Abstract
The diamondback moth (DBM), Plutella xylostella, is an oligophagous pest of cruciferous crops worldwide. Host plants of the DBM possess epicuticular wax, but thick wax is unfavorable to the selection of oviposition sites for female DBMs. How the DBM responds to host plant wax regarding oviposition site selection is largely unknown. The glucosinolates and wax levels in the cotyledons and true leaves of Chinese kale are different, which makes this plant an ideal host for exploring the oviposition behavior of the DBM. We found that although the true leaves contained more glucosinolates and waxy powder than cotyledons of healthy Chinese kale, DBM females preferred to lay eggs on the cotyledons over true leaves. However, the number of eggs laid on true leaves increased significantly when the waxy powder was artificially removed. Furthermore, the hatched larvae greatly preferred to feed and performed better on true leaves. In light of the current results, we propose that DBM females are hindered from laying eggs on the true leaves of Chinese kale by the outer layer of leaf wax powder. However, DBM females and larvae can adapt to the waxy host plant through adaptations: i.e., DBM females first lay eggs on the cotyledons where less wax exists, the hatched larvae then crawl to and spin silk nets on true leaves; the silk nets help subsequent DBM females overcome the obstacle of the wax and oviposit successfully on true leaves. These findings highlight new insights into plant–insect interactions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Key messages
-
Few studies documented the relationship between DBM larval silking and female oviposition.
-
We evaluated how DBM successfully overcomes the influence of host plant wax on oviposition.
-
DBM first lay eggs on cotyledons with less wax and the hatched larvae spin silk on true leaves.
-
The silk helps subsequent DBM oviposition on Chinese kale true leaves.
-
These findings provide new insights into plant–insect interactions.
Introduction
The diamondback moth (DBM), Plutella xylostella, is a serious pest of cruciferous crops worldwide, causing an estimated economic loss of US $4–5 billion per year (Furlong et al. 2013; Li et al. 2016). Suitable oviposition sites on host plants are very important for the growth of herbivorous insect populations. The oviposition site selection of DBMs can be divided into three processes: long-distance locating, short-distance searching, and screening after landing. During long-distance localization, the volatiles emanating from host plants can attract DBM females (Pivnick et al. 1990; Reddy et al. 2004; Ibrahim et al. 2005; Uefune et al. 2017). Olfactory stimulation plays a major role in short-distance searching (Couty et al. 2006), and gustatory and tactile stimuli play a critical role after landing (Justus and Mitchell 1996). However, in order to successfully oviposit, the DBM also requires the recognition of glucosinolates which stimulate oviposition on host plants (Silva and Furlong 2012; Badenes-Pérez et al. 2014, 2020). Experiments using Arabidopsis thaliana mutants with different glucosinolate content and experiments with plant sulfir fertilization have shown that increasing in plant glucosinolate content can be associated with oviposition preference by DBM females (Badenes-Pérez et al. 2010, 2013).
The oviposition preference of the DBM is related not only to the glucosinolate content (Renwick et al. 2006; Sarosh et al. 2010), but also to the wax and trichome density on host plants (Spencer et al. 1999; Silva et al. 2017). In addition, whether host plants have been damaged by phytophagous insects will also affect the oviposition of female adults. DBM females prefer to lay eggs on cabbage plants damaged by conspecific larvae instead of undamaged cabbage plants (Shiojiri and Takabayashi 2003; Silva and Furlong 2012). However, Silva and Furlong (2012) found that DBM females oviposit significantly more frequently on healthy Chinese cabbage Brassica rapa plants than on conspecific larvae-damaged Chinese cabbage plants. Ang et al. (2016) further reported that DBM females display stronger oviposition preference for conspecific larvae-infested common cabbage Brassica oleracea plants than healthy common cabbage plants, but the opposite is true on Chinese cabbage plants. These studies indicate that oviposition by DBM females might be variable and dependent on the host plant species and whether it has experienced herbivory.
The silk of lepidopterous larvae can help the larvae to move between and within plants (Strong et al. 1984; Roden 1993) and protect the larvae and pupae from natural enemy attack (Dias et al. 2012, 2014). Mechanitis isthmai butterfly larvae can spin and form a network of silk scaffolding under the surface of leaves to help them avoid trichomes on spiny host plants (Rathcke and Poole 1975). DBM larvae can escape quickly by spinning and can form cocoons during pupation (Harcourt 1957; Talekar and Shelton 1993). However, it is still not clear whether the silk of lepidopterous larvae facilitate female adult oviposition.
During a preliminary investigation, we found that most DBM eggs were laid on the cotyledons of healthy Chinese kale plants instead of the true leaves. However, after Chinese kale plants were damaged by DBM larvae, more DBM eggs were laid on the true leaves. To explore the internal mechanism of this ecological phenomenon, the present study was designed to investigate the oviposition preference, larval preference, and development time of DBM on Chinese kale leaves of different types, including cotyledons, true leaves, leaves previously infested by conspecific larvae, leaves crawled on, with and without silk, and with and without waxes. In addition, the leaf surface structure and the concentration of glucosinolates in cotyledons and true leaves of Chinese kale were also investigated. This is the first report showing that lepidopterous larvae can help adult females lay eggs on thick waxy leaves by spinning silk, which provides new insights into plant–insect interactions.
