Abstract
Nitrogen (N) plays a vital role in litter decomposition by interacting with microbial activity in temperate and boreal ecosystems. However, in tropical and subtropical forests, where low soil phosphorus (P) availability is widespread, how P affects litter decomposition remains unclear. In the present study, litter-bag method was used to conduct fine root decomposition by P addition experiment in a subtropical natural evergreen broad-leaved forest. Fine roots were collected from two tree species: Cunninghamia lanceolata and Castanopsis carlesii, and their mixture in equal proportion as well. At the end of 2-year experiment, we found an enhancement of soil total P, available P, pH and microbial biomass carbon content, and increased abundance of all the examined microbial groups and total PLFA biomass after P addition. Annual decomposition constants of fine roots of both tree species and the mixture (0.615–1.049) were all elevated, but to different extents by P addition, with fine roots of lower initial P concentration corresponding to higher increase in decomposition rate. Average acid phosphatase activities during decomposition were significantly decreased, but there was a general increase for β-glucosidase (βG), cellobiohydrolase (CBH), phenol oxidase, peroxidase (PerOx) and N-acetyl glucosaminidase (NAG) activities. The response ratios of enzyme activities, which differed for the three experimental fine roots, corresponded to the individual increase in decomposition rates. Correlation analysis demonstrated that fine root decomposition rates were significantly related to initial P concentration, extractives and acid-insoluble fractions of fine roots, and activities of βG, CBH, NAG and PerOx as well. Taken together, our results demonstrate that fine root decomposition can be constrained by P availability through affecting microbial activity in this subtropical natural forest.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fine roots (≤ 2 mm in diameter) account for 33% of annual terrestrial net primary production (Jackson et al. 1997). Fine root decomposition can not only sustain soil fertility through release of plant nutrients, but also influence net ecosystem carbon storage through formation of soil organic matter or release of CO2 to the atmosphere. Due to being somewhat buffered from climate extremes in the soil, root decomposition is likely to be particularly sensitive to nutrient limitations to decomposer organisms (Swift et al. 1979). Furthermore, since roots are belowground, they are hard to study and there are considerably fewer root decomposition experiments than those of leaf litter (Silver and Miya 2001; King et al. 2005; Fujimaki et al. 2008; Berg and McClaugherty 2014).
It has long been believed that nitrogen (N) plays a critical role in litter decomposition, particularly in soil N-poor boreal and temperate ecosystems (Vitousek and Howarth 1991; Cornwell et al. 2008). However, in tropical and subtropical area where highly weathered soil is common, a couple of studies demonstrate that phosphorus (P) availability could be more important than N in litter decomposition (Hobbie and Vitousek 2000; Xu and Hirata 2005; Wieder et al. 2009). For example, a previous study from our group in subtropics of China also reported that fine root decomposition was significantly correlated with initial P-related chemistry of roots in seven forests (Lin et al. 2011), where soil P availability is far below the global average (National Soil Survey Office of China 1998). Nevertheless, there are few studies directly investigating the effect of P addition on litter decomposition, and the results are inconsistent, with some being positive (Hobbie and Vitousek 2000; Kaspari et al. 2008) and others neutral (McGroddy et al. 2004; Cleveland et al. 2006; Barantal et al. 2012). To our knowledge, there is no direct evidence for P limitation on litter decomposition provided by P addition experiments in subtropical forests. Likewise, the importance of microbial activity and the nature and extent of P addition on litter decomposition remain unclear.
While microbial activity is constrained by the availability and quality of carbon (Demoling et al. 2008; Brackin et al. 2014), it can also be limited by P, especially when levels of labile carbon are high and available P are low, such as in highly weathered humid tropical soils (Cleveland et al. 2002; Gnankambary et al. 2008). However, there have been few reports to date on the effects of P addition on soil microbial activity in tropical or subtropical areas (Liu et al. 2012), and much less is known about the links between decomposition and microbial dynamics (Allison and Vitousek 2005). Since extracellular enzymes are produced by microbes to facilitate organic matter decomposition (Burns et al. 2013), studies have been conducted to establish the linkages between microbial extracellular enzymes and litter decomposition (Sinsabaugh and Moorhead 1994; Wright and Reddy 2001; Schimel and Weintraub 2003; Allison and Vitousek 2005; Waring 2013). It is generally accepted that studying extracellular enzymes involved in litter decomposition can inform ecosystem nutrients cycling (Rejmánková and Sirová 2007), and key enzymes activities can be used to indicate microbial nutrient acquisition status (Schimel and Weintraub 2003). For example, response of extracellular enzymes can be used to explain increased and decreased litter decomposition rates after N addition in temperate forest (Carreiro et al. 2000). However, in P-limited tropical and subtropical forests, less is known about the effects of P addition on extracellular enzymes and their effect on litter decomposition. Meta-analysis by Marklein and Houlton (2012) demonstrated that extracellular acid phosphate activity generally decreased with P supply across different ecosystems. Therefore, according to resource allocation model developed by Sinsabaugh and Moorhead (1994), extracellular enzyme production may shift following P addition, and these shifts may have the potential to affect decomposition (Waring 2013).
Subtropical China belongs to a monsoonal climate, where the climax vegetation is evergreen broad-leaved forest developed on highly weathered soils (Huang et al. 2013). Over the decades, most native broad-leaved forests have been harvested, and plantations of more productive tree species, such as Cunninghamia lanceolata (CUL), have been cultivated to satisfy the demand for timber and other forest products (Yang et al. 2009).
