Abstract
The diploid A. glutinosa (L.) Gaertn. is widespread throughout the European continent, except in the Iberian and Balkan Peninsulas where tetraploid populations have been discovered. We focused on the tetraploid species described as A. rohlenae Vít, Douda and Mandák that occupies the western part of the Balkan Peninsula, where it has likely completely replaced the diploid species. While the distribution range of the diploid A. glutinosa s. str. is well known, the exact distribution range of the tetraploid A. rohlenae is unknown. Here, we report the first exact distribution of the tetraploid A. rohlenae and the anticipated hybrid zones in which it is in contact with diploid populations using flow cytometry and morphometrics. Tetraploids are located primarily in the mountainous parts of the study area and towards the lowlands are gradually being replaced by diploids, forming a contact zone. We compare the main morphological characteristics of both species. Due to the geographical proximity of the study species, the morphological differences between them are clear outside the contact zones. However, within the contact zones, we recorded hybridisations that obscure the morphological differences between species, probably due to the presence of triploid hybrids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Alnus Mill. (Betulaceae) contains more than 40 species which are widely distributed across the whole Northern Hemisphere and in South America (Dawson 1990; Liu et al. 2009, 2019; Vít et al. 2017). Most of the species are deciduous trees or shrubs, but there are also a few evergreen species. It is well known that many species of this genus are so-called pioneer trees. They usually occupy disturbed habitats and, because of their mycorrhizal and Frankia symbiotic associations, are able to improve the soil conditions and grow faster than competing tree species (Liu et al. 2009). Alnus glutinosa (L.) Gaertn. is a major component of the deciduous forests of Europe. The species occupies wet and riparian habitats and soils with high levels of ground water. This often results in a patchy distribution for this species but, where it does form stands, it represents an important component of riverine systems. The species is wind-pollinated, the seeds ripen in late autumn and winter and are dispersed by water or wind (McVean 1953, 1956; Beatty et al. 2015).
The phylogeographic history of Alnus glutinosa is well known (King and Ferris 1998; Cubry et al. 2015; Havrdová et al. 2015). Havrdová et al. (2015) showed that the species survived the last Ice Age in multiple refugia located in the Iberian, Apennine and Balkan Peninsulas. From these refugia, the species colonised northern Europe. Expansion times for Iberian, Apennine and Balkan populations were estimated to be 9 270, 8 895 and 10 455 years BP, respectively (Havrdová et al. 2015). The colonising lineages have met several times and have formed secondary contact zones in central and north Europe. In the southern refugia, the occurrence of both diploid and tetraploid populations has been revealed fairly recently (Mandák et al. 2016). These tetraploid populations are located in the putative glacial refugia and have never colonised northern Europe (Mandák et al. 2016). Due to their morphological distinctiveness, allopatric distribution and genetic differentiation, two new tetraploid species of Alnus genus were described by Vít et al. (2017). While in the Iberian Peninsula, the tetraploid A. lusitanica Vít, Douda and Mandák is present; in the west of the Balkan Peninsula, we find another polyploid species, A. rohlenae Vít, Douda and Mandák. Both species are autotetraploids and there are no indications that the closely related A. incana has been involved in their evolutionary history (Mandák et al. 2016).
The anticipated geographical range of the newly discovered autotetraploid (2n = 4x = 56) Alnus rohlenae covers the whole western part of the Balkan Peninsula and can be classified as an endemic tree species. From our observations, A. rohlenae replaces A. glutinosa s. str. in this region and therefore probably carries out the same ecological functions as A. glutinosa. s. str. Alnus rohlenae (2n = 4x = 56) differs from the diploid (2n = 2x = 28) A. glutinosa s. str. in its genome size, morphology and also its very high haplotype diversity (Havrdová et al. 2015; Mandák et al. 2016; Vít et al. 2017). The rate of endemism is generally high in all European peninsulas. In the case of the Balkan Peninsula, this is due both to the complex topography and to its function as an important refugium for many taxa (including A. rohlenae) during the last glacial maximum (Hardion et al. 2014).