Materials and methods
Plants and insects
Seeds of Chinese kale (Brassica oleracea var. alboglabra Bailey cv. Zhonghuajianye) were purchased from the Yangling Nongcheng Seed Supplement Company (Yangling, Shaanxi, China) and sown in plastic pots (10 cm diameter) with a soil mixture (peat moss:perlite:vermiculite = 3:1:1). The pots were maintained at 25 ± 1 °C and 60 ± 5% RH, with a photoperiod of 16 L:8 D in climatic growth chambers (RXZ-600C, Ningbo Jiangnan, China). Chinese kale plants of 3, 4, and 5–6-weeks-old were used in different experiments. When the plants grew for 4 weeks, they had two cotyledons and two expanded true leaves. The cotyledons of Chinese kale plants had completely fallen off after 5–6 weeks of growth.
In 2017, twenty DBM larvae were collected from a cabbage field in Yangling, Shaanxi, China. The larvae were reared on Chinese kale for at least 5 generations in a climate room at 25 ± 2 °C, 60 ± 5% RH and a photoperiod of 16 L:8 D. Pupae of DBM were collected from Chinese kale, and newly emerged adults were used for the experiments. To obtain larvae of the same age, 10 pairs of newly emerged DBM adults were selected and released into a nylon cage (20 × 20 × 30 cm) with a Chinese kale plant. After 12 h, the eggs on the plant were collected and then placed in a Petri dish (10 cm diameter). After 2–3 days, the newly hatched larvae were used for the experiments.
Ovipositional preference of DBM
Ovipositional preference of DBM for true leaves and cotyledons
One four-week-old Chinese kale seedling was placed in a nylon cage (20 × 20 × 30 cm), and 3 pairs of 2-day-old DBM adults were released into the cage. The cage was maintained in the same climate room. After 24 h, the eggs laid on true leaves and cotyledons were counted. The leaf areas were measured by a portable leaf area meter (Li 3000C United States). The egg density was calculated by the following formula: egg density = number of eggs on leaf/leaf area. The experiment was replicated 7 times, i.e., one plant represented one replication.
Ovipositional preference of DBM for larvae-infested Chinese kale
Four 4th instar larvae were put on a 4-week-old Chinese kale for 24 h of feeding, and the larvae were then removed. The oviposition preference of DBM females to the infested versus healthy plants was investigated as described in “Ovipositional preference of DBM for true leaves and cotyledons” section. The experiment was replicated 8 times.
Feeding preference and developmental time of DBM larvae
Feeding preference of DBM larvae for true leaves and cotyledons
Three-week-old Chinese kale seedlings with similar leaf areas of true leaves and cotyledons were used in the experiments. A piece of filter paper was placed at the bottom of each plant when the experiments were executed. Five 1st- or 3rd- instar larvae were placed on filter paper near the stem of Chinese kale, and they were allowed to crawl and feed freely. After 24 h, the number of larvae on the true leaves and cotyledons was counted. A total of 10–13 plants (one plant represented a replication) were used in the experiments.
Developmental times of DBM larvae fed on cotyledons and true leaves
Four-week-old cotyledons of Chinese kale were removed the day before the experiment. One newly hatched larva was introduced on the true leaf to feed freely, and the petiole of this leaf was wrapped in a piece of absorbent cotton to prevent the larva from escaping. The development time and pupal weight were measured. Similarly, these parameters of DBM larvae feeding on cotyledons were recorded on plants whose true leaves were removed the day before the experiment. A total of 38–44 plants (one plant represented one replication) were used in the experiments.
Glucosinolate extraction
Two 4th instar larvae of DBM were placed on the second true leaf of four-week-old Chinese kale plants to feed for 24 h, and the larvae were then removed. The second true leaves of healthy plants were used as a control. The concentration of glucosinolates in cotyledons and true leaves of healthy plants and larvae-infested leaves was assayed according to Cao et al. (2016). Briefly, 200 mg of leaf was placed in a 50 mL centrifuge tube, kept in a 100 °C water bath for 3 min, quickly plugged on ice and ground in a mortar and pestle with 2 mL of Milli-Q water after cooling. The mixture was centrifuged at 12,000g, at 4 °C for 15 min, and the supernatant was then collected and filtered through 0.22 µm syringe filters. Glucosinolates were analyzed using an LTQ XL linear ion trap mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) according to Rochfort et al. (2008). The relative amounts of glucosinolates were calculated in terms of a standard curve that was made by 2-propenyl glucosinolate (sinigrin) (Cao et al. 2016). The results were given as nmol/g fresh weight calculated from the peak areas at 229 nm relative to the peak area of the standard using a relative response factor described according to Brown et al. (2003). The experiment was replicated 10 times.