In this study, we attempted to elucidate whether P availability in a subtropical natural forest serves as a proximate control over fine root decomposition by manipulating endogenous (fine root) and exogenous (soil) P availability. Individual fine roots of CUL and Castanopsis carlesii (CAC), and their mixture (MIX) of equal proportions were determined to have significantly different P concentrations. Furthermore, a P fertilization treatment was added to test the decompositional response of these fine roots to increasing P availability in soils. In order to reveal the link between decomposition and microbial activity, we measured six extracellular enzyme activities during fine root decomposition and determined the soil microbial community composition by phospholipid fatty acid (PLFA) analysis at the end of experiment. Specifically, we tested the following hypotheses: (1) decomposition rates of all three fine roots will increase with P addition, among which the largest increase will be found in CUL fine roots which had the lowest initial P concentration; (2) soil microbial PLFAs and C-mineralizing enzyme activity will increase with P addition, and fine root acid phosphate activity will decrease, because the few studies examining nutrient limitation of microbes in highly weathered humid tropical soils all have concluded that P is the most limiting resource (Gnankambary et al. 2008); and (3) decomposition rate of the three fine roots will be significantly correlated with both initial root chemistry and root enzyme activity after this 2-year experiment. This research will further our understanding of the pattern and control of fine root decomposition, providing new information on the consequences of converting natural evergreen broad-leaved forests to coniferous plantations in a subtropical region with low soil P availability.
Materials and methods
Site description
This study was carried out from 2013 to 2015 in Geshikao Nature Reserve in Sanming, Fujian, China (26°11′N, 117°28′E, 250–500 m a.s.l.). This area borders with Wuyi Mountain on the northwest and experiences a subtropical monsoonal climate with mean annual air temperature of 19.4 °C, relative humidity of 79% and potential evapotranspiration of 1585 mm. Mean annual precipitation is 1700 mm, most of which occurs from March to August. The growing season is relatively long with an annual frost-free period of around 300 days. Soils are ultisols (lateritic red earths) formed from sandstone and are approximately 30–70 cm in depth.
The forest in this Nature Reserve is an old-growth (> 200 years) evergreen, broad-leaved forest dominated by C. carlesii (CAC, 82% of basal area). Other overstory trees include Castanopsis kawakamii, Schima superba, Litsea subcoriacea and Elaeocarpus decipiens. Stand density is about 1995 trees per hectare, with mean DBH and tree height of 20.0 cm and 11.9 m, respectively. Over the past several decades, a large area of C. lanceolata (CUL) plantations was established adjacent to this Nature Reserve after harvesting the natural broad-leaved forest (Liu et al. 2017). CUL is one of the most popular planting tree species, with an area being over 9 million ha and accounting for 30% of all plantations in China (Lei 2005).
Previous study has showed that fine roots of CAC, the dominant tree species in climax vegetation, contained higher concentrations of P and water-soluble carbon fraction decomposed much faster than fine roots of CUL (Lin et al. 2011). In this study, analysis of variance detected significant differences (P < 0.05) in initial root chemistry between both species and the mixture except for initial fine root C concentrations (Table 1). Differences in the initial chemistry of fine roots of two common, subtropical tree species (CAC and CUL) and their roots in mixture (MIX) allowed for an effective test of substrate quality on decomposition in response to P addition in a P-limited, subtropical forest in southern China.
Experiment design
Fine root collection and litterbag preparation
In the Nature Reserve and a nearby CUL plantation, fine roots (≤ 2 mm in diameter) of CAC and CUL trees were collected by sieving soil from the upper 0–20 cm in December 2012, gently washed in tap water to remove adherent soil particles, and air-dried for 24 h. Dead fine roots were discarded, and live fine roots of two tree species were picked out. The way to collect target fine roots was described in detail in our previous study (Lin et al. 2011).
Litterbags (10 cm × 10 cm) were constructed of 0.2-mm nylon mesh at the bottom and 0.3-mm nylon mesh on the top. Each bag was filled in 2 g of air-dried fine roots. There were three fine root litter types used in this experiment, single species roots of CAC and CUL and a bi-species root mixture of equal proportion (MIX). Subsamples of fine roots were taken to determine moisture content and initial chemical composition.
Fine root decomposition
Six 2 × 3 m plots were established in the natural forest. Three of the six plots were randomly assigned as P addition plots which received 100 g P m−2 year−1 in the form of superphosphate, while no P was applied to the remaining three plots. The inorganic P addition was divided into six applications and applied evenly every 2 months starting at the beginning of the study. Each of the six plots was split into three equal parts to bury litterbags of each of the fine root types (CAC, CUL and MIX).
In February 2013, twenty-four bags of each fine root type were inserted into incisions in the soil at a 45° angle to a depth of 10-cm mineral soil. Four replicate bags were retrieved randomly after 3, 6, 9, 12, 15 and 24 months of deployment. Three of the four litterbags from each set collected were placed into individual plastic bags for transport back to the laboratory, while the fourth bag was stored on ice. Once in the laboratory, all litterbags were gently cleaned of all adherent soil and other extraneous materials. The replicate stored on ice was used to measure enzyme activity, and the three remaining replicates were oven-dried at 70 °C to constant mass for the determination of remaining mass and then milled for chemical analysis.
Enzyme assays
The activities of β-glucosidase (βG), cellobiohydrolase (CBH), N-acetyl glucosaminidase (NAG), acid phosphatase (AP), phenol oxidase (PhOx) and peroxidase (PerOx) were assessed for subsamples of fine roots from each plot based on method of Sinsabaugh et al. (1993) with modifications. Briefly, 0.5 g of fine root (cut to about 0.5 cm long) was added to 125 mL of 50 mM acetate buffer (pH = 5.0) and blended for 10 min. Then, 200 μL aliquots were dispensed into 96-well microplates with sixteen replicates per sample per assay. Each replicate contained 200 μL of sample suspension and 50 μL of substrate. Hydrolytic enzyme activity was measured using 10 μM methylumbelliferone (MUB)-linked substrates including 4-MUB-β-D-glucopyranoside, 4-MUB-β-D-cellobioside, 4-MUB-N-acetyl-β-D-glucosaminide and 4-MUB-phosphate for βG, CBH, NAG and AP assays, respectively. The hydrolytic enzyme assays included a blank with 250 μL of acetate buffer, a substrate correcting blank containing 200 μL of acetate buffer and 50 μL of substrate, and a quenching blank containing 200 μL of acetate buffer and 50 μL of standard (10 μM 4-methylumbelliferone). Each of these had eight replicates per plate. In addition, there were eight replicates containing 200 μL of sample suspension and 50 μl of standard for each sample to calculate the quenching coefficient.
