Abstract
The present study has been carried out to evaluate the effect of a composite edible coating of 2 % Sodium alginate and 0.2 % Olive oil with combination of 1 % ascorbic acid and 1 % citric acid on the post harvest nutritional quality and shelf life of Ber fruit stored at 25 ± 2 °C and 65 % R.H. The coatings reduced the decay occurrence, weight loss, accumulation of total soluble solids (TSS) and total sugars in Ber fruit and enhanced the level of antioxidants. The delayed activity of polygalacturonase (PG), Pectate lyase (PL) and Pectin methyl esterase (PME) was noticed in coated fruits than that of the control fruit indicating the reduced softening and ripening process. These findings suggest that the composite edible coating tested under the current study has the potential to control decaying incidence of Ber fruit, extends its storage life and also improves its valuable nutritional characteristics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ber (Ziziphus mauritiana Lamk.) is an indigenous and economically important tropical fruit which is also known as Indian jujube, belonging to the family Rhamnaceae. More than 300 varieties of Ber have been listed in India, but only few of them are commercially important. In Gujarat, ‘Gola’ is one of the most cultivated varieties of Ber, which ripens earlier than others. The richness of the pulp of Ber in nutritive compounds has been widely recognized and it is reported to contain a wide array of phytochemicals and minerals such as amino acids, carbohydrates, ascorbic acid, flavonoids, phenolic acids, vitamins A and C, phosphorus, calcium, and iron (Choi et al. 2011).
However, due to perishable nature and poor shelf life of Ber, high post-harvest losses are major constraints in developing Ber fruit industry (Salunkhe and Kadam 1995). The Ber fruit has extremely short storage life of 2–4 days at ambient temperature and therefore rapid perishability is the main problem. During its peak season, due to the surplus of fruits in the local market, a substantial quantity goes to waste, resulting in heavy postharvest losses (Pareek et al. 2009).
The present study has been undertaken with an aim of minimizing postharvest losses in Ber fruits by extending their shelf life with the application of composite edible coating, due to their economic relevance and the problems occurring in their preservation. Edible coatings have long been used and become one of the most convenient post harvest techniques, which have achieved considerable attention in recent years because of their advantages over synthetic films. The efficacy of edible films in retarding moisture, oxygen, aromas and solute transport can be improved by incorporating additives such as antioxidants, antimicrobials, colorants, flavors, fortifying nutrients and spices in film formulation (Pranoto et al. 2005).
A system integrated with the following four active compounds have been evaluated under the current study: Sodium alginate and olive oil were used as the main components of the coating solution where as ascorbic acid and citric acids were added to it as additives to reduce the browning effect and to improve the quality of fruit. Sodium alginate forms a strong, translucent, glossy film, which is soluble in water, acid and alkali, making them a good choice for coating whole fruits and vegetables (Wang et al. 2007). The most widely used commercial antibrowning formulations available today use calcium salts and ascorbate (Rupasinghe et al. 2005).
Therefore, the present study was designed to evaluate the efficacy of composite coating on the extension of postharvest shelf life and maintaining the quality of Ber fruit by considering the influence of sodium alginate and olive oil on the physico chemical and biochemical attributes of Ber fruit during its post harvest storage.
Materials and methods
Materials
Ber fruits (Ziziphus mauritiana Lamk.) of ‘Gola’ variety were harvested at their commercial maturity stage from an orchard field of Balasinor village belonging to Kheda District of Gujarat state, India. Sodium alginate of Himedia brand, Mumbai (India) and the L-Ascorbic acid and Citric acid of Qualigens Fine Chemicals brand, Mumbai (India) were procured through local chemical suppliers, while the Food-grade refined olive, edible grade oil (92 % purity) was purchased from the local market of Anand town, Gujarat.
Preparation of coatings
The method of Olivas et al. (2007) was followed for preparing the sodium alginate coating suspension. The suspension of alginate (2.0 % w/v) containing glycerol (0.75 % v/v), a plasticizer, was stirred by a magnetic stirrer at 80 °C for 20 min. Then the olive oil (0.2 % v/v) was mixed with alginate solution to form a composite coating solution, and it was further stirred using a magnetic stirrer for 30 min (Martin-Diana et al. 2008) to achieve an emulsion by a complete dispersion of the alginate, glycerol and olive oil. This solution was then allowed to stand for 1 h and used to coat the fruits.
