Abstract
Persian walnut (Juglans regia L.) is a valuable economic plant used worldwide for its wood and nut fruit. In the current study, the genetic variation of 21 selected walnut genotypes from different regions of Iran was described using nut pomological properties, ISSR primers, and DNA analyses based on SSR primers. Nut pomology revealed nut weight of selected genotypes ranged from 12.1 to 17.5 g (OR23). Kernel weight varied from 5.3 to 10.3 g (OR23), and kernel fill percentage ranged from 43.4 to 62.7% (T12). PCR amplification with ISSR primers revealed 112 bands obtained with 91% polymorphism. Numbers of amplified bands ranged from 9 to 14. The highest primer resolving power (Rp), 7.71, was obtained with the UBC.884 primer and highlights the ability of the most informative primers to differentiate between the genotypes. The average band informativeness (AvIb) varied from 0.14 (UBC.886) to 0.62 (UBC.887 and UBC.888). Using the Jaccard coefficient, genetic similarity values among walnut genotypes varied from 0.52 to 0.88. The dendrogram of UPGMA analysis with ISSR primers classified walnuts genotypes in two main groups and three sub-groups. PCoA largely confirmed cluster analysis results. Genotypes collected from Shahrood displayed lower genetic variation and the Tuyserkan and Urmia genotypes were more genetically distant. Unique DNA profiles were established for all studied genotypes based on three distinct microsatellite loci.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Persian walnut (Juglans regia L.) is one of the most important cultivated walnut species in the worldwide. Walnut, as a nut fruit, has economic and nutritional value beneficial to human health (Anderson et al. 2001; Ros and Mataix 2006). Iran is counted as considered the primary origin of Persian walnut genetic diversity and is one of the main walnut producers in the world (Arzani et al. 2008; Jafari Sayadi et al. 2012; Vahdati et al. 2015). There are many promising genotypes in Iran; each with the potential to be introduced as a cultivar for use worldwide (Ebrahimi et al. 2015; Khadivi-Khub and Ebrahimi 2015; Vahdati et al. 2015). Seedling trees and difficulty in identifying superior genotypes in nursery grafted seedlings have hindered walnut orchard establishment in Iran. Morphological markers can be used to identify superior cultivars and genotypes however significant challenges limit the reproducibility of this method. Environmental conditions, insufficient genotypic variation, and developmental stage can confound analyses of resultant data while profiles of traits only apparent years after planting (flowers, fruits) may not be captured at all (Weising et al. 2005). DNA marker-based identification is cost-effective, fast, precise, and reliable for identification of important plant cultivars in agriculture and horticulture as well as for practical breeding and related areas such as plant proprietary rights protection (Carrasco et al. 2012; Potter et al. 2002; Weising et al. 2005). Microsatellites uncover maximal/minimal variation between cultivars, environmental stability and are reproducible making them highly successful for use in accurate identification of specific genotypes (Vieira et al. 2016; Weising et al. 2005). DNA markers have proven effective for identification of grape (Haddou et al. 2018; Migliaro et al. 2017; Zhang et al. 2018), olive (Ben Ayed et al. 2016; Zhan et al. 2015) and walnut (Dangl et al. 2005; Foroni et al. 2007; Pang et al. 2017; Zhao et al. 2017). Molecular marker techniques commonly used to estimate genetic diversity and classify genotypes, are simple sequence repeats (SSR) (Christopoulos et al. 2010; Ebrahimi et al. 2011; Foroni et al. 2006; Vahdati et al. 2015), inter-simple sequence repeats (ISSR), random amplified polymorphic DNA (RAPD) (Fatahi et al. 2010; Potter et al. 2002), amplified fragment length polymorphism (AFLP) (Bayazit et al. 2007) and single nucleotide polymorphisms (SNPs) (Ciarmiello et al. 2013).
Thus, molecular markers represent an ideal tool to accurately and exclusively characterize superior walnut genotypes by detection of DNA polymorphisms and establishment of a unique “fingerprint” for use in rapid screening of plant materials.