PhOx and PerOx activities were measured using L-3,4-dihydroxyphenylalanine (L-DOPA) as the substrate. There were sixteen replicates per sample, and eight replicates for blanks including 250 μL of acetate buffer, 200 μL of acetate buffer and 50 μL of 25 mM L-DOPA, and 200 μL of sample suspension with 50 μL of acetate buffer. For PerOX assays, 10 μL of 0.3% H2O2 solution was added to each sample well. The microplates were incubated at 20 °C in the dark for 4 h for hydrolytic enzyme analysis and 18 h for oxidative enzyme analysis (optimal duration of assay determined prior to experiment). After incubation, 10 μL of 1 M NaOH was added to each well to terminate the reaction. A SpectraMax M5 Microplate Reader (MDS Analytical Technologies, Molecular Devices, USA) was used to quantify absorbance for the oxidative enzyme samples at 450 nm and fluorescence for hydrolytic enzyme samples at 365 nm excitation and 450 nm emission filters. Enzyme activities were calculated as the rate of substrate converted in nmol g−1 h−1 for hydrolytic enzymes and μmol g−1 h−1 for oxidative enzymes (DeForest 2009).
Chemical analyses of fine roots
To express all mass, C-fraction, and nutrient indices, we subsampled the residual fine roots for ash determination (500 °C for 5 h) on an ash-free, dry-mass basis. To assess root carbon fraction concentrations including water-soluble, acid-soluble and acid-insoluble structural components, we used the forest products serial digestion technique (Ryan et al. 1990; Hendricks et al. 2000). Please refer to Lin et al. (2011) for detailed description in terms of the determination of C, N and P.
Soil sampling
In February 2015, five soil cores were taken from each plot to form a composite at a depth of 0–10 cm with a 3.5-cm-diameter soil corer at the time of litterbag collection. All soil samples were kept in sealed plastic bags and taken to the field laboratory within 2 h. Gravel, roots and large organic residues were manually removed before passing through a 2-mm sieve. Each sample was separated into two parts: one stored at 4 °C (< 1 week) for analyses of microbial biomass Carbon (MBC), and the other air-dried for analyses of physical and chemical properties.
Soil MBC in fresh soil samples was determined by the chloroform fumigation–extraction method (Vance et al. 1987). Please refer to our previous study for detailed description of soil MBC assay (Liu et al. 2018).
Soil total C and N were measured in a single analysis using an auto CN analyzer (Elementar Vario MAX, Germany). Soil pH was determined using a pH meter with a soil-to-water ratio of 1:2.5 in weight (Wan et al. 2015). Soil was digested using sulfuric acid and perchloric acid, and total P was analyzed using a Prodigy High Dispersion inductively coupled plasma optical emission spectrometry (ICP-OES) (Teledyne Technologies Incorporated, USA). Sulfuric acid and hydrochloric acid were used to extract and measure available P with a Prodigy High Dispersion ICP-OES (Bao 2000).
PLFA analysis
Microbial community structure was determined for each of the fresh soil samples using the phospholipid fatty acid (PLFA) analysis procedure. Lipid extraction was conducted as described by White et al. (1979) and Bardgett et al. (1996). In summary, an extraction mixture of chloroform, methanol and citrate buffer (1:2:0.8 by volume) was used on 10 g of dried sieved soil. Two phases to this extraction process were used, viz. the chloroform phase followed by the citrate buffer phase. The lipid materials were recovered and evaporated under nitrogen gas during the chloroform phase. These lipids were then re-suspended in chloroform and then fractionated on silicic chromatography acid columns (6 mL volume with 500 mg silicic acid) using 5 mL chloroform to elute neutral lipids, 5 mL acetone to elute glycolipids and 5 mL methanol to elute phospholipids. Nitrogen gas was used to dry the phospholipid fraction before a mild alkaline methanolysis was conducted to prepare fatty acid methyl esters (FAMEs). Nitrogen gas was again used to evaporate off the solvent, and the FAMEs were stored at − 20 °C until gas chromatography analysis (GC). For GC preparation, 200 μl of HPLC-grade ethyl acetate was used to dissolve and separate the individual FAMEs. Samples were run on a GC equipped with a capillary column (SGE 25QC3 BP-5 25 m × 0.32 μm film thickness) and flame ionization detector (run at 150 °C, 4 °C/min to 250 °C, with a helium carrier gas at 2.7 mL min−1; auxiliary gases included nitrogen at 30 mL min−1, hydrogen at 30 mL min−1 and air at 400 mL min−1 for 25 min). A chromographic retention time comparison to bacterial methyl esters was used to identify and quantify the separated FAMEs (Supelco Bacterial Acid Methyl Esters CP Mix 47080-U). Relative nmoles per g of dry soil was used to express the abundance of individual FAMEs using standard nomenclature (Tunlid et al. 1989; Frostegård et al. 1993a, b). FAMEs that have been identified as biomarkers were grouped as follows: gram-positive bacteria (GP) (i14:0, i15:0, a15:0, i16:0, i17:0 and a17:0), gram-negative bacteria (GN) (16:1ω9c, 16:1ω7c, cy17:0ω7c, 18:1ω7c, cy19:0ω7c), fungal (18:2ω6c and 18:1ω9c), actinomycetes (ACT) (10Me16:0, 10Me17:0, 10Me18:0, 10Me17:1ω7c, 10Me18:1ω7c), arbuscular mycorrhizae (VAM) (16:1ω5c) and anaerobes (DMA) (15:0 DMA). The ratio of fungal to bacterial FAMEs (F/B) was used to estimate the relative importance of the bacterial and fungal metabolic presence in the community (Li et al. 2018).