Application of coatings
Harvested fruit were brought to the research laboratory, washed with water, and selected them based on their uniformity in size, shape and color. The fruit having defects, injuries and blemishes were discarded. These selected fruit were dipped in 2 % sodium hypochlorite for 2 min so as to remove the superfluous and infectious impurities, followed by rinsing with tap water and air-dried. After air drying, these disinfected fruits were grouped into four batches with 20 fruits (380 g) in each batch and they were subjected to the following edible coating treatments by dipping batch wise for 5 min: T1- (Sodium alginate 2 % + Olive oil 0.2 %), T2- (Sodium alginate 2 % + Olive oil 0.2 % + Ascorbic acid 1 %), T3- (Sodium alginate 2 % + Olive oil 0.2 % + Citric acid 1 %) and control C (Treated with water). After giving treatment, all the fruits were allowed to dry at room temperature (25 ± 2 °C, 65 % relative humidity) and subsequently stored in food grade storage bags of 42 μm thickness at 25 ± 2 °C and 65 % relative humidity. The storage conditions were selected on the basis of physiology and marketability of ber fruit as the aim of the present study was to extend their shelf life at ambient conditions. The efficacy of these tested treatments for improving the post harvest shelf life and nutritional quality of Ber fruit has been evaluated by analysing the visual, physico-chemical and biochemical attributes using the following parameters at 0 days and at a regular interval of 3 days until the end of storage period of 9 days.
Weight loss percentage (WLP)
Ber fruit was weighed at the beginning of the experiment (just after giving coating treatment) and thereafter at regular interval of 3 days during the storage period. The difference between the initial and final weight of the fruit was considered as the total weight loss and calculated it as a percentage on a fresh weight basis as per the standard method of AOAC (1994).
Storage life
The shelf life was calculated by counting the days required for the Ber fruit to reach the last stage of ripening, but up to the stage of their marketability. The last stage of ripening was considered when the fruit became soft and wrinkles appeared on the surface of fruit. During storage, the fruits having blemishes or mechanical injury were considered as decay.
Physico-chemical analysis of Ber fruit
The pH and TSS content of the Ber fruit were determined as per the method of AOAC (1994). Which was as follow, 1 g of fruit tissue was crushed in the motor-pastel with water and then this homogenized sample was centrifuged. The pH was measured from the supernatant by pH meter (Model Li120, Elico) and the TSS was measured from the same sample by placing few drops of it on the prism of refractometer (Atago Co., Tokyo, Japan) and the direct reading was taken as described in AOAC (1994). Firmness of fruits was measured by using hand-held penetrometer (EFFEGI-FT444 model). The probe of the penetrometer was having 8 mm diameter and 2 cm deep. For this, probe of instrument was inserted into the peel of fruit and reading was noted from meter in lb.
Biochemical analysis of Ber fruit
The reducing sugars and non-reducing sugars were estimated by following di-nitro-salicylic acid method cited by Thimmaiah (1999). The quantitative analysis of ascorbic acid was carried out by using 2, 6- dinitrophenyl hydrazine, as per the method of Roe (1954). Extraction and estimation of total phenols were carried out by FCR method cited by Thimmaiah (1999).
Enzyme extraction and assay
2 g of mesocarpic pulp tissue of Ber fruit was homogenized in Tris-HCL (20 mM, pH 7.0) containing cysteine-HCL (20 mM), EDTA (20 mM) and Triton X-100 (0.05 %). Then this homogenate was centrifuged at 15,000 × 3 g for 30 min at 4 °C in a refrigerated centrifuge (Eppendorf 5430 R) (Lohani et al 2004). The clear supernatant was collected and used for the enzyme assays. The protein content was measured using the Lowry’s method (Lowry et al. 1951).
Assay of pectin methyl esterase
The activity of pectin methyl esterase was measured as described by Hangermann and Austin (1986), with some modifications. The reaction mixture contained 1 ml pectin solution (0.01 % aqueous solution adjusted to pH 7.5 using 0.1 N NaOH), 0.2 ml NaCl (0.15 M), 0.1 ml bromothymol blue solution (0.01 %), 0.2 ml water and 0.1 ml homogenate. The sample of enzyme was added and absorbance was measured immediately at 0 min and again after 3 min at 620 nm. The difference in absorbance between 0 and 3 min was considered as the measure of PME activity. The activity of PME was determined against the standard curve drawn, as described by Hangermann and Austin (1986). One unit of PME enzyme is defined as the amount of the enzyme required for liberating 1 μmol of methyl ester per minute.