As the exact distribution range of this new endemic Balkan species is not yet precisely defined, the first aim of this study was to determine its current distribution range using estimation of ploidy level by flow cytometry. The second aim was based on the discovery that if A. rohlenae is in contact with A. glutinosa s. str., they can hybridise to form hybrid zones (Mandák et al. 2016). The hybridisation of these two species results in the formation of a triploid hybrid, however, the frequency of occurrence of this hybrid is unknown (Mandák et al. 2016). In addition, it is possible that some of the triploid individuals are not hybrids between A. glutinosa and A. rohlenae but some of them may be of autotriploid origin (Šmíd et al. 2020). Therefore, in the context of the contact zone between diploids and tetraploids, it is not appropriate always to speak about hybridization between these two species. Hence, we used a combination of flow cytometry for precise ploidy level determination and morphometric analyses to evaluate the main morphological characteristics useful for determination of the di-, tri- and tetraploid taxa. Specifically, we asked: (1) What is the distribution of A. rohlenae in the Balkan Peninsula; and (2) where is the contact zone with A. glutinosa s. str. situated?; also (3) What is the occurrence frequency of the triploid individuals?; and (4) Are there morphological characters to distinguish triploids/hybrids?
Materials and methods
Field sampling
In 2016 and 2017, samples were collected exclusively from natural forest stands located in the Balkan Peninsula (721 individuals and 43 populations) and in 2018, in a contact zone in Serbia (232 individuals and 26 populations) (see Supplement Table 1).
Wherever possible, 20 individuals per population were collected. In each population, samples were collected along a transect, from individual trees at least 50 m apart, i.e. each population represents a linear transect of at least 1 km in length. From each population, three individuals were chosen at random and set aside as herbarium specimens. In total, 953 individuals from 69 populations were collected and analysed by flow cytometry, and 215 individuals from 66 populations that consisted of all three ploidies were subjected to morphometric analysis. However, in the case of triploid individuals, only five voucher specimens were obtained. Individuals collected for morphometric analysis were about the same age and the voucher specimens were collected from most typical branches usually from the south-facing part of the tree. To map the distribution ranges of the individual Alnus species as precisely as possible, we included in this study 27 populations from a previous study (Mandák et al. 2016) (Supplement Table 1). These additional populations were not included in the flow cytometry or morphometric analyses.
Estimation of DNA ploidy level
Fresh leaves were analysed using DAPI flow cytometry (FCM), with the ploidy of each individual determined according to Doležel et al. (2007). Nuclei were isolated by excising approximately 0.5 cm2 of leaf, together with a similar amount of the common daisy [Bellis perennis L.; 2C = 3.38 pg; Schönswetter et al. (2007)] as an internal standard. Samples were placed in Petri dishes containing 0.5 ml of Otto Ι buffer (0.1 M citric acid, 0.5% Tween-20; Doležel et al. 2007). The nuclear suspension was filtered using a 42 μm nylon mesh and incubated for about 5 min at room temperature. Finally, 1 ml of staining Otto II solution (0.4 M Na2HPO4 × 12 H2O, 2 μl/ml of β-mercaptoethanol and 4 μg/ml of DAPI) was added and the samples were measured using a Partec Space cytometer (Partec GmbH, Münster, Germany) equipped with a 365 nm UV-LED as a UV light source for DAPI excitation. Nuclear fluorescence (sample to standard ratio) was calculated to infer DNA ploidy level. Values of nuclear fluorescence were calibrated against chromosome counts—diploid, triploid, tetraploid as described in Mandák et al. (2016).
Multivariate morphometrics
For the morphometric analyses, 215 voucher specimens were collected across all study areas including all three ploidy levels. Diploids were represented by 44 specimens, tetraploids by 166 specimens and triploids by five specimens. From each voucher specimen, three well-developed leaves were chosen and the seven most discriminant morphological characteristics (including one ratio) were selected according to Vít et al. (2017) and measured to evaluate their morphological variation. These were leaf width (LW), leaf length (LL), distance from lamina base to maximum lamina width (BM), petiole length (PL), ratio between the leaf length and leaf width (RL), hairiness of ventral part of the leaf (VH), hairiness of the dorsal part of the leaf (DH) (see Vít et al. 2017 for more details and a complete list of the morphological characters measured). The morphological characteristics were measured manually from voucher specimens using callipers. Leaf hairiness was evaluated using four discrete categories (hairless, slightly hairy, hairy, very hairy). The morphometric data were analysed using “MorphoTools” R scripts in R version 3.2.2 (R Core Team 2014) following the manual of Koutecký (2015). As mentioned above, three measurements of leave characteristics were performed from each individual and each individual measurement was evaluated separately, not as an average of all three measurements. Therefore, each individual used in the statistical analysis is represented by three independent measurements. Standardisation of characters to zero mean and unit standard deviation were carried out prior to the multivariate analyses. Insights into species structure and relationships among all ploidies were gained using principal component analysis (PCA). Canonical discriminant analysis (CDA) was also employed to test the importance of morphological characters in discriminating between the three ploidy groups (2x, 3x and 4x). The proportion of correctly classified individuals was determined by classificatory discriminant analyses. The optimal k value in k-nearest neighbour classification analysis was selected from values with the highest success rate using a manual of Koutecký (2015). Morphological characters for classificatory discriminant analyses were selected based on the results of CDAs.