Statistical analysis
All data were analyzed with the Statistical Program for Social Science (SPSS) software. Percent dry-weight loss was arcsine square-root-transformed prior to all statistical analyses to approximate the normal distribution of residuals and to reduce variance heterogeneity. Statistical significance was established at P < 0.05, unless otherwise mentioned.
The model for constant potential mass loss is represented by the following equation: x/x0 = exp (− kt), where x is the mass at time t (days), x0 is the initial mass, the constant k is the decomposition coefficient, and t is the elapsed time.
Repeated-measures ANOVA (P addition and root species as main effects and time as a within subject factor) was used to test the enzyme activity among the different P addition treatments and root species. When Mauchly’s test of sphericity was not passed (the variances were not equal), the data were adjusted by the Greenhouse–Geisser method. In order to compare the magnitude of P addition and root species effects in the experiments, we averaged the time-integrated enzyme activity across 2-year decomposition period and calculated the enzyme response ratios after P addition (e.g., Elser et al. 2009; Fatemi et al. 2016) using the following equation:
where Eaddition and Econtrol are the mean enzyme activity in the P addition treatment and the control, respectively.
A nested general linear model (GLM) type III sum of squares was used to test the effects on decomposition rates and enzyme activities of root species (roots of single species and the mixture) and soil treatment (control vs. added P). Treatments (Control and added P) and root species were considered fixed factors. One-way analysis of variance (ANOVA) with Tukey’s HSD test was performed to test for differences of initial fine root chemistry, enzyme response ratios between root species, and PLFA composition between soil treatments.
Relationships between dry mass remaining with the initial litter quality indices such as N, P, extractives, acid-insoluble contents, C/N, acid-insoluble/N and C/P ratios, and also with enzyme activity were explored using Pearson correlation coefficients.
Results
Dry-mass loss
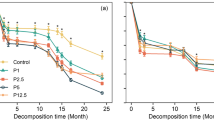
A nested general linear model (GLM) was used to test the effects of root species (roots of single species and the mixture) and soil treatment (control vs. added P) on decomposition rates, which showed significant effect of root species and soil treatment, and also interactive effect of root species and soil treatment on decomposition rate (Table 2, P < 0.001). Over a 2-year period, in the control plots only, the highest decomposition rate was observed in fine root of CAC followed by CUL and then MIX (Table 2). The P addition accelerated mass loss in all three fine root types (Fig. 1); however, decomposition rates between control and P addition increased the most in CUL, and to a lesser degree in the MIX, while fine root of CAC were only marginally enhanced (Table 2).
Fine root extracellular enzyme activity
Extracellular enzyme activities differed significantly according to root species and time (Table 3, P < 0.001). Across the three fine roots in the control, root of CAC generally showed the highest enzyme activity during the experiment time, followed by MIX root, with the root of CUL showing the lowest enzyme activity. There was also significant effect on all the six measured extracellular enzyme activities in terms of P addition and time (Table 3, P < 0.001; Fig. 2). Across 2 years of decomposition, time-integrated acid phosphatase (AP) activities in the added P treatment decreased significantly, while the activities of β-1,4-glucosidase (βG), cellobiohydrolase (CBH), β-1,4-N-acetylglucosaminidase (NAG) and phenol oxidase (PhOx) significantly increased, and phenol oxidase (PhOx) activity was unchanged (Fig. 2). The response ratio of enzyme activity to P addition differed for the three fine root types (Fig. 3). Response ratios of AP, CBH and PhOx activity for MIX and CUL roots were significantly higher than those of CAC root (P < 0.05). The highest response ratio of PerOx activity was seen in the root of CUL, while there were no significant differences in the response ratios of βG and NAG activities among the three root types (Fig. 3).
Averaged enzyme activities for individual fine roots of Castanopsis carlesii (CAC) and C. lanceolata (CUL) and their mixtures (MIX) in the control (CT) and added P treatments during 2-year decomposition in subtropical natural evergreen broad-leaved forest. Error bar is ± se (n = 3). Different letters indicate significant differences between control and added P treatment (P < 0.05)
Response ratios of enzyme activities for individual fine roots of Castanopsis carlesii (CAC) and Cunninghamia lanceolata (CUL) and their mixtures (MIX) following P addition (n = 3). Error bar is ± se (n = 3). Acid phosphatase (AP); β-glucosidase (βG); cellobiohydrolase (CBH); N-acetyl glucosaminidase (NAG); phenol oxidase (PhOx); peroxidase (PerOx). Different letters indicate significant differences among fine root species (P < 0.05)
Correlation between the rate of mass loss, initial root chemistry and enzyme activity
Data from all three fine root types in the control plots were pooled together to examine the correlation between mass loss rate (k), initial root chemistry and average enzyme activity after 2 years of decomposition. As shown in Table 5, k was positively correlated with initial root litter concentrations of P and extractive fraction and average enzyme activities of CBH and PerOx, and negatively correlated with initial root litter concentration of acid-insoluble fraction (P < 0.05).
Under P addition alone, the response ratios of CBH, PerOx and PhOx activities showed significant negative correlations with the initial P concentration of fine roots, while the response ratio of AP activity was positively correlated (P < 0.01, Table 6).
Soil physicochemical properties and microbial community analysis
After 2 years of P addition, there were no significant differences in soil organic carbon (SOC), total N and C/N ratio between the control versus P treatments, but there were significantly higher concentrations of total P, available P and pH. Dissolved organic C (DOC) nearly doubled and microbial biomass C (MBC) increased 40% by the end of the experiment under P addition compared to the controls (Table 4).