Observation of leaf surface structure
Epicuticular wax of leaves in Chinese kale
The epicuticular wax on the adaxial leaf surface was observed by a scanning electron microscope (FEI, Nova Nano SEM-450, Hillsboro, OR, USA). One fresh true leaf and cotyledon of a four-week-old Chinese kale plant were cut from their petioles, rinsed gently, and then put in an oven at 30 °C for 48 h. A total of three plants (i.e., three replications) were used in the experiments. Thereafter, each leaf was cut into 5 × 10 mm rectangle pieces without cutting the veins. The leaf samples (two rectangle pieces of each leaf) were mounted on specimen stubs and coated with superfine gold particles using 90-s bursts from a sputter coater (Hitachi E-1045, Tokyo, Japan). After coating, the leaf samples were photographed with a Nova Nano SEM-450 operated at 5 kV.
Two 4th instar DBM larvae were allowed to feed on a Chinese kale leaf for 24 h, and the larvae were then removed. The infested leaves and the preinfested leaves with eggs deposited (from the experiment described in “Ovipositional preference of DBM for larvae-infested Chinese kale” section) were detached from the plants (a total of 8 plants, i.e., 8 replications) and photographed as described above.
Morphological characteristics of leaves infested by larvae and chosen for oviposition
The morphological characteristics of leaves infested by DBM larvae and selected by females for oviposition were observed using an SDPTOP-SZN71 microscope system (Sunny, Hangzhou, Zhejiang, China). Digital images were acquired using a Panasonic DMC-GH4 digital camera (Panasonic, Osaka, Japan). Each treatment was replicated 10 times.
Effect of silk and wax on ovipositional behavior of DBM females
Oviposition selectivity of DBM females on leaves with and without waxy powder
The waxy powder on one side of the main vein of a leaf of 4-week-old Chinese kale was carefully removed according to the Silva et al. (2017) method using wet absorbent cotton with chloroform. All the expanded true leaves were treated in the same way. The ovipositional selectivity of DBM females on the treated plants was investigated as described in “Ovipositional preference of DBM for true leaves and cotyledons” section. The experiment was replicated 8 times.
Oviposition selectivity of DBM females to the leaves of different treatments
Fully expanded leaves were detached from 5 to 6-week-old Chinese kale, and the petioles were each dipped into water in a 2 mL centrifuge tube. Leaves with similar leaf areas were selected for each treatment. The leaves were assigned one of the following four treatments: (1) a healthy leaf (HL); (2) a leaf infested by DBM larvae (LID), where two 4th instar larvae were placed on the leaf to feed for 24 h and were removed after feeding; (3) a leaf crawled on by DBM larvae (LCD), where four 4th instar larvae were kept on the leaf to crawl for 1 h, during which the larvae were continuously touched with a dissecting needle to prevent them from feeding on the leaf; and (4) a leaf with silk removal (LSR). The silk of the leaf infested by DBM larvae was carefully removed with a dissecting needle under a dissecting microscope. Six groups of detached leaves were tested: (a) HL versus LID; (b) HL versus LCD; (c) HL versus LSR; (d) LID versus LCD; (e) LSR versus LCD; (f) LSR versus LID. All groups were separately maintained in nylon cages (20 × 20 × 30 cm), and 3 pairs of DBM adults were then introduced to the cage. The cages were maintained in the same climate room. After 24 h, the eggs laid on each leaf were counted separately. Each experiment was replicated 25–31 times.
Data analysis
The oviposition preference of DBM females, the developmental duration of DBM larvae, pupal weight, and glucosinolate content were compared using a t-test at P = 0.05. The feeding preference of DBM larvae between true leaves and cotyledons was compared with a Chi square test. The effects of waxy powder and leaf silk on the ovipositional preference of DBM females were analyzed by Wilcoxon matched-pair signed-rank tests for each pairwise treatment. The experimental data were analyzed using the package IBM SPSS Statistics 22.0 (SPSS, Inc., Chicago, IL, USA).
Results
Ovipositional preference of DBM females
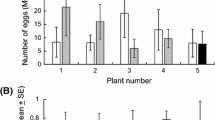
The number of DBM eggs laid on cotyledons was significantly greater than that on true leaves (Fig. 1a; t = 5.463, P = 0.001). More eggs were found on DBM larvae-infested Chinese kale than on healthy Chinese kale (Fig. 1b; t = 3.582, P = 0.009). The number of eggs laid on DBM larvae-infested true leaves was 54.6 times higher than that on healthy true leaves (Fig. 1b; t = 6.781, P < 0.001).