Because a significant proportion of the populations had their origins on the contact zone, we divided the data set into two sets. The first set (215 individuals, 66 populations) included all the individuals and populations used for morphometric evaluation and the second set (99 individuals, 35 populations) included only populations outside contact zones (“pure”). This was to eliminate samples originating from species crosses (Supplement Table1).
Results
Cytotype composition
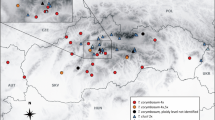
Flow cytometry identified individuals of all three ploidy levels in the study area (Supplement Fig. 1, Supplement Table 1). These are described as belonging to two different species and their putative hybrid, i.e. diploid (2n = 2x = 28; Alnus glutinosa s. str.), triploid (2n = 3x = 42; putative hybrid) and tetraploid (2n = 4x = 56; A. rohlenae) (Fig. 1). Of the 69 populations and 953 individual trees sampled, diploids (A. glutinosa s. str.) occurred in 19.0% (181 individuals), triploids in 1.9% (18 individuals) and tetraploids (A. rohlenae) in 79.1% (754 individuals). The majority of populations sampled (81.2%, 774 individuals from 58 populations) consisted exclusively of one ploidy, the rest of the populations contained ploidy-mixed populations, i.e. two ploidy levels were present (12.8%, 122 individuals from eight populations) or three (6.0%, 57 individuals from three populations). Triploids were found in 10.1% of all collected populations (Fig. 1, Supplement Table 1). The average sample to standard ratios ± S.D. of samples analysed for diploids, triploids, and tetraploid was 0.316 ± 0.036, 0.475 ± 0.023, and 0.602 ± 0.058, respectively. The coefficient of variation (CV) for the diploids was 4.10% ± 1.1 SD, for triploids 3.66% ± 0.99 SD and for tetraploids 2.84% ± 0.58 SD.
Alnus rohlenae distribution range together with the triploid individuals and with adjacent populations of diploid A. glutinosa s. str. Combined map of the data collected for this study and that published by Mandák et al. (2016) (circled in black; Supplement Table 1)
The distribution of tetraploid A. rohlenae is strictly related to the Dinaric Alps and the north-western Pindos Mountains in Greece. Only a few populations were found in lowland areas in Bosnia and Herzegovina and Serbia (Fig. 1). Rarely, A. rohlenae also occurred in Mediterranean areas, such as in Croatia at the mouth of the Neretva River. The contact zone is defined by the occurrence of diploid and tetraploid parents together with their putative triploid hybrid. The contact zones with mixed-ploidy populations were found in Bosnia and Herzegovina in the catchment area of the Neretva River, southern Serbia and northern Greece. The distributions of different ploidies are largely allopatric, with the diploid A. glutinosa s. str. distributed mostly outside the area of the Dinaric Alps, except for the western parts in Bosnia and Herzegovina and Croatia (Fig. 1).
Morphometry
Firstly, to obtain a general picture, not influenced by the expected hybridisation, we excluded from our analysis the samples from the Serbian contact zone (Supplement Table 1). We carried out a PCA analysis of samples out of the contact zones (only pure diploid or pure tetraploid populations) (Fig. 2 A, Supplement Table 1). In this dataset, 81 individuals of A. rohlenae from 29 populations and 18 individuals of A. glutinosa s. str. from six populations were used. This PCA accounts for 49.7% of the total variance in the first component axis and 22.1% in second component axis and is mostly correlated with leaf width (LW), leaf length (LL) and the distance from lamina base to maximum lamina width (BM). Canonical discriminant analysis histogram of pure populations clearly separated diploids from tetraploids (Fig. 3A). The most tightly correlated characters in the first component axis were petiole length (PL), hairiness of ventral part of leaf (VH) and hairiness of dorsal part of leaf (DF) (Supplement Table 2).