Soil microbial biomass and community composition differed significantly between added P and control soils. Added P soils were characterized by higher total PLFAs and a greater abundance of compounds associated with gram-positive bacteria (GP), gram-negative bacteria (NP), arbuscular mycorrhizal (VAM), actinomycetes (ACT) and fungal (F) compared with control soils. The ratio of GP/GN was significantly lower in added P plots, whereas we did not observe a significant difference in the ratio of F/B between soil treatments (Fig. 4).
Discussion
Litter substrate quality such as organic fractions and original nutrient status is of particular importance in controlling decomposition rate (Cornu et al. 1997; Hobbie 2000; Berg and McClaugherty 2014), especially under a given microclimatic condition (Vitousek and Sanford 1986; Yang and Chen 2009). A previous study which decomposed fine roots in their original stands demonstrated that fine root decomposition rates were strongly correlated with initial P-related parameters in mid-subtropical forests (Lin et al. 2011). Unsurprisingly, the present study also showed that fine root decomposition rates were strongly correlated with initial fine root P concentration, extractive and acid-insoluble fractions in multiple root types in the same forest (Table 5). Together with the previous study, results again indicate that the initial substrate P could limit decomposition in subtropical forests with low soil P availability.
In addition to fine root quality, ambient nutrient levels have been shown to be important in determining fine root decomposition in forests (Guerrero-Ramírez et al. 2016). Faster decomposition rates have been reported in response to increased nitrogen, phosphorus and both nutrients combined (Ostertag 2001). In contrast, some studies have reported no change in root decomposition rate in response to nutrient enrichment (Snyder and Rejmánková 2015). However, it is not clear how initial chemistry of fine roots can potentially mediate the effect of ambient nutrients on fine root decomposition. In this study, we directly tested the effect of added P on the decomposition of roots with different qualities (i.e., carbon quality and nutrient content) and found a significant increase in decomposition rates for all of the experimental fine roots (Fig. 1), which clearly supports the hypothesis that P is a limiting factor for fine root decomposition in this subtropical forest. Furthermore, the increase in decomposition rate under P addition differed among the three fine root types and was inversely correlated with initial fine root P concentration (Table 1), with the highest increase observed in CUL, followed by MIX, and the smallest increase in decomposition rate being seen in CAC (Table 2). Under P addition, higher increase rate of decomposition in roots with lower P concentration may imply that short supply of P to decomposer in these roots is more pronounced than that in roots with higher P concentration when the different fine roots were decomposed in the same environment (Güsewell and Gessner 2009), which could further evidence the constraint of P in the decomposition.
Soil microbes play a key role in forest litter decomposition and associated nutrient release (Weand et al. 2010). In turn, recent studies have demonstrated the importance of P in regulating soil microbial biomass and community composition in tropical forests (Cleveland et al. 2002; Treseder 2008; Liu et al. 2012; Li et al. 2015). The present study was carried out in a natural forest, which had been protected for more than 200 years with extremely low soil P availability (2 mg kg−1). As expected, P addition increased soil MBC and pH (Table 4), which was in line with previous studies in both secondary forest (Li et al. 2015) and old-growth forest (Liu et al. 2012) with low soil available P (lower than 4 mg kg−1) in southern China. Together, these studies indicate that P is a potential limiting factor for microbial growth in this subtropical forest.
P addition not only increased the soil MBC but also altered soil microbial community composition in the present study (Fig. 3). We found an increase in the abundance of GP, GN, VAM, ACT, fungi, F/B ratio and total microbial PLFAs with P addition (Fig. 3). Interestingly, VAM are expected to decrease with nutrient input (Read 1999), and it is frequently stated that high soil P markedly reduces or eliminates mycorrhizal colonization (Smith and Read 2008). While it is well known that P availability does influence colonization and the formation of arbuscules, the magnitude of the effect is strongly influenced by the host species and environmental factors. When soil P availability is very low, mycorrhizal colonization may actually be inhibited so that small additions result in increased values. An increase in VAM and F/B ratio was also reported by Li et al. (2015) in a tropical secondary forest, where the P availability is always low and microbial utilization of C is constrained by P. Likewise, P fertilization was a key factor in controlling microbial abundance, and abundance of soil fungi was a more sensitive indicator of soil fertility than soil bacterial abundance in red soils of this region (He et al. 2008). Soil available P in the present study was even lower than that in the study of Li et al. (2015), which could explain why we also observed increased microbial PLFAs under P addition. Moreover, an decrease in the ratio of GP/GN was consistent with previous studies, suggesting that GP bacteria were associated with oligotrophic communities and the utilization of more recalcitrant C resources (Wan et al. 2015). With P addition, there was increase in DOC and pH, which could also contribute to the alteration of microbial community (Huang et al. 2016). All in all, P addition may create an environment favoring microbial activity in this subtropical forest with very low soil P availability.
Because decomposition requires the expression of extracellular enzymes that break down the structural components of plant litter, and enzyme measurements can directly follow the functional responses of the microbial community to litter quality and other environmental factors, enzyme activities can therefore provide a more intimate connection between nutrient availability and litter decomposition (Sinsabaugh and Moorhead 1994; Sinsabaugh et al. 2002). The present study correlated fine root decomposition in the control plots with extracellular enzyme activity, which showed that decomposition rates were significantly correlated with activities of CBH and PerOx (Table 5). This result demonstrates that other than substrate quality parameters, selected enzyme activities might serve as indices for the complex hydrolytic and oxidative reactions that underlie microbial decomposition, which is in conformity with the study of Sinsabaugh and Moorhead (1994). Moreover, we observed a consistent decrease in acid phosphatase activity and overall increase in the five other N- or C-mineralizing enzyme activities when P was added to the system (Fig. 4). A meta-analysis by Marklein and Houlton (2012) demonstrates that extracellular acid phosphatase activities are highly responsive to P supply, among plant roots and bulk soils, and across a wide array of terrestrial ecosystems. Increased soil pH value under P addition (Table 4) may favor the decrease in acid phosphatase activity, which agrees with Sinsabaugh et al. (2008) who found that P-mineralizing enzyme activities declined with pH in a global study across a broad range of biomes and soil pH levels. Likewise, inorganic P input reduces microbial dependence on organic P mineralization via acid phosphatase (Olander and Vitousek 2000).
