Abstract
Recognition of cabbage as a host plant for the diamondback moth (DBM) has previously been shown to depend on compounds that are extracted by soaking intact foliage in chloroform. Analysis of such chloroform extracts by open column chromatography has now resulted in the isolation of highly active fractions that elicit oviposition on treated filter papers. Further separation of these fractions by high-performance liquid chromatography revealed the presence of two distinct groups of active compounds that may be classified as volatile and non-volatile. The two prominent volatile components were separated and identified by mass spectrometry as the isothiocyanates, iberin (3-methylsulfinylpropyl isothiocyanate) and sulforaphane (4-methylsulfinyl-3-butenyl isothiocyanate). Subsequent bioassays of a range of isothiocyanates showed that iberin and sulforaphane were the most active of those tested. Other isothiocyanates with sulfur in the side chain were also active, whereas alkyl and phenyl isothiocyanates had only limited activity. In electrophysiological experiments, electroantennograms (EAGs) indicated positive responses of moth antennae to the isothiocyanates that were most active in behavioral assays. Since sulforaphane has been identified as a major inducer of anticarcinogenic activity in mouse tissue, a synthetic analog (exo-2-acetyl-5-isothiocyanatonorbornane) that shows similar inducer activity was tested on DBM. This bicyclic analog was highly active in both behavioral and EAG assays, suggesting similarity in receptor sites for the two types of biological activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The diamondback moth (DBM), Plutella xylostella L. (Lepidoptera: Plutellidae), is a major pest of cruciferous crops on a worldwide basis and has developed resistance to most insecticides, including Bacillus thuringiensis (Talekar and Shelton, 1993). Efforts to find new natural approaches to control this insect have focused on understanding the plant chemistry that plays a major role in acceptance or rejection of potential host plants. The involvement of glucosinolates in host recognition has been suggested by several studies (Gupta and Thorsteinson, 1960; Reed et al., 1989; Renwick and Radke, 1990). Furthermore, a synergistic effect of leaf waxes with a glucosinolate or with cabbage homogenates was demonstrated by Spencer (1996) and Spencer et al. (1999). Also, the involvement of plant volatiles in attracting moths to their host plants was shown by Palaniswamy et al. (1986) and by Pivnick et al. (1990), but no attempts to identify active compounds were reported.
More recent studies have shown that potent oviposition stimulants for the DBM are extracted from cabbage foliage by soaking the intact leaves in chloroform (Hughes et al., 1997). A bioassay was developed whereby thousands of eggs were laid on filter papers treated with the extracts or with active fractions from an open column separation. However, sufficient separation was not obtained for identification of the active constituents at that time. Here, we report on the identification of two volatile compounds that account for much of the activity. Tests were performed to examine structure–activity relationships, and electrophysiological studies were conducted to determine the sensory mode of detection of these compounds by the moths.
Methods and Materials
Plants and Extracts
Cabbage plants, Brassica oleracea L. var. Golden Acre (Agway, Inc., Syracuse, NY, USA) were grown from seed in an air-conditioned greenhouse at 23/19°C day/night with a 16 hr photophase. Supplemental lighting was provided by 400-W multivapor, high-intensity discharge lamps (General Electric MV 400/VBU). Plants were generally 4–5 wk old when used for extraction. One kg of intact leaves was immersed in approximately 3 l of chloroform for a period of 1.5 hr. The resulting extract was separated from leaf water and evaporated to a small volume for chromatography.
Insects
P. xylostella moths used for behavioral assays were from a laboratory colony maintained in Ithaca, NY, as previously described (Hughes et al., 1997). For the electrophysiological recordings in Switzerland, the insects came from the laboratory culture at Wädenswil that was established with approximately 200 individuals during spring of 2000 and reared as described by Marazzi and Städler (2004). Briefly, an equal number of male and female mature moths (approximately 100 per cage) was placed in mesh cages (50 × 45 × 45 cm) in a climate-controlled room (21 ± 1°C, 70% RH and 16 hr photophase), where they were allowed to mate and oviposit only on the leaves of potted B. napus cv CC-Cross F1 at the prebolt stage. The moths had access to a source of water and to 10% sugar water.