Assay of polygalacturonase
Polygalacturonase activity was assayed by following the method described by Pathak and Sanwal (1998). The reaction mixture contained 0.2 ml sodium acetate (200 mM, pH 4.5), 0.1 ml NaCl (200 mM), 0.3 ml polygalacturonic acid (PGA, 1 % aqueous solution adjusted to pH 4.5), and 0.05 ml of enzyme extract in a total volume of 1.0 ml. The reaction was initiated by the addition of PGA substrate. The mixture was incubated at 37 °C for 1 h and followed by addition of DNS. The reaction was terminated by heating the reaction mixture in a boiling water bath for 5 min. In control tubes, the substrate was added after the heat treatment. The formation of reducing groups was estimated against D-galacturonic acid as the standard after measuring the absorbance at 540 nm. One unit of PG enzyme is defined as the amount of enzyme required to liberate 1 nmol of galacturonic acid per min under the conditions of the enzyme assay (Miller 1951).
Assay of pectate lyase
Pectate lyase activity was measured by using the method described by Moran et al. (1968) with some modifications. The assay carried out in a mixture containing 4 mM sodium acetate buffer (pH 4.5), 0.3 ml polygalacturonic acid (PGA, 1 % aqueous solution adjusted to pH 4.5) and 0.1 ml enzyme preparation in 1 ml total reaction volume. The tubes containing the reaction mixture were incubated at 37 °C for 30 min followed by boiling in a water bath for 2 min to stop the reaction. The absorbance of the reaction mixture was measured at 235 nm. The increase in the absorbance against the control with pre-boiled enzyme was taken as a measure of the pectate lyase activity. All the calculations were carried out according to Moran et al. (1968) and 1 unit of pectate lyase activity was expressed as the amount of enzyme required to liberate 1 nmol of aldehyde groups from PGA per minute under the conditions of the enzyme assay.
Statistical analysis
The study consists of a Randomized Block Design, with three replicates. The data presented in this paper was statistically analysed by SPSS 17 software and the mean and standard deviation (SD) were calculated. The statistical significance of the data was assessed by one way Analysis of variance and LSD test. Mean comparisons were performed using HSD of Tukey’s test to examine if differences between treatments and storage time were significant at P < 0.05. The overall least significance difference (LSD; p ≤ 0.05) was calculated and used it to detect significant differences among all the treatments and control set. Relationships among measurement variables were studied by using the correlation coefficient (Bico et al. 2009).
Results and discussion
Effect on weight loss percentage (WLP) and shelf life
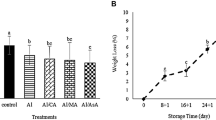
The weight loss of the fruit is mainly associated with respiration and moisture evaporation through their skin. The rate at which water is lost depends on the water pressure gradient between the fruit tissue and the surrounding atmosphere, and the storage temperature (Hernandez-Munoz et al. 2008). The occurrence of weight loss during the fruit storage is normally due to its respiratory process, the transference of humidity and some process of oxidation. Data presented in the Fig. 1 shows that the weight loss of all the treated as well as control Ber fruit studied under the present study had increased gradually throughout the storage period. However, at the end of storage period, the least WLP (22 %) was noticed in the fruit treated with T2 (sodium alginate 2 %, olive oil 0.2 % and ascorbic acid 1 %) and treatment T3 (28 %) whereas the higher WLP (60 %) was observed in control and fruits treated with T1- sodium alginate 2 % and olive oil 0.2 % (50 %). On 6th day of storage period, control fruits were classified as decayed. However, the weight of decayed fruits have been taken only to compare with the treated fruits.These results are in accordance with the findings of Zapata et al. (2008) who showed the beneficial effects of polysaccharide-based alginate coating in retarding the weight loss and maintaining the quality of stored tomatoes. This retardation in weight loss is due to the edible coating created a barrier on the surface of the fruit which reduces the transpiration rate. In this regard, Debeaufort (1998) also stated that the edible coatings are selective barriers that modify internal atmosphere and help in retarding the respiration rate of fruit which leads to the reduction in the weight loss. Moreover, in the present study, sodium alginate, ascorbic acid, olive oil and citric acid excellently proved their ability to lengthen the storage life of Ber fruits. The storage life of control fruits was 3 days, whereas the fruits treated with T2 (sodium alginate + olive oil + ascorbic acid) could maintain their marketable acceptability till 10 days followed by T1 (sodium alginate and olive oil) and T3 (Sodium alginate + olive oil + citric acid) which showed storage life of 9 days for both. Maftoonazad et al. (2008) also reported the shelf-life extension of peaches through sodium alginate..