Performance of Plutella xylostella adults and larvae on Chinese kale. a Eggs of P. xylostella laid on Chinese kale. b Eggs of P. xylostella laid on conspecific preinfested Chinese kale. c Preference of P. xylostella larvae for true leaves and cotyledons. d Developmental period of P. xylostella larvae on true leaves and cotyledons. e Pupal weight of P. xylostella from true leaves and cotyledons. *indicates a significant difference at P < 0.05; **indicates a significant difference at P < 0.01; ***indicates a significant difference at P < 0.001. The mean amounts are presented with the standard error of the mean (± SE)
Feeding preference and developmental time of DBM larvae
The 1st or 3rd instar DBM larvae markedly preferred feeding on true leaves to cotyledons (Fig. 1c; 1st: χ2 = 13.000, df = 1, P < 0.001; 3rd: χ2 = 30.421, df = 1, P < 0.001). The developmental times of DBM larvae feeding on cotyledons were significantly longer than those of larvae feeding on true leaves (Fig. 1d; t = 9.446, P < 0.001). The pupal weight from the true leaves was notably greater than that from the cotyledons (Fig. 1e; t = 6.864, P < 0.001).
Glucosinolate analysis
Nine and five types of glucosinolates were detected in healthy true leaves and cotyledons, respectively (Fig. 2a). The levels of three glucosinolates, i.e., gluconapin (t = − 3.005, P = 0.015), glucobrassicin (t = − 7.955, P < 0.001) and neoglucobrassicin (t = − 3.191, P = 0.011), were significantly greater in true leaves than those in cotyledons, but the 4-hydroxyglucobrassicin content in cotyledons was markedly greater than that in true leaves (t = 2.934, P = 0.014). The glucobrassicin (t = 11.197, P < 0.001) and neoglucobrassicin (t = 11.197, P < 0.001) concentrations were significantly greater in the DBM larvae-infested true leaves than in healthy true leaves. The content of total glucosinolates in healthy true leaves was significantly greater than that in healthy cotyledons (Fig. 2b; t = − 4.605, P < 0.001). However, the content of total glucosinolates between the healthy and DBM larvae-infested true leaves was not significantly different (t = − 1.118, P = 0.278).
Glucosinolate content. a Individual glucosinolate content in healthy and P. xylostella larvae-infested leaves of Chinese kale. b Total glucosinolate content in healthy and P. xylostella larvae-infested leaves of Chinese kale. *indicates a significant difference at P < 0.05; **indicates a significant difference at P < 0.01; ***indicates a significant difference at P < 0.001; ns indicates no significant difference at P = 0.05. The mean amounts are presented with the standard error of the mean (± SE). Note: 2OH3B: Progoitrin; 2-Propenyl: Sinigrin; 3-Butenyl: Gluconapin; 4OHI3M: 4-hyrdroxyglucobrassicin; Benzyl: Glucotropaeolin; I3M: Glucobrassicin; 1MOI3M: 4-methoxyglucobrassicin; 2PE: Gluconasturtiin; 4MOI3M: Neoglucobrassicin
Leaf surface structure of Chinese kale
The cotyledons were smooth and bright green, and no waxy powder was observed on the surface. In contrast, the true leaves were light blue and covered with rod- and flake-shaped waxy powder (Fig. 3A). Furthermore, there were substantial amounts of silk on the leaves infested by DBM larvae, and DBM eggs were laid on the silk (Fig. 4).
Leaf surface structure of Chinese kale and the oviposition preference of Plutella xylostella for a dewaxed leaf of Chinese kale. a Leaf surface structure of Chinese kale. (a) Abaxial surface of a cotyledon. (b) Enlarged view of the delineated box in (a). (c) Abaxial surface of a true leaf. (d) Enlarged view of the delineated box in (c). b Oviposition preference of P. xylostella for a dewaxed leaf of Chinese kale. (a) Eggs of P. xylostella laid on the abaxial surface of Chinese kale. (b) The hydrophilicity of a Chinese kale leaf after the waxy powder is removed. (c) Eggs of P. xylostella laid on the adaxial surface of Chinese kale. (d) Eggs of P. xylostella laid on a leaf of Chinese kale. Different lowercase letters indicate significant differences at P < 0.05. The mean amounts are presented with the standard error of the mean (± SE)
Silk spun by Plutella xylostella larvae and eggs of P. xylostella laid on preinfested Chinese kale. a Silk “net” on leaf. b Enlarged view of the delineated box in (a). c Damaged parts by P. xylostella larvae. d Enlarged view of the delineated box in (c). e P. xylostella egg deposition site. f Enlarged view of the delineated box in (e)
DBM larvae produced a silk “ladder” when crawling on the leaves and wove a “net” before feeding (video S1 and video S2). During the process of crawling and feeding, the larvae continued spinning (video S3). At the beginning of feeding, DBM larvae stood on the net and firmly grasped the net with their thoracic legs (video S4).
