveying carnivores. J Wildl Manag 71(6):2018–2025
Lynch AB, Brown MJF, Rochford JM (2006) Fur snagging as a method of evaluating the presence and abundance of a small carnivore, the pine marten (Martes martes). J Zool 270:330–339
Manzo E, Bartolommei P, Rowcliffe JM, Cozzolino R (2012) Estimation of population density of European pine marten in central Italy using camera trapping. Acta Theriol 57:165–172
Merrick MJ, Koprowski JL (2017) Should we consider individual behavior differences in applied wildlife conservation studies? Biol Conserv 209:34–44
Met Office (2013) Western Scotland: climate [online]. Available at: <http://www.metoffice.gov.uk/climate/uk/regional-climates/ws> [Accessed 29 Jan 2018]
Miller CR, Joyce P, Waits LP (2005) A new method for estimating the size of small populations from genetic mark–recapture data. Mol Ecol 14:1991–2005
Mills LS, Citta JJ, Lair KP, Schwartz MK, Tallmon DA (2000) Estimating animal abundance using noninvasive DNA sampling: promise and pitfalls. Ecol Appl 10(1):283–294
Mullins J, Statham MJ, Roche T, Turner PD, O’Reilly C (2010) Remotely plucked hair genotyping: a reliable and non-invasive method for censusing pine marten (Martes martes, L. 1758) populations. Eur J Wildl Res 56:443–453
Mumma MA, Zieminski C, Fuller TK, Mahoney SP, Waits LP (2015) Evaluating noninvasive genetic sampling techniques to estimate large carnivore abundance. Mol Ecol Resour 15:1133–1144
Murphy MA, Kendall KC, Robinson AP, Waits LP (2007) The impact of time and field conditions on brown bear (Ursus arctos) faecal DNA amplification. Conserv Genet 8:1219–1224
Natali C, Banchi E, Ciofi C, Manzo E, Bartolommei P, Cozzolino R (2010) Characterization of 13 polymorphic microsatellite loci in the European pine marten Martes martes. Conserv Genet Res 2:397–399
O’Mahony DT, Powell C, Power J, Hanniffy R, Marnell F, Turner P, O’Reilly C (2017) Non-invasively determined multi-site variation in pine marten Martes martes density, a recovering carnivore in Europe. Eur J Wildl Res 63(48). https://doi.org/10.1007/s10344-017-1108-3
O'Mahony DT (2014) Socio-spatial ecology of pine marten (Martes martes) in conifer forests, Ireland. Acta Theriol 59:251–256
O'Mahony D, Turner P, O’Reilly C (2015) Pine marten (Martes martes) abundance in an insular mountainous region using non-invasive techniques. Eur J Wildl Res 61(1):103–110
Otis DL, Burnham KP, White GC, Anderson DR (1978) Statistical inference from capture data on closed animal populations. Wildl Monogr 62:3–135
Power J (2015) Non-invasive genetic monitoring of pine marten (Martes martes) and stone marten (Martes foina) in and around the Nietoperek bat hibernation site, Poland. PhD thesis: Waterford Institute of Technology, Ireland
Putman RJ (1995) Ethical considerations and animal welfare in ecological field studies. Biodivers Conserv 4:903–915
Riddle AE, Pilgrim KL, Mills LS, McKelvey KS, Ruggiero LF (2003) Identification of mustelids using mitochondrial DNA and non-invasive sampling. Conserv Genet 4:241–243
Riley M, Soutyrina S, Miquelle D, Hayward G, Goodrich G, Buskirk S (2017) Comparison of methods for estimating Amur tiger abundance. Wildl Biol 2017:wlb.00253. https://doi.org/10.2981/wlb.00253
Robinson L, Cushman SA, Lucid MK (2017) Winter bait stations as a multispecies survey tool. Ecol Evol 7:6826–6838
Royle JA, Chandler RB, Sollmann R, Gardner B (2013) Spatial capture-recapture. Waltham, Elsevier
Sheehy E, Lawton C (2014) Population crash in an invasive species following the recovery of a native predator: the case of the American grey squirrel and the European pine marten in Ireland. Biodivers Conserv 23(3):753–774
Sheehy E, O’Meara DB, O’Reilly C, Smart A, Lawton C (2013) A non-invasive approach to determining pine marten abundance and predation. Eur J Wildl Res 60(2):223–236
Sheehy E, Sutherland C, O’Reilly C, Lambin X (2018) The enemy of my enemy is my friend: native pine marten recovery reverses the decline of the red squirrel by suppressing grey squirrel populations. Proc R Soc B 285:20172603. https://doi.org/10.1098/rspb.2017.2603
Summers RW, Willi J, Selvidge J (2009) Capercaillie Tetrao urogallus Nest loss and attendance at Abernethy Forest, Scotland. Wildl Biol 15(3):319–327
Velli E, Bologna MA, Silvia C, Ragni B, Randi E (2015) Non-invasive monitoring of the European wildcat (Felis silvestris silvestris Schreber, 1777): comparative analysis of three different monitoring techniques and evaluation of their integration. Eur J Wildl Res 61:657–668
Wilson GJ, Delahay RJ (2001) A review of methods to estimate the abundance of terrestrial carnivores using field signs and observation. Wildl Res 28:151–164
Witmer GW (2005) Wildlife population monitoring: some practical considerations. Wildl Res 32:259–263
Zalewski A (1999) Identifying sex and individuals of pine marten using snow track measurements. Wildl Soc Bull 27(1):28–31
Zalewski A, Jędrzejewski W (2006) Spatial organisation and dynamics of the pine marten Martes martes population in Białowieża Forest (E Poland) compared with other European woodlands. ECOGRAPH. 29:31–43
Acknowledgments
We are grateful to Andrew Jarrott, Colin Hossack and Kenny Kortland for the continued support of this work. We thank the following volunteers who assisted with fieldwork: Trina Barratt, Jacob Graham, Sammy Grey, Kevin Heywood and Jenni Mouat. We are especially grateful to Shirley Martin for her significant contributions to fieldwork, logistical and administrative support.
Funding
This study was funded by Forestry Commission Scotland.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Croose, E., Birks, J.D.S., Martin, J. et al. Comparing the efficacy and cost-effectiveness of sampling methods for estimating population abundance and density of a recovering carnivore: the European pine marten (Martes martes). Eur J Wildl Res 65, 37 (2019). https://doi.org/10.1007/s10344-019-1282-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10344-019-1282-6