Total PLFA biomass and abundance of microbial groups in soils of the control (CT) and added P treatments after 2-year decomposition. Total: total microbial PLFAs; fungi: fungal PLFAs; F/B: the ratio of fungal to bacterial PLFAs; GP: gram-positive bacterial PLFAs; GN: gram-negative bacterial PLFAs; GP/GN: the ratio of gram-positive bacterial to gram-negative bacterial PLFAs; VAM: arbuscular mycorrhizal fungi PLFAs; ACT: actinomycetes PLFAs. Values are mean ± SD (n = 5). Different letters indicate significant differences between control and P addition treatment (P < 0.05)
Microbial production of C-, N- and P-mineralizing enzymes is energetically expensive (Allison and Vitousek 2005); therefore, microbial decomposers may reallocate their energy following satisfaction of P supply (Sinsabaugh et al. 2013) and energetic investment in C- and/or N-mineralizing enzymes should theoretically increase with enhanced P availability (Sinsabaugh and Moorhead 1994). Our results corroborate these previous findings as we saw increased activities of five C- and N-mineralizing enzyme, in three root types with P addition. However, the response ratios of enzyme activity were different for the three root types (Fig. 2). Correlation analysis revealed that there were significant relationships between fine root initial P concentration and response ratios of enzyme activities of AP, CBH, PerOx and PhOx (Table 6). This indicates that enzyme activity of the fine roots with lower initial P concentration, such as CUL (0.33 g kg−1), responded more strongly to P addition and contributed to a higher increase in decomposition rate. In other words, with P addition, greater reduction in AP may lead to greater increases in C-mineralizing enzymes of fine root with lower initial P concentration and eventually lead to higher increased rates of decomposition.
Conclusion
Significant increases in soil total P, available P, pH and MBC following P addition may contribute to a general decrease in acid phosphatase activity and significant increases in five other enzyme activities in fine roots of two tree species and their mixture. However, differences in enzyme response ratios under P addition may reflect the contrasting substrate quality of individual fine root types, notably initial P concentration, which leads to increased decomposition rates for fine roots with lower initial P concentration. Taken together with the strong correlation between mass loss rates and initial substrate quality and enzyme activity, the present study demonstrates that P can constrain fine root litter decomposition and some key enzyme activities can be related to decomposition rate in this subtropical natural forest with low soil P availability.
References
Allison SD, Vitousek PM (2005) Responses of extracellular enzymes to simple and complex nutrient inputs. Soil Biol Biochem 37:937–944
Bao S (2000) Soil and agricultural chemistry analysis, 3rd edn. China Agriculture Press, Beijing
Barantal S, Schimann H, Fromin N, Hattenschwiler S (2012) Nutrient and carbon limitation on decomposition in an Amazonian moist forest. Ecosystems 15:1039–1052
Bardgett RD, Hobbs PJ, Frostegård Å (1996) Soil fungal:bacterial ratios following reductions in the intensity of management of an upland grassland. Biol Fertil Soils 22:261–264
Berg B, McClaugherty C (2014) Plant litter: decomposition, humus formation, carbon sequestration. Springer, Berlin
Brackin R, Robinson N, Lakshmanan P, Schmidt S (2014) Soil microbial responses to labile carbon input differ in adjacent sugarcane and forest soils. Soil Res 52(3):307–316
Burns RG, Deforest JL, Marxsen J, Sinsabaugh RL, Stromberger ME, Wallenstein MD, Weintraub MN, Zoppini A (2013) Soil enzymes in a changing environment: current knowledge and future directions. Soil Biol Biochem 58:216–234
Carreiro MM, Sinsabaugh RL, Repert DA, Parkhurst DF (2000) Microbial enzyme shifts explain litter decay responses to simulated nitrogen deposition. Ecology 81:2359–2365
Cleveland CC, Townsend AR, Schmidt SK (2002) Phosphorus limitation of microbial processes in moist tropical forests: evidence from short-term laboratory incubations and field studies. Ecosystems 5:680–691
Cleveland CC, Reed SC, Townsend AR (2006) Nutrient regulation of organic matter decomposition in a tropical rain forest. Ecology 87:492–503
Cornu S, Luizao F, Rouiller J, Lucas Y (1997) Comparative study of litter decomposition and mineral element release in two Amazonian forest ecosystems: litter bag experiments. Pedobiologia 41:456–471
Cornwell WK, Cornelissen JHC, Amatangelo K, Dorrepaal E, Eviner VT, Godoy O, Hobbie SE, Hoorens B, Kurokawa H, Pérez-Harguindeguy N, Quested HM, Santiago LS, Wardle DA, Wright IJ, Aerts R, Allison SD, van Bodegom P, Brovkin V, Chatain A, Callaghan TV, Díaz S, Garnier E, Gurvich DE, Kazakou E, Klein JA, Read J, Reich PB, Soudzilovskaia NA, Vaieretti MV, Westoby M (2008) Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol Lett 11:1065–1071
DeForest JL (2009) The influence of time, storage temperature, and substrate age on potential soil enzyme activity in acidic forest soils using MUB-linked substrates and L-DOPA. Soil Biol Biochem 41:1180–1186
Demoling F, Nilsson LO, Baath E (2008) Bacterial and fungal response to nitrogen fertilization in three coniferous forest soils. Soil Biol Biochem 40:370–379