Effects of waxy powder and silk on the ovipositional preference of the DBM
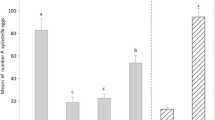
After removing the wax, the hydrophilicity of the true leaves was significantly enhanced, and the egg density was greatly increased (video S5 and Fig. 3B). The number of eggs laid on dewaxed leaves was much greater than that on normal leaves (t = 6.525, P = 0.001). Furthermore, the density of DBM eggs was highest on the cotyledons and lowest on the normal true leaves (Fig. 3b; F = 32.763, P < 0.001).
Compared with the healthy leaves (HL), the amount of eggs laid on the leaves infested by DBM larvae (LID), the leaves crawled on by DBM larvae (LCD), and the leaves with silk removal (LSR) were significantly increased (Fig. 6; HL vs LID: Z = − 4.623, P < 0.001; HL vs LCD: Z = − 4.704, P < 0.001; HL vs LSR: Z = − 4.065, P < 0.001). No significant difference in egg numbers was found between LID and LCD treatments (Fig. 6; LID vs LCD: Z = − 0.854, P = 0.443). The number of eggs laid in the LCD treatment (LCD vs LSR: Z = − 3.810, P < 0.001) and the LID treatment (LID vs LSR: Z = − 4.362, P < 0.001) was substantially greater than that in the LSR treatment (Fig. 6). In addition, the number of eggs laid by DBM females in places with silk distribution was much greater than that in places without silk distribution (Fig. 6; silk vs without silk: Z = − 4.861, P < 0.001).
Discussion
It is critical for female adult phytophagous insects to find a suitable plant oviposition site (Gripenberg et al. 2010). Glucosinolate concentration, waxy powder, and trichome density in host plants have been identified as the main host plant features for DBM oviposition (Sarosh et al. 2010; Silva et al. 2017; Badenes-Pérez et al. 2020). However, it was not clear how DBM females overcame the obstacles of the wax and successfully oviposited on waxy plants. Here, we demonstrate adaptations of DBM females and larvae on the waxy host plant Chinese kale: i.e., DBM females first lay eggs on the cotyledons of Chinese kale with less wax, even though the cotyledons are not suitable for their larvae; afterwards, the hatched larvae may have changed the surface structure of the true leaves by crawling and spinning, which is conducive to subsequent oviposition on the true leaves by DBM females. These results further suggest why DBM females prefer to lay eggs on host plants damaged by conspecific larvae.
Glucosinolates are the main secondary metabolites in cruciferous plants and are also stimulating substances for oviposition by DBM females (Gupta and Thorsteinson 1960; Reed et al. 1989; Renwick and Radke 1990; Furlong et al. 2013). Badenes-Pérez et al. (2014, 2020) reported that the ovipositional preference of DBM females in cruciferous plants is positively correlated with the glucosinolate content of the plant species. However, in this study, we found that the content of glucosinolates in the true leaves of Chinese kale was significantly higher than that in the cotyledons (Fig. 2), whereas DBMs significantly preferred the cotyledons to the true leaves for oviposition (Fig. 1). Information on the relationship between the oviposition preference of DBM in different parts of a plant and the content of glucosinolates is rather limited. Apart from glucosinolates, plant surface wax also had a significant effect on the oviposition preference of DBM. Spencer et al. (1996,1999) reported that the wax on the surface of plants acts synergistically with glucosinolates to help DBM lay eggs. However, we found that the waxy powder in the true leaves of Chinese kale hindered DBM oviposition, and the number of eggs of DBM laid on the true leaves was significantly increased when the waxy powder was removed (Fig. 3). Similar to our results, several studies have shown that wax on the surface of plants hinders DBM oviposition (Justus et al. 2000; Silva et al. 2017; Rahardjo and Tarno 2018; Rahman et al. 2019). Furthermore, DBM larvae performed better when fed on the true leaves of Chinese kale (Fig. 1). These results are consistent with previous reports on rape (Brassica napus) seedlings in which true leaves were more suitable as food in the DBM larval stage than cotyledons (Uematsu 1996).
Gustatory stimuli without olfactory stimuli do not affect the oviposition behavior of female DBMs, and female moths do not lay eggs with or without olfactory stimulation if gustatory stimuli are lacking (Justus and Mitchell 1996). In the current study, two-choice tests of DBM oviposition indicated that gustatory and tactile stimuli might play a decisive role in oviposition selection in DBM. Interestingly, DBM females have been shown to prefer laying most of their eggs on a centrifuge tube rather than on leaves, which suggests that even if DBM females are exposed to oviposition-stimulating substances on leaves, they will not lay eggs on the leaves. However, when the wax on leaves was removed, DBM oviposition was significantly higher in Chinese kale leaves with wax removal than in those without wax removal. We speculate that wax removal might change the physical properties of the Chinese kale leaf surface, leaving it more attractive and vulnerable to DBM females. This further confirmed our conclusion that the waxy powder on leaves was the main factor that hindered DBM oviposition on the true leaves of Chinese kale.