PCA (A–B) analyses of Alnus glutinosa s. str., A. rohlenae and triploid individuals. A PCA analysis of pure diploid and tetraploid populations, where diploids are represented by white triangles and tetraploids by grey circles. B PCA analysis including all samples, where diploids are represented by white triangles, tetraploids by grey circles and triploids by black squares
CDA (A–B) analyses of Alnus glutinosa s. str., A. rohlenae and triploids. A CDA histogram of pure populations of A. glutinosa s. str. (white columns) and A. rohlenae (black columns). Grey columns indicate species overlap. B CDA with an overlay of taxon based on cytotype of the individuals in the anticipated contact zone (contact zone in the legend is marked as “hybr.”) (pure diploids white triangles; pure tetraploids white circles; diploids from contact zones black triangles; tetraploids from contact zones grey circles; triploids grey squares)
Next, we analysed the whole dataset including samples from the Serbian contact zone and the mixed populations. Leaf width (LW), leaf length (LL), distance from lamina base to maximum lamina width (BM) are the characters most tightly correlated with the first component axis of PCA analysis, which represents 48.6% of explained variation in the whole dataset. The second component axis explained another 19.3% (Fig. 2B; Supplement Table 2). The CDA analysis of individuals from the contact zones was overlaid by species designations based on ploidy levels. These five groups were defined on the basis of their ploidy. The first and second discriminant axes explained 94.7% and 5.3% of total variance, respectively (Fig. 3B; Supplement Fig. 2; Supplement Table 2). The most tightly correlated characters in the first component axis were distance from lamina base to maximum lamina width (BM), hairiness of ventral part of leaf (VH) and hairiness of dorsal part of leaf (DF) (Supplement Table 2).
The nonparametric k-nearest neighbour approach was used for classificatory discriminant analysis. The data of the three most closely correlated characters (BM, VH, DF; see Supplement Table 2) in the CDA analysis were used in the classificatory discriminant analysis of A. glutinosa s. str., A. rohlenae and triploid individuals. The analysis resulted in poor separation of A. glutinosa s. str. and A. rohlenae (29.5% and 42.8%, respectively). One triploid individual was classified as A. glutinosa s. str. and the remaining four as A. rohlenae. On the other hand, the exclusion of the anticipated hybrid zone from the dataset significantly increased the separation of A. glutinosa s. str. and A. rohlenae (94.4% and 71.6%, respectively) (Supplement Table 3).
Discussion
Distribution range of Alnus rohlenae
So far, it has been reported that A. rohlenae occurs only in a small area of the western part of the Balkan Peninsula in mountain valleys and deep canyons (Mandák et al. 2016; Vít et al. 2017). However, extending the sample collections demonstrates the occurrence of the species in a larger part of the Western Balkan Peninsula both near the sea (the mouth of the Neretva River into the Adriatic Sea) and in the lowlands of southern Serbia. The probability of the species distribution in other areas than those identified is rather low (Šmíd, unpublished data). We did not detect the species in the southern part of Albania or Greece as expected, as the climate in the Mediterranean region is not suitable for this species. Only in the northern area of its occurrence in Serbia and Bosnia can it be assumed that the occurrence of tetraploids may be several tens of kilometres north of the already discovered localities.