Materials and Method
Plant Materials and Evaluation
More than 400 walnut genotypes were collected from the major walnut regions of Iran (Tuyserkan, Urmia, Karaj, and Khorasan). They were cultured in a walnut collection at Shahrood Agricultural and Natural Resources Research and Education Centre, Shahrood, Iran (longitude: 54°57′ E, latitude 36°25′ N, 1367 m elevation) in 1998. Shahrood has a cold and dry climate with an average annual temperature of 14.4 °C, relative humidity of 63%, and average rainfall is about 160 mm per year. Genotypes used in the study originated from seed material obtained from trees within the major walnut production regions of Iran (Karaj, Khorasan, Shahrood, Tuyserkan, and Urmia) that previously produced superior fruit. Horticulture practices such as irrigation, fertigation and foliar application, pruning, pest management and harvesting were similarly performed on all studies genotypes before being assessed for commercial bearing from 2011 to 2016. Twenty-one superior genotypes, grafted on seedling rootstocks, were selected based on their pomological characteristics (International Plant Genetic Resources 1994) and adaptation to the Shahrood climate (Table 1). Measurements of each nut and kernel traits were based on 30 replicates and the mean values were used. The identification and location of walnut genotypes used in the study were reported and mapped (Table 1, Fig. 1).
DNA Extraction and Amplification
Leaves of the 21 selected genotypes were collected in spring, immediately frozen in liquid nitrogen, and stored at −80 °C. Genomic DNA was extracted from leaf tissue using the CTAB method (Doyle and Doyle 1987) with minor modifications. Quality and concentration of the extracted DNA were assessed by Nanodrop (Implen EN N‑60) and visualized on a 0.8% agarose gel.
SSR and ISSR Primers and PCR Amplification
Six black walnut (Juglans nigra L.) SSR primer pairs were developed for black walnut (Dangl et al. 2005; Wang et al. 2008). Ten ISSR primers were selected based on polymorphism information content (PIC), heterozygosity rate, and number of observed alleles (Christopoulos et al. 2010; Ebrahimi et al. 2011; Foroni et al. 2006; Vahdati et al. 2015) (Tables 2 and 3). Polymerase chain reaction (PCR) amplifications were performed in 15 μl reactions: 7.5 μl of 1X PCR master mix (Sinaclon, Iran), 1 μl primer pair (10 pM), 1 μl (100 ng) template DNA and 5.5 μl ddwater. Touchdown PCR reactions 5 min at 95 °C, then 35 cycles of 1 min at 95 °C, 1 min at annealing temperature (Tables 2 and 3), and 2 min at 72 °C, followed by a final extension of 72 °C for 10 min. PCR products from ISSR and SSR primers were visualized on 2% MetaPhor™ agarose gels in 1X TAE buffer. Alleles of SSRs were sized and scored visually using a transcription ruler alongside a reference DNA ladder (Jena Bioscience GmbH). PCR products were stored at 4 °C until needed.
Molecular Data Analysis
Amplified band range, total number of bands, number of polymorphic bands, polymorphic percentage, average band informativeness (AvIb), and resolving power (Rp) were determined for each locus. Band informativeness (Ib), [Ib = 1 − (2 × |0.5 − p|], where p is the proportion of total genotypes containing the band, and resolving power (Rp), [Rp = Σ Ib], the sum of band informativeness, were calculated according to published formulas (Prevost and Wilkinson 1999).
A phonogram for the 21 selected genotypes was constructed using UPGMA (unweighted pair-group method using arithmetic average) based on the Jaccard’s similarity index of ISSR data by simple matching using Ntsys 2.1 software (http://www.exetersoftware.com/cat/ntsyspc/ntsyspc.html). GenAlex6.5 software (http://biology-assets.anu.edu.au/GenAlEx/Welcome.html) was used to generate the principal coordinate analysis (PCoA) based on the distance genetic matrix (Peakall and Smouse 2006). Probability of exclusion (P1) for each SSR loci was calculated with GenAlEx 6.5 software.