Elser JJ, Kyle M, Steger L, Nydrick K, Baron JS (2009) Nutrient availability and phytoplankton nutrient limitation across a gradient of atmospheric nitrogen deposition. Ecology 90:3062–3073
Fatemi FR, Fernandez IJ, Simon KS, Dail DB (2016) Nitrogen and phosphorus regulation of soil enzyme activities in acid forest soils. Soil Biol Biochem 98:171–179
Frostegård A, Bååth E, Tunlid A (1993a) Shifts in the structure of soil microbial communities in limed forests as revealed by phospholipid fatty acid analysis. Soil Biol Biochem 25:723–730
Frostegård A, Tunlid A, Bååth E (1993b) Phospholipid fatty acid composition, biomass, and activity of microbial communities from two soils types experimentally exposed to different heavy metals. Appl Environ Microbiol 59:3605–3617
Fujimaki R, Takeda H, Wiwatiwitaya D (2008) Fine root decomposition in tropical dry evergreen and dry deciduous forests in Thailand. J For Res 13:338–346
Gnankambary Z, Ilstedt U, Nyberg G, Hien V, Malmer A (2008) Nitrogen and phosphorus limitation of soil microbial respiration in two tropical agroforestry parklands in the south-Sudanese zone of Burkina Faso: the effects of tree canopy and fertilization. Soil Biol Biochem 40:350–359
Guerrero-Ramírez NR, Craven D, Messier C, Potvin C, Turner BL, Tanya Handa I (2016) Root quality and decomposition environment, but not tree species richness, drive root decomposition in tropical forests. Plant Soil 404:125–139
Güsewell S, Gessner MO (2009) N: P ratios influence litter decomposition and colonization by fungi and bacteria in microcosms. Funct Ecol 23:211–219
He JZ, Zheng Y, Chen CR, He YQ, Zhang LM (2008) Microbial composition and diversity of an upland red soil under long-term fertilization treatments as revealed by culture-dependent and culture independent approaches. J Soil Sediment 8:349–358
Hendricks JJ, Aber JD, Nadelhoffer KJ, Hallett RD (2000) Nitrogen controls on fine root substrate quality in temperate forest ecosystems. Ecosystems 3:57–69
Hobbie SE (2000) Interactions between litter lignin and soil nitrogen availability during leaf litter decomposition in a Hawaiian montane forest. Ecosystems 3:484–494
Hobbie SE, Vitousek PM (2000) Nutrient limitation of decomposition in Hawaiian forests. Ecology 81:1867–1877
Huang WJ, Liu JX, Wang YP, Zhou GY, Han TF, Yin Li (2013) Increasing phosphorus limitation along three successional forests in southern China. Plant Soil 364:181–191
Huang JS, Hu B, Qi KB, Chen WJ, Pang XY, Bao WK, Tian GL (2016) Effects of phosphorus addition on soil microbial biomass and community composition in a subalpine spruce plantation. Eur J Soil Biol 72:35–41
Jackson RB, Mooney HA, Schulze ED (1997) A global budget for fine root biomass, surface area, and nutrient contents. Proc Natl Acad Sci USA 94:7362–7366
Kaspari M, Garcia MN, Harms KE, Santan M, Wright SJ, Yavitt JB (2008) Multiple nutrients limit litterfall and decomposition in a tropical forest. Ecol Lett 11:35–43
King JS, Pregitzer KS, Zak DR, Holmes WE, Schmidt K (2005) Fine root chemistry and decomposition in model communities of north-temperate tree species show little response to elevated atmospheric CO2 and varying soil resource availability. Oecologia 146:318–328
Lei JF (2005) Forest resources in China. China Forestry Press, Beijing, p 172 (in Chinese)
Li J, Li ZA, Wang FM, Zou B, Chen Y, Zhao J, Mo QF, Li YW, Li XB, Xia HP (2015) Effects of nitrogen and phosphorus addition on soil microbial community in a secondary tropical forest of China. Biol Fertil Soils 51:207–215
Li YQ, Qing YX, Lyu MK, Chen SD, Yang ZJ, Lin CF, Yang YS (2018) Effects of artificial warming on different soil organic carbon and nitrogen pools in a subtropical plantation. Soil Biol Biochem 124:161–167
Lin CF, Yang YS, Guo JF, Chen GS, Xie JS (2011) Fine root decomposition of evergreen broadleaved and coniferous tree species in mid-subtropical China: dynamics of dry mass, nutrient and organic fractions. Plant Soil 338:311–327
Liu L, Gundersen P, Zhang T, Mo JM (2012) Effects of phosphorus addition on soil microbial biomass and community composition in three forest types in tropical China. Soil Biol Biochem 44:31–38
Liu XF, Lin TC, Yang ZJ, Vadeboncoeur MA, Lin CF, Xiong DC, Lin WS, Chen GS, Xie JS, Li YQ, Yang YS (2017) Increased litter in subtropical forests boosts soil respiration in natural forests but not plantations of Castanopsis carlesii. Plant Soil 418:141–151
Liu XF, Chen SD, Yang ZJ, Lin CF, Xiong DC, Lin WS, Xu C, Chen GS, Xie JS, Li YQ, Yang YS (2018) Will heterotrophic soil respiration be more sensitive to warming than autotrophic respiration in subtropical forests? Eur J Soil Sci. https://doi.org/10.1111/ejss.12758
Marklein AR, Houlton BZ (2012) Nitrogen inputs accelerate phosphorus cycling rates across a wide variety of terrestrial ecosystems. New Phytol 193:696–704
McGroddy ME, Silver WL, de Oliveira RC Jr (2004) The effect of phosphorus availability on decomposition dynamics in a seasonal lowland Amazonian forest. Ecosystems 7:172–179
National Soil Survey Office of China (1998) Soils of China. Chinese Agriculture Press, Beijing, pp 483–486 (in Chinese)
Olander LP, Vitousek PM (2000) Regulation of soil phosphatase and chitinase activity by N and P availability. Biogeochemistry 49:175–191
Ostertag R (2001) Effects of nitrogen and phosphorus availability on fine-root dynamics in Hawailan montane forests. Ecology 82:485–499