Alnus rohlenae and A. glutinosa s. str. grow only in the vicinity of rivers, on the shores of lakes and in floodplain forests. The environmental conditions throughout most of the distribution range of the tetraploid species are different from those of the diploid species. The Balkan Peninsula, and especially its mountainous western parts, has a diversity of climatic conditions, depending both on elevation and on proximity to the Mediterranean Sea (Griffiths et al. 2004). Therefore, the distribution of tetraploids is limited by the presence of non-drying rivers and streams and by increasing elevations. As already mentioned, A. rohlenae is confined to the bottoms of deep canyons. There are likely specific microclimatic conditions that favour the presence of the tetraploids but prevent the diploids from expanding there. Polyploids generally occupy different ecological niches than diploids and are very well adapted to them (Mandák et al. 2016). The adaptation of polyploids to a specific microclimatic condition is known from many examples (e.g. Maherali et al. 2009 or Ramsey 2011). The polyploids for example might be better adapted to drier and warmer climate by larger stomata, leaf thickness or leaf hairiness (Madlung 2013; Bomblies 2020). These adaptations can allow them to grow where diploids are not able to survive and also prevent them to expand from these peripheral areas of species occurrence (Madlung 2013). Therefore, we hypothesise that the adaptation processes in A. rohlenae have resulted in an array of different morphological and ecological traits. One such adaptation could be, for example, greater leaf hairiness of the tetraploids, which could reduce water evaporation from the leaves during the hot summer periods. These have turned the species into an endemic plant and have prevented their expansion out of their current distribution range. This specialisation is further suggested by the observation that most of the area occupied by the tetraploids is not separated from that of the diploids by any obvious geographical barrier. The only obvious geographical barrier separating diploids from tetraploids is the Neretva River canyon in Bosnia. This also forms a barrier for some other taxa (Sotiropoulos et al. 2007; Kučera et al. 2008; Surina et al. 2014). Therefore, climatic conditions related to the area of Dinaric Alps may be responsible for species distribution and expansion. The Balkan Peninsula, which served as an important European refugium during the glaciations, has similarly influenced the development of many other species of plants and animals (Surina et al. 2011; Cakovic et al. 2015; Sotiropoulos et al. 2007).
Possible hybridisation with Alnus glutinosa s. str.
In addition to diploid and tetraploid populations, Mandák et al. (2016) also found three populations containing triploid individuals (of 209 populations sampled, triploids occurred in 1.9%). The proportion of triploids in this study is significantly lower than in ours, because they were discovered by chance and the existence of a new species (A. rohlenae) was not foreseen. The triploids never seemed to form separate populations but to always occur among diploids or tetraploids. This suggests the triploids are the result of a number of ongoing process (Šmíd et al., 2020). The hybrid zone, where the triploids occur, was determined as a tension hybrid zone. This zone is constantly moving depending on the climate changes in past or in recent times (Šmíd et al. 2020).
Hence, the determination of the triploid ploidy level in an individual does not necessarily mean that its evolution was by the hybridisation of diploid and tetraploid species. Instead, other processes may have played important roles, such as the fusion of reduced and unreduced gametes from one species and introgressive hybridisation. One such process (Mandák et al. 2016) is illustrated by the triploid individuals occurring in Austria. These lie outside the distribution range of the tetraploids. Based on an analysis of microsatellite variation, it has been shown these individuals originated by hybridisation of reduced and unreduced gametes of A. glutinosa s. str. Šmíd et al. (2020) found that triploid individuals produced triploid progeny in addition to diploid and tetraploid. This means that not all triploids are necessarily hybrids between A. glutinosa s. str. and A. rohlenae even in the contact zones. Another possibility of triploid origin is hybridisation between diploid A. glutinosa s. str. and tetraploid A. rohlenae.
Triploid individuals occupy intermediate habitats in the contact areas between diploids and tetraploids and the frequency of their occurrence is generally low. Several processes can account for this. The distribution ranges of both species are largely allopatric, so the chances of hybrid development are low. Nevertheless, Alnus species are anemophilous and known for their high rates of pollen production. Hence, hybridisation is not necessarily limited to areas where the distribution ranges overlap.
Šmíd et al. (2020) described the germination ability of triploid seeds across the discovered contact zone in southern Serbia. The germination varied greatly between individuals, even within the same population (from zero to 71.7%). The reasons for the low triploid occurrence may therefore be explained in several ways. Some of the triploids have lower germinability caused by an inappropriate combination of genes (Yang et al. 2000; Aleza et al. 2009, 2010, 2012). While individuals derived from a triploid mother plant with high seed germination ability may be disadvantaged during initial growth by selection or less competitiveness. Of course, the underlying question also remains unanswered—are the triploids even able to be distinguished in the field, i.e. morphologically?