Results and Discussion
Pomology for Selected Juglans regia L. Genotypes
Pomological trait evaluation indicated that average nut and kernel weights among genotypes were 14.34 and 7.33 g, respectively (Table 4). The highest nut weight, 17.55 g, was recorded in in OR23 genotype. Nut weights collected in this study were greater than those from central Iran (15.25 g) (Arzani et al. 2008), east Anatolia, Turkey (17.04 g) (Yarilgac et al. 1999), north-eastern Anatolia, Turkey (16.01 g) (Aslantaş 2006), but lower than those reported by Ebrahimi et al. (2011) for walnuts in Fars province, Iran (17.7 g). Higher nut weights were also reported for Azadshar, Iran (19.79 g) (Shamlo et al. 2017), Adilcevaz, Turkey (23.81 g) (Sen and Tekintas 1990), Kamal-Abad, Iran (20 g) (Atefi 2001), Himachal Pradesh, India (18.60 g) (Sharma and Sharma 1998), and the Oltenia region of Romania (18.40 g) (Cosmulescu and Botu 2012). Ideal kernel weights for walnut range between 6–10 g and have at least 50% kernel fill percentage (Khadivi-Khub et al. 2015). Twelve of our 21 studied genotypes neared the preferred kernel weight and fill range. Kernel weights for 20 of 21 selected genotypes were greater than 6 g. Average kernel fill percentage was 51.17 and ranged from 43.4% in KH31 to 62.70% in T12 (Table 4). Nine genotypes (KH4, KH31, KH34, OR4, OR26, R1G6, R1G7, R2G4, T1) had kernel fill percentages of less than 50% (Table 4). Kernel pigmentation for all 21 genotypes was considered light and 13 genotypes (K28, KH31, KH34, OR23, R1G6, R1G7, R2G1, R2G5, R2G8, SH1, T1, T9) had easily extracted kernels (Fig. 3). Previous research by Arzani et al. (2008); Cosmulescu and Botu (2012); Yarilgac et al. (1999) and Yarilgac et al. (2001) indicated these two traits were consistent in superior walnut selections.
Molecular Analysis
ISSR Markers
Ten ISSR primers were used to assay the genetic variation of 21 superior Iranian walnut genotypes from Shahrood, Iran (Fig. 2). A total of 112 bands were obtained from the PCR reaction, which showed 102 polymorphic bands, representing approximately 91% of the polymorphism (Table 5). Pollegioni et al. (2004) observed 31 of 54 (57.4%) bands from 48 walnut genotypes were polymorphic using 8 ISSR primers. Study of germplasm diversity and genetic relationships among walnut cultivars and Greek walnut varieties with ISSR revealed 82.8% polymorphism among amplified bonds (Christopoulos et al. 2010). In a study of 104 walnut genotypes from China, Li et al. (2011) reported 481 polymorphic bands from 485 amplified DNA fragments (91.1%) using 121 ISSR primers. Doğan et al. (2014) reported 69.1% polymorphism with 25 ISSR primers on 59 Turkish walnut genotypes. Array of DNA bands and polymorphism percentages in ISSR markers is dependent upon rate of genetic diversity and numbers of studied genotypes. Furthermore, incorporation of degenerative primers increased the likelihood that more bands would be amplified from genomic DNA.
Number of amplified bands ranged from 9 to 14 with the percentage of polymorphic bands ranging from 78 to 100% (Table 5). Primer UBC.884 was the most polymorphic with 14 bands and also had the highest Rp value (7.71). The lowest Rp value, (1.24), was recorded for UBC.886 while AvIb varied from 0.14 (UBC.886) to 0.62 (UBC.887 and UBC.888) (Table 5). The highest and lowest Rp values for Greek walnut germplasm were represented by UBC.814 (18.8) and UBC.830 (0.86) while the Rp value of AFLP markers in walnut genotypes was reported to be 16.2 (Bayazit et al. 2007; Christopoulos et al. 2010).