Effect of composite edible coatings on the weight loss percentage in Ber during its storage at 25 ± 2 °C. [T1- (Sodium alginate 2 % + Olive oil 0.2 %), T2- (Sodium alginate 2 % + Olive oil 0.2 % + Ascorbic acid 1 %), T3- (Sodium alginate 2 % + Olive oil 0.2 % + Citric acid 1 %) and C (Control). Different letters on the bars means significantly different at p ≤ 0.05]
Effect on pH and titratable acidity (TA)
The values of pH of coated and uncoated Ber fruits increased gradually over storage time, with the most significant changes after 3 days of storage, as shown in the Table 1. However, lesser pH values were observed in the coated fruit than that of the control fruit. Coating reduces respiratory and metabolic rates, and thereby the lesser utilization of organic acids, reported by Baraiya et al. (2012). As per the data shown in the Table 1, the value of pH increased from 5.06 (at 0 day) to the highest 6.21 at the end of the storage. On the 6th day, the least pH values (5.57) and (5.17) were noticed in the fruit treated with T2 and T3 respectively due to the addition of ascorbic acid and citric acid which increased the acidity. These results indicate that the treated fruits could retain more acidity than that of the control fruit because the metabolic activities were slowed down due to coating application.
The data summarized in the Table 1 reveals that a gradual decrease in TA was occurred in Ber fruit throughout the storage period. The probable reason for decline in the acidity may be the utilization of organic acids in the respiration and metabolic processes of the fruit. Srinivasa et al. (2002) also suggested that the decrease in acidity has been attributed towards the conversion of organic acids into sugars and their further utilization in the metabolic process of the fruit. The correlation between pH and titrable acidity during storage is significant (at 0.01 level) (Table 3). According to Vyas et al. (2014), the TA values in both coated and uncoated papaya fruit had decreased with the passage of storage time. However, the results of the present study suggest that the TA values in the control fruits were significantly lower as compared to that of sodium alginate combined with olive oil coated fruits. The higher values of the TA were observed in the fruits coated with sodium alginate containing ascorbic acid (T2) (0.54) and citric acid (T3) (0.63). The results from this study are in agreement with those of Debeaufort (1998) who used edible coating to preserve strawberry and found that the edible coating could reduce the transpiration rate due to declining of availability of organic acids for enzymatic reaction of respiration.
Effect on total soluble solids (TSS) and total sugars
The present study further reveals that the level of TSS and Total sugars of Ber has gradually increased gradually during their storage (Table 2). The increase in TSS and total sugars during fruit ripening has been attributed to the hydrolysis of starch to sugars (Biale 1960). The data presented in the Table 2 reveals higher TSS levels in untreated fruit in comparison to that of the treated fruit throughout the storage, which indicates that the edible coating has efficacy in delaying the process of ripening. However, at the 3rd day of the storage period, the occurrence of higher accumulation of total soluble solids was noticed in the control sample (C) (2.5 %) as compared to that of the treated fruit. On 6th day, lower accumulation of TSS was noticed in the fruit treated with T3 (2.60 %) followed by T1 (2.63 %) and T2 (3.47 %). The lower accumulation of TSS in the coated fruit may be due to the barrier of coating which reduces the rate of respiration by preventing the gaseous exchange. As suggested by Ali et al. (2011), the slower respiration can lead to the delayed synthesis and use of metabolites resulting in lower TSS.
The correlation between TSS and total sugars during storage is significant (at 0.05 level) (Table 3). Total sugar of the fruit is believed as one of the fundamental criteria to evaluate the fruit ripening. The remarkable increase in total sugars during their storage is attributed to the increase in the activity of enzymes responsible for starch hydrolysis and for the decline in the rate of sugar breakdown by respiration. Campestre et al. (2002) also gave an appropriate explanation for such trend as the polysaccharides get converted into soluble sugar through the hydrolytic conversion process. More or less similar tendency was observed in the course of the present study as the level of total sugars was very low initially (50.63 mg/g), but as the ripening process of Ber fruit continued, the sugar levels increased till the end of the storage period. On the 6th day of storage, the level of total sugars in the Ber fruits of T1, T2 and T3 were 152.31 mg/g, 304.24 mg/g and 306.39 mg/g respectively (Table 2). The reasons for this significant increase may be attributed to the fact that in the beginning of the experiment Ber fruit was in mature stage and during this stage the rate of metabolic activities remain considerably slow. As the storage period increases and ripening begins which intern causes increase in the levels of sugars. Thus lower levels of sugars were observed during initial stages of ripening, whereas the sugar content had increased gradually and significantly as the storage period advanced.