Morphometric evaluation
The results of the PCA and CDA analyses of pure diploids and tetraploids show the two species are morphologically differentiated. This confirms the distinctness of Alnus rohlenae from A. glutinosa s. str. as shown by Vít et al. (2017). If we compare the same measured characteristics with the research of Vít et al. (2017), we find that the most important characteristic distinguishing both species is hairiness of ventral part of leaf (VH) and hairiness of dorsal part of leaf (DH). From our observations, these characteristics were the most important for rapid visual recognition of both species. In both cases, the leaves of A. rohlenae are more covered with hairs than leaves of A. glutinosa s. str. This is confirmed by a study by Vít et al. (2017), which states that both species are morphologically similar, but differ in hairiness of dorsal sides of leaf lamina (as well as annual shoots and buds). The presence of more hairs on the leaves can be associated with the adaptation of plants to higher temperatures, more sunlight or protection against insects. Higher hairiness is quite common in species occurring in drier and warmer areas (Ackerly et al. 2000; Šingliarová et al. 2011; Madlung 2013).
Despite the fact that the two species were morphologically distinguished from each other on the basis of selected morphological characteristics, diploids and tetraploids are still morphologically very similar to each other, especially in the places of their contact. The CDA analysis of pure populations together with a species designation overlay based on cytotype of the contact zone also showed a large overlap between the individual groups (Fig. 3B). These overlaps are largely based on the differentiation between A. glutinosa and A. rohlenae (Fig. 3A). The differentiation between the two species is not so great. Therefore, if the hypothetical hybrids can have both intermediate and parental traits, they can significantly overlap. This overlap can be affected, for instance, by bidirectional gene flow or by the presence of fertile triploid hybrids (and autotriploids).
Hence, a similarity in the morphological features of autotetraploids to their diploid parents is common, rather than rare (Ramsey and Ramsey 2014). A similarity of morphological characters between diploids and polyploids has been described previously, for example in hybrid zones between Centaurea jacea and Ranunculus parnassifolius (Vanderhoeven et al. 2002; Cires et al. 2009). These plants are morphologically similar to their parents but genetically quite different from them, as in our case with Alnus species in the contact zone.
We did not find enough triploid individuals for a proper morphometric evaluation, as only five voucher specimens were examined, of which two were collected without fully developed leaves, thus distinguishing taxa by morphological differences was not possible. We also found that triploids may arise via several routes and their genetic composition (and likely also morphologies) may depend strongly on the parent from which they arose (Šmíd et al. 2020). For these reasons, it can be difficult or even impossible to distinguish them morphologically from either parent.
References
Ackerly DD, Dudley SA, Sultan SE et al (2000) The evolution of plant ecophysiological traits: recent advances and future directions. Bioscience 50:979–995. https://doi.org/10.1641/00063568(2000)050[0979:TEOPET]2.0.CO;2
Aleza P, Juárez J, Ollitrault P, Navarro L (2009) Production of tetraploid plants of non-apomictic citrus genotypes. Plant Cell Rep 28:1837–1846. https://doi.org/10.1007/s00299-009-0783-2
Aleza P, Juárez J, Cuenca J, Ollitrault P, Navarro L (2010) Recovery of citrus triploid hybrids by embryo rescue and flow cytometry from 2x × 2x sexual hybridisation and its application to extensive breeding programs. Plant Cell Rep 29:1023–1034. https://doi.org/10.1007/s00299-010-0888-7
Aleza P, Juárez J, Cuenca J, Ollitrault P, Navarro L (2012) Extensive citrus triploid hybrid production by 2x × 4x sexual hybridizations and parent-effect on the length of the juvenile phase. Plant Cell Rep 31:1723–1735. https://doi.org/10.1007/s00299-012-1286-0