Although some host plants utilize waxy powder to prevent DBM from laying eggs, DBM females have evolved adaptations to resist this plant defense. DBM females first oviposited on the less waxy parts of plants, and the hatched larvae then produced a silk “ladder” and a silk “net” while crawling and feeding. These structures, formed on the surface of the plant leaves, helped subsequent DBM females oviposit on thick waxy leaves (Fig. 5; video S1 and video S2). Therefore, the oviposition obstacle of DBM females was conquered by a larvae-made silk “ladder” and “net”. Moreover, the silk ladder helped the larvae crawl freely on the leaf surface, which was similar to findings from Roden (1993) regarding the gypsy moth. More interestingly, our results showed that when DBM larvae fed on host plants, the silk gland also connected to the leaf by silk (video S3), which might explain why DBM larvae can escape quickly by silk when responding to danger (Harcourt 1957). Although DBM larvae possess ring toe hooks on their abdominal legs, their thoracic legs are very thin. Therefore, it is difficult for them to grasp smooth leaves directly, especially away from the edges of the leaves. DBM larvae wove a silk “net” before feeding and then held onto the “net” to feed (video S2 and video S4). With the expansion of the feeding site, the larvae crawled along the edge of the feeding site and then either returned to the “net” to rest or formed a new “net”. We speculate that spinning might be the main auxiliary tool for DBM larvae crawling and feeding on smooth leaves. Shiojiri et al. (2002) reported that DBM females prefer ovipositing on Pieris rapae larvae-infested plants instead of healthy plants. The reason might be related to the silk that P. rapae larvae spin. In fact, we also observed that DBM laid more eggs on leaves damaged by P. rapae larvae than on healthy leaves (unpublished data).
Silk spun by Plutella xylostella larvae. a, b The larvae of P. xylostella grasping on the silk net to feed. c The silk net produced by P. xylostella larvae when crawling on the smooth blackboard. d, e, g The eggs laid by a P. xylostella female on the silk net. f Larvae feeding along the edge of a leaf accompanied by spinning. h The larva of P. xylostella grasping onto the silk net to crawl
It has been reported that DBM females prefer to lay eggs on cabbage plants damaged by conspecific larvae instead of undamaged plants (Shiojiri and Takabayashi 2003; Silva and Furlong 2012; Ang et al. 2016). Our results indicated that this was caused by the silk vomited by DBM larvae when feeding on plants. However, Badenes-Pérez et al. (2013) documented that oviposition preference by DBM females is unaffected by feeding by conspecifics or Helicoverpa armigera larvae on the wild-type and mutants of Arabidopsis thaliana. This could be a reason that there is no or very little wax powder on these plants. In the present study, most DBM eggs were laid on the silk spun by its larvae, but the amount of eggs was significantly decreased when the silk on the damaged leaves was removed compared to the damaged leaves with silk. Furthermore, compared with the leaves that were only crawled on by the larvae, the amount of eggs was significantly decreased when the silk was removed (Fig. 6). These results suggest that the larvae and females of DBM overcome the adverse effects of plant wax through adaptation to complete the reproduction of the population.
Oviposition selectivity of Plutella xylostella females on leaves subjected to different treatments. The leaves of different treatments were offered to P. xylostella in two-choice tests. The numbers of eggs laid overnight by P. xylostella females were used to quantify oviposition preferences. Horizontal lines in boxes show medians, boxes contain the 25th–50th percentiles, whiskers show the upper and lower quartiles and points show outliers. Statistical differences within pairwise treatment combinations were carried out with the Wilcoxon matched-pair signed-rank test at P < 0.05. The mean amounts are presented with the standard error of the mean (± SE). Note: HL: healthy leaf; LID: leaf infested by P. xylostella larvae; LCD: leaf crawled on by P. xylostella larvae; LSR: leaf with silk removal
In this study, the silk on the leaf surface of Chinese kale was removed by a fine dissecting needle, but other forms of manipulation may also alter the surface of leaves, for example, by removing waxes. This could be a reason that more DBM eggs were laid on the leaves with silk removal than on the healthy leaves (Fig. 6). On the other hand, the plants used for culturing DBM and for experiments were grown under controlled conditions in growth chambers, and the chemical substance and leaf structure such as the outer wax layer in these plants might differ from those grown outdoors where they are exposed to the complicated natural environment. These changes will inevitably affect the performance of DBMs. Therefore, further research is needed to explore the behavior of DBMs under natural conditions.
In conclusion, thick waxy powder, acting as a physical barrier, is found on true leaves of Chinese kale and impedes oviposition by DBM females. However, it is proposed that DBM females have evolved an adaptation to deal with this waxy powder on Chinese kale. Female DBM adults first laid eggs on cotyledons of Chinese kale, which are not suitable for their larvae, but hatched larvae change the surface structure of the leaves by spinning silk in the process of crawling and feeding: i.e., they produced a silk “ladder” and “net”. These structures significantly increased the subsequent oviposition of DBM females on true leaves. This study represents the first comprehensive report on why female DBM adults lay eggs on the cotyledons of healthy cruciferous plants rather than on true leaves and why DBM females prefer to lay eggs on conspecifically damaged cruciferous plants. Moreover, this is the first report of a new function of lepidopterous larval silk, i.e., helping female adults lay eggs. These findings thus provide new insights into plant–insect interactions.