ISSR-based Genetic Relatedness
Genetic similarity values varied from 0.52 to 0.88. Significantly higher similarities were observed between R1G2 and R2G1 and between R1G2 and R1G7 with significantly lower similarities observed between OR37 and T9 (Data not shown). Genotypes collected from Shahrood were most similar while those from Tuyserkan and Urmia had the maximum genetic distance, making them more suitable for use in breeding programs. ISSR primers are useful in a wide range of studies, including genetic variation, phylogeny, genetic mapping, and evolutionary biology (Christopoulos et al. 2010; Li et al. 2011; Prevost and Wilkinson 1999) and for tree species such as walnut (Li et al. 2011), olive (Sesli and Dilsat Yegenoglu 2018), apricot (Zhang et al. 2018) and plum (Carrasco et al. 2012). The dendrogram based on UPGMA analysis showed the relationship between Persian walnut genotypes based on the Jaccard coefficient index (Fig. 3).
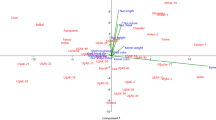
The 21 selected walnut genotypes were classified in two main groups based on the 10 ISSR markers. Group I (GI) was composed of 16 genotypes and Group II (GII) was populated by only 5 genotypes. Also, with a similarity coefficient of 0.77, GI was divided into three sub-groups (Fig. 3). The majority of genotypes within subgroup one (GIA), except KH4 and OR37, originated from Shahrood. Higher levels of genetic similarity were observed between R1G2 and both R1G7 and R2G1. The second subgroup (G1B) included genotypes KH34, OR26, and SH1. Genotypes K26, K28, OR23, R2G8, and T12 were classified in the third subgroup (G1C) (Fig. 3). Genotypes with greater genetic distances (KH31, OR4, R2G4, T1 and T9) were all in GII. Principal component analysis (PCoA) showed a division between the 21 genotypes into three groups (A, B, C) (Fig. 4). The majority of genotypes in Group A matched those from GI and originated in Shahrood. Groups B and C each held a single Shahrood genotype. Genotypes in Group C, with the exception of OR23, were all GII. Thus, PCA analysis largely supported cluster analysis results. Approximately 18.6%, 24.1%, and 20% of variation from Groups A, B, and C respectively account for greater than 62% of overall variation. These data indicate the ISSR primers worked well.
SSR Markers
All SSR primers used here were polymorphic and amplified 31 polymorphic alleles ranging from 110 bp in WGA054 to 260 bp in WGA032. Allelic frequency amplification of six microsatellite loci in 21 walnut genotypes revealed more than 32% of alleles have frequencies greater than 0.25 and 19% have frequencies lower than 0.05 (Fig. 5). Number of different alleles (Na) varied from 4 to 8, with an average of 5.17 alleles per locus (Table 6). WGA009 had the highest number of effective alleles (Ne = 5.8) and the highest Shannon’s information index value (I = 1.88) while WGA089 had the lowest Ne (3.22) and I (1.23). Observed heterozygosity (Ho) ranged from 0.00 in WGA089 and WGA001 to 0.95 for WGA009 and WGA054 with an average value of 0.58 for all studied SSR primers. Expected heterozygosity (He) ranged from 0.69 in WGA089 to 0.83 for WGA009 with an average value of 0.73 (Table 6). Differences between Ho and He for WGA089 and WGA001 may be associated with the DNA separating properties of MetaPhor™ agarose as it can only separate DNA fragments greater than 4 bp apart. In contrast, Dangl et al. (2005) reported Ho values of 0.57 and 0.61 for WGA001 and WGA089 in 44 walnut genotypes.
Fingerprinting With SSR Primers
WGA009 displayed maximum probably of exclusion (Table 6). DNA fingerprint analyses at 6 loci indicated that 3 loci (WGA009, WGA032, and WGA276) were unique across the 21 selected genotypes (Fig. 6, Table 7). WGA009 exhibited a unique profile for five genotypes (K26, KH34, OR23, R1G6, R2G3), but when combined with WGA276, 14 of the 21 genotypes were unique. The third loci WGA032, was used with R2G4 and T12 genotypes to produce an individualized profile (Table 7). DNA profiles were compiled for each of the 21 genotypes (Table 7).