Beatty GE, Montgomery WI, Tosh DG, Provan J (2015) Genetic provenance and best practice woodland management: a case study in native alder (Alnus glutinosa). Tree Genet Genomes 11(5):92. https://doi.org/10.1007/s11295-015-0919-1
Bomblies K (2020) When everything changes at once: finding a new normal after genome duplication. Proc R Soc B Biol Sci 287(1939):20202154. https://doi.org/10.1098/rspb.2020.2154
Caković D, Stešević D, Schönswetter P, Frajman B (2015) How many taxa? Spatiotemporal evolution and taxonomy of Amphoricarpos (Asteraceae, Carduoideae) on the Balkan Peninsula. Org Divers Evol 15:429–445. https://doi.org/10.1007/s13127-015-0218-6
Cires E, Cuesta C, Peredo EL, Revilla MÁ, Prieto JAF (2009) Genome size variation and morphological differentiation within Ranunculus parnassifolius group (Ranunculaceae) from calcareous screes in the Northwest of Spain. Plant Syst Evol 281:193–208. https://doi.org/10.1007/s00606-009-0201-9
Cubry P, Gallagher E, O’Connor E, Kelleher CT (2015) Phylogeography and population genetics of black alder (Alnus glutinosa (L.) Gaertn.) in Ireland: putting it in a European context. Tree Genet Genomes 11:1–15. https://doi.org/10.1007/s11295-015-0924-4
Dawson JO (1990) Interactions among actinorhizal and associated plant species. In: Schwintzer CR, Tjepkema JD (eds) The biology of Frankia and actinorhizal plants. Academic Press, San Diego, California, pp 299–316
Doležel J, Greilhuber J, Suda J (2007) Estimation of nuclear DNA content in plants using flow cytometry. Nat Protoc 2:2233–2244. https://doi.org/10.1038/nprot.2007.310
Douda J, Doudová J, Drašnarová A et al (2014) Migration patterns of subgenus Alnus in Europe since the last glacial maximum: a systematic review. PLoS ONE 9(2):1–14. https://doi.org/10.1371/journal.pone.0088709
Griffiths HI, Kryštufek B, Reed JM (2004) Balkan biodiversity - pattern and process in the European hotspot. Springer, Berlin
Hardion L, Verlaque R, Saltonstall K, Leriche A, Vila B (2014) Origin of the invasive Arundo donax (Poaceae): a trans-Asian expedition in herbaria. Ann Bot 114:455–462. https://doi.org/10.1093/aob/mcu143
Havrdová A, Douda J, Krak K et al (2015) Higher genetic diversity in recolonized areas than in refugia of Alnus glutinosa triggered by continent-wide lineage admixture. Mol Ecol 24:4759–4777. https://doi.org/10.1111/mec.13348
King AR, Ferris C (1998) Chloroplast DNA phylogeography of Alnus glutinosa (L.) Gaertn. Mol Ecol 7:1151–1161
Koutecký P (2015) MorphoTools: a set of R functions for morphometric analysis. Plant Syst Evol 301:1115–1121. https://doi.org/10.1007/s00606-014-1153-2
Kučera J, Tremetsberger K, Vojta J, Marhold K (2008) Molecular study of the Cardamine maritima group (Brassicaceae) from the Balkan and Apennine Peninsulas based on amplified fragment length polymorphism. Plant Syst Evol 275:193–207. https://doi.org/10.1007/s00606-008-0061-8
Liu J, Ren B-Q, Luo P, Ren Z-L (2009) Karyotype Analysis of Alnus Mill. (Betulaceae) species originating from northeastern Asia. Silvae Genet 59:219–223. https://doi.org/10.1515/sg-2010-0026
Liu K, Meng W, Zheng L, Wang L, Zhou S (2019) Cytogeography and chromosomal variation of the endemic east Asian herb Lycoris radiata. Ecol Evol 9(12):6849–6859. https://doi.org/10.1002/ece3.5252
Madlung A (2013) Polyploidy and its effect on evolutionary success: old questions revisited with new tools. Heredity 110:99–104. https://doi.org/10.1038/hdy.2012.79
Maherali H, Walden AE, Husband BC (2009) Genome duplication and the evolution of physiological responses to water stress. New Phytol 184:721–731. https://doi.org/10.1111/j.1469-8137.2009.02997.x
Mandák B, Vít P, Krak K et al (2016) Flow cytometry, microsatellites and niche models reveal the origins and geographical structure of Alnus glutinosa populations in Europe. Ann Bot 117:107–120. https://doi.org/10.1093/aob/mcv158
McVean DN (1953) Account of Alnus glutinosa (L.) Gaertn. for the biological flora of the British Isles. J Ecol 41:447–466. https://doi.org/10.2307/2257070