References
Ang GCK, Zalucki MP, Furlong MJ (2016) Temporal changes in olfactory and oviposition responses of the diamondback moth to herbivore induced host plants. Entomol Exp App 160:28–39
Badenes-Pérez FR, Gershenzon J, Heckel DG (2014) Insect attraction versus plant defense: young leaves high in glucosinolates stimulate oviposition by a specialist herbivore despite poor larval survival due to high saponin content. PLoS ONE 9:e95766
Badenes-Pérez FR, Gershenzon J, Heckel DG (2020) Plant glucosinolate content increases susceptibility to diamondback moth (Lepidoptera: Plutellidae) regardless of its diet. J Pest Sci 93:491–506
Badenes-Pérez FR, Reichelt M, Gershenzon J, Heckel DG (2013) Interaction of glucosinolate content of Arabidopsis thaliana mutant lines and feeding and oviposition by generalist and specialist lepidopterans. Phytochemistry 86:36–43
Badenes-Pérez FR, Reichelt M, Heckel DG (2010) Can sulfur fertilisation improve the effectiveness of trap crops for diamondback moth, Plutella xylostella (L.) (Lepidoptera: Plutellidae)? Pest Manag Sci 66:832–838
Brown PD, Tokuhisa JG, Reichelt M, Gershenzon J (2003) Variation of glucosinolate accumulation among different organs and developmental stages of Arabidopsis thaliana. Phytochemistry 62:471–481
Cao HH, Liu HR, Zhang ZF, Liu TX (2016) The green peach aphid Myzus persicae perform better on pre-infested Chinese cabbage Brassica pekinensis by enhancing host plant nutritional quality. Sci Rep 6:21954
Couty A, Van Emden H, Perry JN, Hardie J, Pickett JA, Wadhams LJ (2006) The roles of olfaction and vision in host-plant finding by the diamondback moth, Plutella xylostella. Physiol Entomol 31:134–145
Dias FM, Carneiro E, Casagrande MM, Mielke OH (2012) Biology and external morphology of immature stages of the butterfly, Diaethria candrena candrena. J Insect Sci 12:9
Dias FM, Casagrande MM, Mielke OH (2014) Biology and external morphology of the immature stages of the butterfly Callicore pygas eucale, with comments on the taxonomy of the genus Callicore (Nymphalidae: Biblidinae). J Insect Sci 14:91
Furlong MJ, Wright DJ, Dosdall LM (2013) Diamondback moth ecology and management: problems, progress, and prospects. Annu Rev Entomol 58:517–541
Gripenberg S, Mayhew PJ, Parnell M, Roslin T (2010) A meta-analysis of preference-performance relationships in phytophagous insects. Ecol Lett 13:383–393
Gupta PD, Thorsteinson AJ (1960) Food plant relationships of the diamond-back moth [Plutella maculipennis (Curt.)] II. Sensory regulation of oviposition of the adult female. Entomol Exp App 3:241–250
Harcourt DG (1957) Biology of the diamondback moth, Plutella maculipennis (Curt.) (Lepidoptera: Plutellidae), in Eastern Ontario. II. Life-history, behaviour, and host relationships. Can Entomol 89:554–564
Ibrahim MA, Nissinen A, Holopainen JK (2005) Response of Plutella xylostella and its parasitoid Cotesia plutellae to volatile compounds. J Chem Ecol 31:1969–1984
Justus KA, Dosdall LM, Mitchell B (2000) Oviposition by Plutella xylostella (Lepidoptera: Plutellidae) and effects of phylloplane waxiness. J Econ Entomol 93:1152–1159
Justus KA, Mitchell BK (1996) Oviposition site selection by the diamondback moth, Plutella xylostella (L.) (Lepidoptera: Plutellidae). J Insect Behav 9:887–898
Li ZY, Feng X, Liu SS, You MS, Furlong MJ (2016) Biology, ecology, and management of the diamondback moth in China. Annu Rev Entomol 61:277–296
Pivnick KA, Jarvis BJ, Gillot C, Slater GP, Underhill EW (1990) Daily patterns of reproductive activity and the influence of adult density and exposure to host plants on reproduction in the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). Environ Entomol 19:587–593
Rahardjo BT, Tarno H (2018) Diamondback moth, Plutella xylostella (Linnaeus) responses on Chinese kale (Brassica oleracea Var. alboglabra) treated by plant growth promoting rhizobacteria. Asian J Crop Sci 10:73–79
Rahman M, Zalucki MP, Furlong MJ (2019) Diamondback moth egg susceptibility to rainfall: effects of host plant and oviposition behavior. Entomol Exp App 167:701–712
Rathcke BJ, Poole RW (1975) Coevolutionary race continues: butterfly larval adaptation to plant trichomes. Science 187:175–176