Effect on ascorbic acid and total phenols
Ascorbic acid is said to have the ability to scavenge the superoxide and hydroxyl radicals, as well as regenerate a-tocopherol (Davey et al. 2000). As an antioxidant, ascorbic acid prevents browning of tissue, which is an oxidation reaction, directly and indirectly (Smirnoff 1996). Table 4 shows that the level of ascorbic acid in Ber fruit was found to be maintained with post harvest application of composite coating of sodium alginate, ascorbic acid and olive oil. The result of the fruit treated with T2 (sodium alginate + olive oil + ascorbic acid) showed a better effect on ascorbic acid content as compared to that of other treatments and control (Table 4). In the beginning, ascorbic acid content was 115.5 mg/100 g (i.e. 0 day), but gradually it decreased in treated as well as untreated Ber fruit during their storage. After 3 days of storage, the ascorbic acid content was found to be least in the untreated fruit (43.13 mg/100 g), while the higher level of ascorbic acid 75.42 mg/100 g, 123.53 mg/100 g and 64.166 mg/100 g were found in the Ber fruits treated with T1, T2 and T3 respectively. Togrul et al. (2004) stated that the coatings serve as a protective layer and control the permeability of O2 and CO2, thus decreasing the autoxidation potential of the fruit. Zhu et al. (2008) reported a similar finding that the use of edible coatings of different types of polysaccharides significantly reduced the loss of vitamin C in mango. In view of Ayranci and Tunc (2004), the antioxidants like ascorbic acid or citric acid can help in controlling the oxygen permeability (OP) of edible films. These authors reported the reduced loss of vitamin C in apricots and green peppers using these antioxidants.
Data regarding the total phenol content (Table 4) reveals that the level of phenolic content declines gradually as the ripening progresses. Initially (i.e. 0 day), the content of total phenols was 3.566 mg/g, but gradually it decreased in fruits of all the treatments during their storage. However, after 3 days, the level of total phenol was found to be least in the control fruit (1.612 mg/g), whereas the higher level (3.334 mg/g) of phenols was found in the fruits treated with sodium alginate, olive oil and citric acid. These results are in agreement with the results reported by Meng et al. (2008) regarding the decrease of total phenolic compounds with the increase of storage time and that postharvest chitosan treatment significantly inhibited the decrease of phenols in the table grape fruit stored at 20 °C. Gol et al. (2013a) also noticed that the carambola fruit treated with sodium alginate exhibited higher levels of phenols than that of the control and other coated fruit.
Effect on softening related enzymes
The softening of fruits during ripening is one of the major limiting factors in the transportation and storage of freshly harvested commodities (Brady 1987). Pectins are the lone cell wall polysaccharides that are easily soluble in water and due to this property; they can be de-esterified and depolymerized mostly by enzymatic reactions. PG catalyses the hydrolysis of α 1, 4- galacturonan linkage of demethylated pectins and releases shorter chains, thereby causing the depolymerization and dissolution of pectins (Singh and Dwivedi 2008).
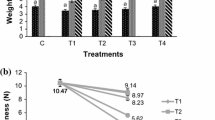
The firmness of ber fruit was 6 lb. initially (Fig. 2), but then after it decreased gradually till the end of the storage period. However, on 3rd day of storage, the higher firmness was noticed in fruits treated with T2 (Sodium alginate 2 % + Olive oil 0.2 % + Ascorbic acid 1 %) i.e. 5 lb. followed by T1 (4.5 lb) and T3 (4 lb) and the least firmness was noticed in control fruits i.e. 3.5 lb. Likewise, on 6th day of storage, fruits treated with T2 showed higher firmness i.e. 4.5 lb. whereas the lower firmness was noticed in T1 (4 lb) and T3 (3.5 lb) treated fruits. Theses results are in accordance with the findings of Qiupinga and Wenshui (2007) who reported that the polysaccharide based chitosan coating delayed the decline of firmness of ber fruit compared to the control.