McVean DN (1956) Ecology of Alnus glutinosa (L.) Gaertn. III. Seedling establishment V. Notes of some British Alder populations. J Ecol 44:115–218. https://doi.org/10.2307/2256824
R Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/
Ramsey J (2011) Polyploidy and ecological adaptation in wild yarrow. Proc Natl Acad Sci 108:7096–7101. https://doi.org/10.1073/pnas.1016631108
Ramsey J, Ramsey TS (2014) Ecological studies of polyploidy in the 100 years following its discovery. Philos Trans R Soc Lond B Biol Sci 369(1648):20130352. https://doi.org/10.1098/rstb.2013.0352
Schönswetter P, Lachmayer M, Lettner C et al (2007) Sympatric diploid and hexaploid cytotypes of Senecio carniolicus (Asteraceae) in the eastern Alps are separated along an altitudinal gradient. J Plant Res 120(6):721–725. https://doi.org/10.1007/s10265-007-0108-x
Šingliarová B, Hodálová I, Mráz P (2011) Biosystematic study of the diploid-polyploid Pilosella alpicola group (Asteraceae) with variation in breeding system: patterns and processes. Taxon 60:450–470. https://doi.org/10.1002/tax.602014
Šmíd J, Douda J, Krak K, Mandák B (2020) Analyses of population genetic differentiation and hybrid viability across a hybrid zone between two Alnus species using microsatellites and cpDNA markers. Genes 11(7):770. https://doi.org/10.3390/genes11070770
Šmíd J, Douda J, Krak K, Mandák B, Phylogeography of Alnus glutinosa and A. rohlenae (Betulaceae) on the Balkan Peninsula, inferred from chloroplast DNA sequences, microsatellites and niche modelling. (unpublished; manuscript in preparation).
Sotiropoulos K, Eleftherakos K, Džukić G et al (2007) Phylogeny and biogeography of the alpine newt Mesotriton alpestris (Salamandridae, Caudata), inferred from mtDNA sequences. Mol Phylogenetics Evol 45:211–226. https://doi.org/10.1016/j.ympev.2007.03.012
Surina B, Schönswetter P, Schneeweiss GM (2011) Quaternary range dynamics of ecologically divergent species (Edraianthus serpyllifolius and E. tenuifolius, Campanulaceae) within the Balkan refugium. J Biogeogr 38:1381–1393. https://doi.org/10.1111/j.1365-2699.2011.02493.x
Surina B, Pflanzelt S, Einzmann HJR, Albach DC (2014) Bridging the Alps and the middle east: evolution, phylogeny and systematics of the genus Wulfenia Jacq. (Plantaginaceae). Taxon 63:843–858. https://doi.org/10.12705/634.18
Vanderhoeven S, Hardy O, Vekemans X et al (2002) A morphometric study of populations of the Centaurea jacea complex (Asteraceae) in Belgium. Plant Biol 4:403–412. https://doi.org/10.1055/s-2002-32327
Vít P, Douda J, Krak K, Havrdová A, Mandák B (2017) Two new polyploid species closely related to Alnus glutinosa in Europe and north Africa – an analysis based on morphometry, karyology, flow cytometry and microsatellites. Taxon 66(3):567–583. https://doi.org/10.12705/663.4
Yang XH, Yang JH, Luo CJ (2000) Review and prospect of mulberry polyploidy breeding. Agric Sci Zhejiang 6:304–306
Acknowledgements
We would like to especially thank to Jakub Polák, Eva Hodková, Vít Dvořák, Petr Chajma and Kateřina Machynková for their help with data collection, data analysis and laboratory work. Sandy Lang and Gabrielle A. Filippi are thanked for helping with the language of the manuscript. This study was supported by the Czech Science Foundation (18-03028S) and is part of long-term research development project RVO 67985939. Other financial support was provided by Grant agency of CULS (CIGA00002052).
Funding
Czech Science Foundation (number 18-03028S); Grant agency of CULS (CIGA00002052).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Code availability
Not applicable.
Data availability
Data are available from the corresponding author on reasonable request.
Additional information
Communicated by Oliver Gailing.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Šmíd, J., Vít, P., Douda, J. et al. Distribution, hybridisation and morphological variation in Alnus rohlenae (Betulaceae) an endemic species of the Balkan Peninsula. Eur J Forest Res 141, 641–648 (2022). https://doi.org/10.1007/s10342-022-01466-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10342-022-01466-4