Reddy GVP, Tabone E, Smith MT (2004) Mediation of host selection and oviposition behavior in the diamondback moth Plutella xylostella and its predator Chrysoperla carnea by chemical cues from cole crops. Biol Control 29:270–277
Reed DW, Pivnick KA, Underhill EW (1989) Identification of chemical oviposition stimulants for the diamondback moth, Plutella xylostella, present in three species of Brassicaceae. Entomol Exp App 53:227–286
Renwick JAA, Haribal M, Gouinguené S, Stadler E (2006) Isothiocyanates stimulating oviposition by the diamondback moth, Plutella xylostella. J Chem Ecol 32:755–766
Renwick JAA, Radke CD (1990) Plant constituents mediating oviposition by the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). Phytophaga 3:37–46
Rochfort SJ, Trenerry VC, Imsic M, Panozzo J, Jones R (2008) Class targeted metabolomics: ESI ion trap screening methods for glucosinolates based on MSn fragmentation. Phytochemistry 69:1671–1679
Roden DB (1993) Laddering: climbing behavior of the gypsy moth (Lepidoptera: Lymantriidae). Ann Entomol Soc Am 3:379–383
Sarosh BR, Wittstock U, Halkier BA, Ekbom B (2010) The influence of metabolically engineered glucosinolates profiles in Arabidopsis thaliana on Plutella xylostella preference and performance. Chemoecology 20:1–9
Shiojiri K, Takabayashi J, Yano S, Takafuji A (2002) Oviposition preferences of herbivores are affected by tritrophic interaction webs. Ecol Lett 5:186–192
Shiojiri K, Takabayashi J (2003) Effects of specialist parasitoids on oviposition preference of phytophagous insects: encounter-dilution effects in a tritrophic interaction. Ecol Entomol 28:573–578
Silva GA, Pereira RM, Rodrigues-Silva N, Souza TC, Ferreira DO, Silva GAR et al (2017) Wax removal and diamondback moth performance in collards cultivars. Neotrop Entomol 46:571–577
Silva R, Furlong MJ (2012) Diamondback moth oviposition: effects of host plant and herbivory. Entomol Exp Appl 143:218–230
Spencer JL, Pillai S, Bernays EA (1999) Synergism in the oviposition behavior of Plutella xylostella: sinigrin and wax compounds. J Insect Behav 12:483–500
Spencer JL (1996) Waxes enhance Plutella xylostella oviposition in response to sinigrin and cabbage homogenates. Entomol Exp Appl 81:165–173
Strong DR, Lawton JH, Southwood TR (1984) Insects on plants. Harvard Univ Press, Cambridge, MA
Talekar NS, Shelton AM (1993) Biology, ecology, and management of the diamondback moth. Annu Rev Entomol 38:275–301
Uefune M, Shiojiri K, Takabayashi J (2017) Oviposition of diamondback moth Plutella xylostella females is affected by herbivore-induced plant volatiles that attract the larval parasitoid cotesia vestalis. Arthropod-Plant Inte 11:235–239
Uematsu H (1996) Inter-leaf movement of larvae of diamondback moth, Plutella xylostella L. (Lepidoptera: Yponomeutidae) on rape (Brassica napus) seedlings. Jap J Appl Entomol Z 40:35–38
Acknowledgements
This work was supported by the Natural Science Foundation of China (31470484), the National Key R&D Program of China (No. 2017YFD0201000) and China Agriculture Research System (No. CARS-23-D-06).
Author information
Authors and Affiliations
Contributions
JY and SZ designed the research; JY and ZW performed research; LN and TX provided assistance; JY and SZ analyzed data; JY, SZ and TX wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Communicated by Antonio Biondi.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file 1. Video S1. Fourth instar larvae of Plutella xylostella produce a “ladder” on the leaf by spinning to help them crawl. (MOV 3657 kb)
Supplementary file 2. Video S2. The “net” helps Plutella xylostella larvae stand for feeding. (MOV 2329 kb)
Supplementary file 3. Video S3. Fourth instar larvae of Plutella xylostella feed on a leaf. The feeding process was accompanied by spinning, and the thoracic legs clawed the fresh silk. (MOV 2090 kb)
Supplementary file 4. Video S4. The larvae of Plutella xylostella stand on the “net” to feed. (MOV 3407 kb)
Supplementary file 5. Video S5. The hydrophobicity of Chinese kale leaf before and after removing waxy powder. (MOV 8762 kb)
Rights and permissions
About this article
Cite this article
Zhu, JY., Xiang, ZW., Zhang, SZ. et al. Adaptations of Plutella xylostella adult females and larvae to waxy host plants. J Pest Sci 95, 203–214 (2022). https://doi.org/10.1007/s10340-021-01366-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-021-01366-3