Conclusion
Climate change can affect morphological traits in plants however in collection establishment morphology is influenced more by genotype and generic differences than environmental impacts. Initial evaluation of Persian walnut cultivars at Shahrood Agricultural and Natural Resources Research and Education Center (Shahrood, Iran) resulted in selection of 21 walnut genotypes with superior pomological properties for study. Kernel and nut pomology for the genotypes studied indicated the greatest nut weight (17.55 g) was observed in OR23 with all genotypes having kernel weights greater than 6 g. Evaluation of kernel fill showed an average of 51.17% and ranged from 43.4% in KH31 to 62.7% in T12. Twelve genotypes neared the preferred kernel weight and fill range. UPGMA analysis separated the 21 study genotypes into two groups (G1, G2) with G1 being further divided into three sub-groups (G1A, G1B, G1C), a result very similar to the PCoA. Six SSR primers were used in our effort to individually identify each genotype and three in particular (WGA001, WGA009, WGA276) allowed for accurate identification of all 21 genotypes which this information are useful for walnut growers for credentials of true to type promising walnut genotypes in new orchards establishment. Numerous bands were produced in this study and highlighted the high levels of diversity within the genotypes chosen for study. The genotypes collected from Urmia and Tuyserkan regions have a higher genetic distance than other genotypes and which according to their superior characteristics; they can be used as parent along with selected walnut genotypes from the Shahrood area in a breeding program. The results of this study showed that the SSR and ISSR markers described here are able to reliably differentiate between numerous walnut genotypes.
Abbreviations
- AvIb::
-
average band informativeness,
- ISSR::
-
inter-simple sequence repeats,
- PcoA::
-
principle coordinate analysis.
- Rp::
-
resolving power,
- SSR::
-
simple sequence repeats,
- UPGMA::
-
unweighted pair group method with arithmetic mean,
References
Anderson KJ, Teuber SS, Gobeille A, Cremin P, Waterhouse AL, Steinberg FM (2001) Walnut polyphenolics inhibit in vitro human plasma and LDL oxidation. J Nutr 131:2837–2842
Arzani K, Mansouri-Ardakan H, Vezvaei A, Roozban MR (2008) Morphological variation among Persian walnut (Juglans regia) genotypes from central Iran. N Z J Crop Hortic Sci 36:159–168. https://doi.org/10.1080/01140670809510232
Aslantaş R (2006) Identification of superior walnut (Juglans regia) genotypes in north-eastern Anatolia, Turkey. N Z J Crop Hortic Sci 34:231–237. https://doi.org/10.1080/01140671.2006.9514412
Atefi J (2001) Comparison of some promising Iranian walnut clones and foreign varieties. Acta Hortic 544:51–59. https://doi.org/10.17660/ActaHortic.2001.544.5
Bayazit S, Kazan K, Gülbitti S, Çevik V, Ayanoğlu H, Ergül A (2007) AFLP analysis of genetic diversity in low chill requiring walnut (Juglans regia L.) genotypes from Hatay, Turkey. Sci Hortic 111:394–398. https://doi.org/10.1016/j.scienta.2006.11.006
Ben Ayed R, Ben Hassen H, Ennouri K, Ben Marzoug R, Rebai A (2016) OGDD (Olive Genetic Diversity Database): a microsatellite markers’ genotypes database of worldwide olive trees for cultivar identification and virgin olive oil traceability. Database (Oxford) 2016:bav90. https://doi.org/10.1093/database/bav090
Carrasco B, Díaz C, Moya M, Gebauer M, García-González R (2012) Genetic characterization of Japanese plum cultivars (Prunus salicina) using SSR and ISSR molecular markers. Cienc Inv Agr 39:533–543