Effect of composite edible coatings on firmness of Ber during its storage at 25 ± 2 °C. [T1- (Sodium alginate 2 % + Olive oil 0.2 %), T2- (Sodium alginate 2 % + Olive oil 0.2 % + Ascorbic acid 1 %), T3- (Sodium alginate 2 % + Olive oil 0.2 % + Citric acid 1 %) and C (Control). Different letters on the bars means significantly different at p ≤ 0.05]
In the current study, the specific activity of PG increased gradually due to the continuous softening process in the Ber fruit of the treated as well as control sets during their post harvest storage. Yadav et al. (2012) also found during their study that the activities of cell wall-degrading enzymes in Ber fruit increased linearly during ripening. Paull et al. (1999) reported that the activity of PG increases during the ripening process in fruits and vegetables which lead to the de-esterification of pectin and degradation of pectic substances during the ripening process. The results of the present study reveal that initially (at 0 day storage), the activity of PG was low (0.012 U/mg proteins) but subsequently it increased and reached to its peak on 9th day of storage period. During the storage period, significantly increased activity of PG was noticed in the control fruit, while all the treatments and their combinations were strong inhibitors of this enzyme. On the 3rd day of storage, the higher activity of PG was found in the control fruit (0.032 U/mg protein), while the reduced activity was found in the fruits treated with T1 (0.026 U/mg proteins), T2 (0.018 U/mg proteins) and T3 (0.017 U/mg proteins). At the end of the storage period, the activity of PG in fruits treated with T2 and T3 was i.e. 0.070 U/mg and 0.050 U/mg proteins respectively (Fig. 3a). Zhou et al. (2011) reported that the relatively lower activities of PE and PG in the shellac-coated pears contributed to the enhanced retention of brittleness and firmness during storage.
Effect of composite edible coatings on the specific activity of a PG, b PME and c PL in Ber fruit during its storage at 25 ± 2 °C. [T1- (Sodium alginate 2 % + Olive oil 0.2 %), T2- (Sodium alginate 2 % + Olive oil 0.2 % + Ascorbic acid 1 %), T3- (Sodium alginate 2 % + Olive oil 0.2 % + Citric acid 1 %) and C (Control). Different letters on the bars means significantly different at p ≤ 0.05]
The results of the current study have established an increased trend in the activity of PME of all the treated and untreated fruits throughout the storage period (Fig. 3b). At the beginning of storage (i.e. at 0 day), the activity of PME of freshly harvested Ber fruit was found to be less (0.005 U/mg proteins). But as the storage period increases, the activity of PME also gradually increased. On the 3rd day of storage, the activity of PME in untreated fruit was higher (0.020 U/mg proteins), but the reduced activity of PME was noticed in fruits treated with T1 (0.014 U/mg proteins), T2 (0.017 U/mg proteins) and T3 (0.010 U/mg proteins). The fruits treated with the composite coating of sodium alginate and olive oil reduced the activities PG and PME that may be attributed to the reduction in the gaseous exchange by coating layer created on the surface of the fruit. Moreover, the retardation of the activity of enzymes responsible for softening of the fruit facilitated the reduced softening and lesser ripening process in the Ber fruits. Gol et al. (2013b) also noticed the relatively lower activity of PME in the coated strawberry fruit that contributed to the enhanced retention firmness during storage.
Pectate lyase (PL) catalyses the cleavage of de-esterified or esterified galacturonate units by a trans β-elimination of hydrogen from the C-4 and C-5 positions of galacturonic acid. During the storage of Ber, as the fruit ripens, the activity of the pectate lyase was found to be decreased. Initially, the activity of pectate lyase of freshly harvested Ber fruit was higher (1.85 U/mg of protein on 0 day), but subsequently the activity of PL dropped, as the storage period advances. The results of the present study thus suggest that the activity of PL was higher in the control fruits than that of the fruits treated with composite coatings of sodium alginate and olive oil (Fig. 3c). On the 3rd day of storage period, the activity of pectate lyase was found to be higher in fruits treated with T1 (1.634 U/mg proteins) and T2 (1.741 U/mg proteins), whereas the activity of PL falls further in the fruits treated with T3 (1.460 U/mg proteins) and C (1.054 U/mg proteins). Coating creates a barrier on the surface of the fruit which reduces the gaseous exchange between fruit and environment thereby reducing the availability of O2 for the activity of enzymes. Thus, coating reduces the enzyme activity. Lin and Zhao (2007) also reported that the coating provides sufficient gas barrier for controlling gas exchange between the fresh produce and its surrounding atmosphere, which would slow down respiration and delay deterioration. The gas-barrier function could in turn retard the enzymatic oxidation and protect the fresh produce from browning discoloration and texture softening during storage.
Conclusion
The current study revealed that the composite coating of sodium alginate and olive oil was effective in retaining quality of Ber fruit. The use of this composite coating enriched with ascorbic acid and citric acid improved the levels of antioxidants and maintained the lower activities of cell wall hydrolases such as PG, PME and PL in Ber fruits during their storage than that of the control set of fruits. The treatment of Xanthan gum and olive oil incorporated with ascorbic acid (T2) showed best effect on nutritional quality compounds (i.e. total phenols and ascorbic acid) and shelf life of Ber fruit followed by T1 and T3. In the light of these results, it can be assumed that the composite edible coating on Ber fruit tested under the present study may be a useful and promising eco-friendly postharvest technique for enhancement of postharvest shelf life and quality maintenance of perishable fruit like Ber.