Read DJ (1999) Mycorrhiza—the state of the art. In: Varma A, Hock B (eds) Mycorrhiza. Springer, Berlin
Rejmánková E, Sirová D (2007) Wetland macrophyte decomposition under different nutrient conditions: relationships between decomposition rate, enzyme activities and microbial biomass. Soil Biol Biochem 34:526–538
Ryan MG, Melillo JM, Ricca A (1990) A comparison of methods for determining proximate carbon fractions of forest litter. Can J For Res 20:166–171
Schimel JP, Weintraub MN (2003) The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: a theoretical model. Soil Biol Biochem 35:549–563
Silver WL, Miya RK (2001) Global patterns in root decomposition: comparisons of climate and litter quality effects. Oecologia 129:407–419
Sinsabaugh RL, Moorhead DL (1994) Resource-allocation to extra-cellular enzyme-production—a model for nitrogen and phosphorus control of litter decomposition. Soil Biol Biochem 26:1305–1311
Sinsabaugh RL, Antibus RK, Linkins AE, McClaugherty CA, Rayburn L, Repert D, Weiland T (1993) Wood decomposition: nitrogen and phosphorus dynamics in relation to extracellular enzyme activity. Ecology 74:1586–1593
Sinsabaugh RL, Carreiro MM, Repert DA (2002) Allocation of extracellular enzymatic activity in relation to litter composition, N deposition, and mass loss. Biogeochemistry 60:1–24
Sinsabaugh RL, Lauber CL, Weintraub MN, Ahmed B, Allison SD, Crenshaw C, Contosta AR, Cusack D, Frey S, Gallo ME, Gartner TB, Hobbie SE, Holland K, Keeler BL, Powers JS, Stursova M, Takacs-Vesbach C, Waldrop MP, Wallenstein MD, Zak DR, Zeglin LH (2008) Stoichiometry of soil enzyme activity at global scale. Ecol Lett 11:1252–1264
Sinsabaugh RL, Manzoni S, Moorhead DL, Richter A (2013) Carbon use efficiency of microbial communities: stoichiometry, methodology and modeling. Ecol Lett 16:930–939
Smith SE, Read D (2008) Colonization of roots and anatomy of arbuscular mycorrhizas. In: Smith S, Read D (eds) Mycorrhizal symbiosis, 3rd edn. Academic Press, Cambridge, pp 42–90
Snyder JM, Rejmánková E (2015) Macrophyte root and rhizome decay: the impact of nutrient enrichment and the use of live versus dead tissue in decomposition studies. Biogeochemistry 124:45–59
Swift MJ, Heal OW, Anderson JM (1979) Decomposition in terrestrial ecosystems, vol 5. University of California Press, Berkerley
Treseder KK (2008) Nitrogen additions and microbial biomass: a meta-analysis of ecosystem studies. Ecol Lett 11:1111–1120
Tunlid A, Hoitink HAJ, Low C, White DC (1989) Characterization of bacteria that suppress Rhizoctonia damping-off in bark compost media by analysis of fatty acid biomarkers. Appl Environ Microbiol 55:1368–1374
Vance E, Brookes P, Jenkinson D (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Vitousek PM, Howarth RW (1991) Nitrogen limitation on land and in the sea -how can it occur. Biogeochemistry 13:87–115
Vitousek PM, Sanford RJ (1986) Nutrient cycling in moist tropical forest. Annu Rev Ecol Syst 17:137–167
Wan XH, Huang ZQ, He ZM, Yu ZP, Wang MH, Davis Murray, Yang YS (2015) Soil C: N ratio is the major determinant of soil microbial community structure in subtropical coniferous and broadleaf forest plantations. Plant Soil 387:103–116
Waring BG (2013) Exploring relationships between enzyme activities and leaf litter decomposition in a wet tropical forest. Soil Biol Biochem 64:89–95
Weand MP, Arthur MA, Lovett GM, McCulley RL, Weathers KC (2010) Effects of tree species and N additions on forest floor microbial communities and extracellular enzyme activities. Soil Biol Biochem 42:2161–2173
White DC, Davis WM, Nickels JS, King JD, Bobbie RJ (1979) Determination of the sedimentary microbial biomass by extractible lipid phosphate. Oecologia 40:51–62
Wieder WR, Cleveland CC, Townsend AR (2009) Controls over leaf litter decomposition in wet tropical forests. Ecology 90(12):3333–3341
Wright AL, Reddy KR (2001) Phosphorus loading effects on extracellular enzyme activity in everglades wetland soils. Soil Sci Soc Am J 65:588–595
Xu XN, Hirata E (2005) Decomposition patterns of leaf litter of seven common canopy species in a subtropical forest: N and P dynamics. Plant Soil 273:279–289
Yang XD, Chen J (2009) Plant litter quality influences the contribution of soil fauna to litter decomposition in humid tropical forests, southwestern China. Soil Biol Biochem 41:910–918
Yang YS, Guo JF, Chen GS, Yin YF, Gao R, Lin CF (2009) Effects of forest conversion on soil labile organic carbon fractions and aggregate stability in subtropical China. Plant Soil 323:153–162
Acknowledgements
The research was financially supported by the National Natural Science Foundation of China (Nos. 31770663 and 31270584). We are grateful to Mr. Jim Nelson for providing valuable comments and doing proofreading on the manuscript and Dr. Daheng He for good suggestions in doing statistics analysis.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by Agustín Merino.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lin, C., Lin, W., Chen, S. et al. Phosphorus addition accelerates fine root decomposition by stimulating extracellular enzyme activity in a subtropical natural evergreen broad-leaved forest. Eur J Forest Res 138, 917–928 (2019). https://doi.org/10.1007/s10342-019-01211-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10342-019-01211-4