Christopoulos MV, Rouskas D, Tsantili E, Bebeli PJ (2010) Germplasm diversity and genetic relationships among walnut (Juglans regia L.) cultivars and Greek local selections revealed by Inter-Simple Sequence Repeat (ISSR) markers. Sci Hortic 125:584–592. https://doi.org/10.1016/j.scienta.2010.05.006
Ciarmiello LF, Pontecorvo G, Piccirillo P, De Luca A, Carillo P, Kafantaris I, Woodrow P (2013) Use of nuclear and mitochondrial single nucleotide polymorphisms to characterize English walnut (Juglans regia L.) genotypes. Plant Mol Biol Rep 31:1116–1130
Cosmulescu S, Botu M (2012) Walnut biodiversity in south-western Romania resource for perspective cultivars. Pak J Bot 44:307–311
Dangl GS, Woeste K, Aradhya MK, Koehmstedt A, Simon C, Potter D, Leslie CA, McGranahan G (2005) Characterization of 14 microsatellite markers for genetic analysis and cultivar identification of walnut. J Am Soc Hortic Sci 130:348–354
Doyle J, Doyle J (1987) Genomic plant DNA preparation from fresh tissue-CTAB method. Phytochem Bull 19:11–15
Doğan Y, Kafkas S, Sütyemez M, Akça Y, Türemiş N (2014) Assessment and characterization of genetic relationships of walnut (Juglans regia L.) genotypes by three types of molecular markers. Sci Hortic 168:81–87. https://doi.org/10.1016/j.scienta.2014.01.024
Ebrahimi A, Fatahi R, Zamani Z (2011) Analysis of genetic diversity among some Persian walnut genotypes (Juglans regia L.) using morphological traits and SSRs markers. Sci Hortic 130:146–151. https://doi.org/10.1016/j.scienta.2011.06.028
Ebrahimi A, Khadivi-Khub A, Nosrati Z, Karimi R (2015) Identification of superior walnut (Juglans regia) genotypes with late leafing and high kernel quality in Iran. Sci Hortic 193:195–201
Fatahi R, Ebrahimi A, Zamani Z (2010) Characterization of some Iranians and foreign walnut genotypes using morphological traits and RAPD markers. Hortic Environ Biotechnol 51:51–60
Foroni I, Rao R, Woeste K (2006) Molecular characterization of Juglans regia L. cultivars with SSR markers. Acta Hortic 705:207–213. https://doi.org/10.17660/ActaHortic.2005.705.25
Foroni I, Woeste K, Monti L, Rao R (2007) Identification of ‘Sorrento’ walnut using simple sequence repeats (SSRs). Genet Resour Crop Evol 54:1081–1094
Haddou LA, Charafi J, Abdelali B, Messaoudi Z, Outghouliast H (2018) Molecular characterization and study of genetic relationships among local Cultivars of the Moroccan fig (Ficus carica L.) using microsatellite and ISSR markers. Int J Environ Agric Biotechnol 3:18–27. https://doi.org/10.22161/ijeab/3.1.4
International Plant Genetic Resources (IPGR) (1994) Descriptors for walnut (Juglans Spp.). International Plant Genetic Resources Institute, Rome
Jafari Sayadi MH, Vahdati K, Mozafari J, Mohajer MRM, Leslie CA (2012) Natural Hyrcanian populations of Persian walnut (Juglans regia L.) in Iran. Acta Hortic 948:97–101. https://doi.org/10.17660/ActaHortic.2012.948.10
Khadivi-Khub A, Ebrahimi A (2015) The variability in walnut (Juglans regia L.) germplasm from different regions in Iran. Acta Physiol Plant 37:1–11
Khadivi-Khub A, Ebrahimi A, Mohammadi A, Kari A (2015) Characterization and selection of walnut (Juglans regia L.) genotypes from seedling origin trees. Tree Genet Genomes 11:1–10
Li GT, Ai CX, Zhang LS, Wei HR, Liu QZ (2011) ISSR analysis of genetic diversity among seedling walnut (Juglans spp.) populations. J Plant Genet Resour 12:640–645
Migliaro D, De Nardi B, Vezzulli S, Crespan M (2017) An upgraded core set of 11 SSR markers for grapevine cultivar identification: the case of Berry-color mutants. Am J Enol Vitic 68:496–498