References
Ali A, Muhammad MTM, Sijam K, Siddiqui Y (2011) Effect of chitosan coating on the physicochemical characteristics of eksotika II papaya (Carica papaya L.) fruit during cold storage. Food Chem 124:620–625
AOAC (Association of Official Analytical Chemists) (1994) Official methods of analysis, 16th edn. Virginia, USA
Ayranci E, Tunc S (2004) The effect of edible coatings on water and vitamin C loss of apricots (Armeniaca vulgaris Lam.) and green peppers (Capsicum annuum L.). Food Chem 87:339–342
Baraiya NS, Gol NB, Rao TVR (2012) Influence of polysaccharide-based edible coatings on the shelf life and nutritional quality of tomato fruit. Food 6(1):22–27
Biale JB (1960) The post-harvest biochemistry of tropical and subtropical fruits. Adv Food Res 10:293–354
Bico SLS, Raposo MFJ, Morais RMSC, Morais AMMB (2009) Combined effects of chemical dip and/or carrageenan coating and/or controlled atmosphere on quality of fresh cut banana. Food Control 20:508–514
Brady CJ (1987) Fruit ripening. Annu. Rev Plant Physiol 38:155–178
Campestre C, Marsilio V, Lanza B, Iezzi C, Bianchi G (2002) Phenolic compounds and organic acids change in black oxidized table olives. ISHS Acta horticulturae. 586: IV International Symposium on Olive Growing. ISBN: 978–90–66057–56–2
Choi SH, Ahn JB, Kozukue N, Levin CE, Friedman M (2011) Distribution of free amino acids, flavonoids, total phenolics, and antioxidative activities of jujube (Ziziphus jujuba) fruits and seeds harvested from plants grown in Korea. J Agric Food Chem 59:6594–6604
Davey MW, Van Montagu M, Inze D, Sanmartin M, Kanellis A, Smirnoff N (2000) Plant L-ascorbic acid: chemistry, function, metabolism, bioavailability and effects of processing. J Sci Food Agric 80:825–860
Debeaufort FJ, Quezada-Gallo A, Voilley A (1998) Edible films and coatings: tomorrow’s packaging: a review. Crit Rev Food Sci Nutr 38:299–313
Gol NB, Chaudhari ML, Rao TVR (2013a) Effect of edible coating on quality and shelf-life of carambola (Averrhoa carambola L.) fruit during storage. J Food Sci Technol 52(1):78–91
Gol NB, Patel PR, Rao TVR (2013b) Improvement of quality and shelf life of strawberry with edible coatings enriched with chitosan. Postharvest Biol Technol 85:185–195
Hangermann AE, Austin PJ (1986) Continuous spectrophotometric assay for plant pectin methyl esterase. J Agric Food Chem 34:440–444
Hernandez-Munoz EA, Valle VD, Velez D, Gavara R (2008) Effect of chitosan coating with postharvest calcium treatment on strawberry (Fragaria x ananassa) quality during refrigerated storage. Food Chem 110:428–435
Lin D, Zhao Y (2007) Innovations in the development and application of edible coatings for fresh and minimally processed fruits and vegetables. Compr Rev Food Sci F 6:60–75
Lohani S, Trivedi PK, Nath P (2004) Changes in activities of cell wall hydrolases during ethylene-induced ripening in banana: effect of 1-MCP, ABA and IAA. Postharvest Biol Technol 31:119–126
Lowry OH, Roserbrough NJ, Farr AL, Randall RJ (1951) Protein measurement with folin phenol reagent. J Biol Chem 193:262–267
Maftoonazad N, Ramaswamy HS, Marcotte M (2008) Shelf-life extension of peaches through sodium alginate and methyl cellulose edible coatings. Int J Food Sci Technol 43:951–957
Martin-Diana AB, Rico D, Barry-Rayn C (2008) Green tea extract as natural antioxidant to extend the shelf life of fresh cut lettuce. Innov Food Sci Emerg Technol 9:593–603
Meng X, Boqiang L, Liu J, Shiping T (2008) Physiological responses, and quality attributes of table grape fruit to chitosan preharvest spray and postharvest coating during storage. Food Chem 106:501–508
Miller GL (1951) Use of dinitro salicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Moran F, Nasuno S, Starr MP (1968) Extracellular and intracellular polygalacturonic acid trans-eliminase of Erwinia carotovora. Arch Biochem Biophys 123:298–306