Pang K, Woeste K, Michler C (2017) Cultivar identification and genetic relatedness among 25 black walnut (Juglans nigra) clones based on microsatellite markers. In: Proceedings of the 20th Central Hardwood Forest Conference GTR-NRS‑P, 2017, p 167
Peakall R, Smouse PE (2006) GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecol Resour 6:288–295
Pollegioni P, Major A, Bartoli S, Ducci F, Proietti R, Malvolti M (2004) Application of microsatellite and dominant molecular markers for the discrimination of species and interspecific hybrids in genus Juglans. In: V International Walnut Symposium 705, 2004, pp 191–197
Potter D, Gao F, Aiello G, Leslie C, McGranahan G (2002) Intersimple sequence repeat markers for fingerprinting and determining genetic relationships of walnut (Juglans regia) cultivars. J Am Soc Hortic Sci 127:75–81
Prevost A, Wilkinson M (1999) A new system of comparing PCR primers applied to ISSR fingerprinting of potato cultivars. Theor Appl Genet 98:107–112
Ros E, Mataix J (2006) Fatty acid composition of nuts-implications for cardiovascular health. Br J Nutr 96:S29–S35
Sen SM, Tekintas FE (1990) A study on the selection of Adilcevaz walnuts. Fruit Breed Genet 317:171–174
Sesli M, Dilsat Yegenoglu E (2018) Assessment of genetic relations among cultivated olives by ISSR markers. J Agric Sci Technol 19(6):1333–1343
Shamlo F, Rezaei M, Biabani A, KhanAhmadi AR (2017) Morphological diversity among walnut genotypes of in Azadshar, Iran. Iran Hortic Sci J 30:469–479
Sharma SD, Sharma OC (1998) Studies on the variability in nuts of seedling walnut (Juglans regia L.) in relation to the tree age. Fruit Var Journal 51(1):20–23
Vahdati K, Pourtaklu SM, Karimi R, Barzehkar R, Amiri R, Mozaffari M, Woeste K (2015) Genetic diversity and gene flow of some Persian walnut populations in southeast of Iran revealed by SSR markers. Plant Syst Evol 301:691–699
Vieira MLC, Santini L, Diniz AL, Munhoz CD (2016) Microsatellite markers: what they mean and why they are so useful. Genet Mol Biol 39:312–328
Wang H, Pei D, Gu R, Wang B (2008) Genetic diversity and structure of walnut populations in central and southwestern China revealed by microsatellite markers. J Am Soc Hortic Sci 133:197–203
Weising K, Nybom H, Pfenninger M, Wolff K, Kahl G (2005) DNA fingerprinting in plants: principles, methods, and applications. CRC press, Boca Raton
Yarilgac T, Koyuncu F, Koyuncu M, Kazankaya A, Sen S (1999) Some promising walnut selections (Juglans regia L.). In: IV International Walnut Symposium 544, 1999, pp 93–96
Yarilgac T, Koyuncu F, Koyuncu MA, Kazankaya A, Sen SM (2001) Some promising walnut selections (Juglans regia L.). Acta Hortic 544:93–96. https://doi.org/10.17660/ActaHortic.2001.544.10
Zhan MM et al (2015) Genetic relationships analysis of olive cultivars grown in China. Genet Mol Res 2:5958–5969
Zhang SL, Zhao H, Liu MP, Zhang YL, Wuyun TN, Zhu GP (2018) Genetic diversity of primary core kernel-apricot germplasms using ISSR markers. Int J Agric Biol 20:839–845
Zhao P, Zhou H, Coggeshall MV, Reid B, Woeste KE (2017) Discrimination and assessment of black walnut (Juglans nigra L.) nut cultivars using phenology and microsatellite markers (SSRs). Can J Plant Sci 98(3):616–627. https://doi.org/10.1139/cjps-2017-0214
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
F. Davoodi, M. Rezaei, P. Heidari, H. Hokmabadi and S. Lawson declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Davoodi, F., Rezaei, M., Heidari, P. et al. Identification and DNA Fingerprinting of Some Superior Persian Walnut Genotypes in Iran. Erwerbs-Obstbau 63, 393–402 (2021). https://doi.org/10.1007/s10341-021-00597-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10341-021-00597-z