Olivas GI, Mattinson DS, Barbosa-Canovas GV (2007) Alginate coatings for preservation of minimally processed “gala” apples. Postharvest Biol Technol 45:89–96
Pareek S, Kitinoja L, Kaushik RA, Paliwal R (2009) Postharvest physiology and storage of Ber. Stewart Posthar Rev. doi:10.2212/spr.2009.5.5
Pathak N, Sanwal GG (1998) Multiple forms of polygalacturonase from banana fruits. Phytochem 48:249–255
Paull RE, Gross K, Qiu Y (1999) Changes in papaya cell walls during fruit ripening. Postharvest Biol Technol 16(1):79–89
Pranoto Y, Rakshit V, Salokhe M (2005) Enhancing antimicrobial activity of chitosan films by incorporating garlic oil, potassium sorbate and nisin. LWT Food Sci Technol 38:859–865
Qiupinga Z, Wenshui X (2007) Effect of 1-methylcyclopropene and/or chitosan coating treatments on storage life and quality maintenance of Indian jujube fruit. LWT 40:404–411
Roe J (1954) Chemical determination of ascorbic acids. Meth Biochem Anal 1:115
Rupasinghe HPV, Murr DP, Deell JR, Odumeru J (2005) Influence of 1-methylcyclopropene and nature seal on the quality of fresh-cut “empire” and “Crispin” apples. J Food Qual 28:289–307
Salunkhe DK, Kadam SS (1995) Handbook of fruit science and technology. Marcel Dekker, New York, p. 611
Singh P, Dwivedi UN (2008) Purification and characterisation of multiple forms of polygalacturonase from mango (Mangifera indica cv. Dashehari) fruit. Food Chem 111(2):345–349
Smirnoff N (1996) The function and metabolism of ascorbic acid in plants. Ann Bot 78:661–669
Srinivasa P, Baskaran CR, Ramesh MN, Prashantand KVH, Tharanthan RN (2002) Storage studies of mango packed using biodegradable chitosan film. Eur Food Res Technol 215:504–508
Thimmaiah SK (1999) Standards methods of Bio-chemical analysis. Kalyani publishers, New Delhi, India
Togrul H, Arslan N (2004) Carboxymethyl cellulose from sugar beet pulp cellulose as a hydrophilic polymer in coating of mandarin. J Food Eng 62:271–279
Vyas PB, Gol NB, Rao TVR (2014) Postharvest quality maintenance of papaya fruit by using polysaccharide based edible coatings. Int J Fruit Sci 14(1):81–94
Wang LZ, Liu L, Holmes J, Kerry JP (2007) Assessment of film forming potential and properties of protein and polysaccharide-based biopolymer films. Int J Food Sci Technol 42(9):1128–1138
Yadav P, Kumar S, Jain V, Malhotra S (2012) Cell wall metabolism of two varieties of ber (Ziziphus mauritiana Lam.) fruit during ripening. Food Technol Biotechnol 50(4):467–472
Zapata PJ, Guillén F, Martínez-Romero D, Castillo S, Valero D, Serrano M (2008) Use of alginate or zein as edible coatings to delay postharvest ripening process and to maintain tomato (Solanum lycopersicon mill) quality. J Sci Food Agric 88:1287–1293
Zhou A, Yunfei LB, Liping YC, Jing XA (2011) Effect of edible coatings on enzymes, cell-membrane integrity, and cell-wall constituents in relation to brittleness and firmness of huanghua pears (Pyrus pyrifolia nakai, cv. Huanghua) during storage ran. Food Chem 124:569–575
Zhu X, Wang Q, Cao J, Jiang W (2008) Effects of chitosan coating on postharvest quality of mango (Mangifera indica) fruits. J Food Process Press 32:77–83
Acknowledgments
The authors are grateful to the Head, B. R. Doshi School of Biosciences, Sardar Patel University, Gujarat, India for providing necessary facilities for carrying out this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ramana Rao, T.V., Baraiya, N.S., Vyas, P.B. et al. Composite coating of alginate-olive oil enriched with antioxidants enhances postharvest quality and shelf life of Ber fruit (Ziziphus mauritiana Lamk. Var. Gola). J Food Sci Technol 53, 748–756 (2016). https://doi.org/10.1007/s13197-015-2045-3
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-015-2045-3