Behavioral Assays
Extracts and fractions were tested for activity using recently emerged moths (approximately 150 mixed sexes) in 31 × 31 × 31 cm screened cages. Moths in each cage were provided with a 10% sucrose solution. Test material representing 1 g leaf equivalent (gle) was applied in chloroform solution to 5.5-cm-diam. filter paper disks, and chloroform alone was applied to control disks. After air drying, the treated filter papers were weighed (±0.0001 g), and two treated and two control disks were placed on small plastic supports in opposite corners of each cage. After overnight exposure to the moths (approx. 15 hr), test filter papers were weighed to provide an estimate of the number of eggs laid, based on an average weight of 0.0000195 g per egg. Active fractions generally received more than 3000 eggs in this time period.
Known isothiocyanates were tested in the same way, using 250 μl of a 0.1 μg/μl chloroform solution to deliver 25 μg of test compound per disk. Relative activity of each isothiocyanate was determined in choice assays, using allyl isothiocyanate (ANCS) as control. Based on these comparative assays, activity was expressed as an oviposition preference index (OPI), where OPI = 100 (T − ANCS) / (T + ANCS), where T is the number of eggs laid on the test compound and ANCS is the number of eggs laid on the control.
Chemicals
All of the common aliphatic and aromatic isothiocyanates for behavioral studies (Fig. 1) were obtained from Sigma-Aldrich Corp (St. Louis, MO, USA). Iberin and sulforaphane were from LKT Laboratories (St. Paul, MN, USA) and the sample of bicyclic ketal (Posner #23) was a gift from Dr. Gary H. Posner of Johns Hopkins University, Baltimore, MD, USA. For electroantennograms recorded in the Wädenswil Laboratory, analytical quality allyl (2-propenyl) as well as benzyl, butyl, and methyl isothiocyanates were obtained from Fluka Chemicals, Basel, Switzerland.
Isolation and Identification of Active Compounds
Chloroform extracts of cabbage were separated by open column and medium pressure chromatography on silica gel as previously described (Hughes et al., 1997). Using stepwise elution with increasing concentrations of chloroform in hexane, followed by methanol in chloroform, two active fractions, A and B, were obtained. Fraction A eluted in 30:70 chloroform/hexane, whereas B eluted in 4:96 methanol/chloroform. The active fractions were subjected to high-performance liquid chromatography (HPLC) using a Waters instrument fitted with a Phenomenex analytical column, Luna 5 μ silica, 1.0 × 250 cm, with a flow rate of 1.0 ml/min. A diode array detector was used to monitor elution at 254 nm and to provide UV spectra of detected compounds. A solvent gradient of methanol in chloroform was used as follows: 0% methanol from 0 to 5 min, 3.0% MeOH at 17 min, 10.0% MeOH at 35 min, and 10.0% MeOH at 45 min.
The high-resolution electron impact mass spectra (HREIMS) and MS–MS were recorded on an AutoSpec (VG Analytical Instruments, Manchester, UK) instrument. The sample was introduced through a direct probe insert, and mass spectral data were collected at a temperature gradient of 70–350°C. The positive low-resolution electron spray ionization mass spectra (LRESIMS) and MS–MS were recorded on a VG Quattro (Micromass UK) instrument. The sample was introduced directly through a syringe pump at a flow rate of 200 μl/hr.
Electrophysiological Recordings
All isothiocyanates were dissolved in hexane (Fluka, GC grade, Buchs) to prepare six concentrations (1 × 10−5, 1 × 10−4, 1 × 10−3, 1 × 10−2, 0.1, 1 mg/ml).
Freshly emerged females were cooled in a refrigerator (5.5°C) for 15 min to reduce their activity. The wings and legs of the cooled insects were amputated and the body mounted ventral side up in the groove of a Plexiglas® holder and positioned so that the antennae were attached to a sticky wax layer, using strips of adhesive tape. The preparation was mounted under a stereomicroscope and continuously humidified with a water-saturated air stream (1 m s−1, room temperature ≈ 22°C). These preparations showed good longevity, and it was possible to obtain strong responses for over 1.5 hr, allowing plenty of recording time. For the stimulation we used basically the same method as Guerin and Visser (1980). In brief, the airflow was split into continuous and stimulatory air streams in a 9:1 ratio, which converged prior to reaching the preparation. The stimulatory air stream passed through a Pasteur pipette (20 mm tip diameter) containing the test compound in 100 μl paraffin oil spread on a folded filter paper (15 × 50 mm, from Schleicher and Schuell). The amount of an individual isothiocyanate in the air stream reaching the antenna is dependent on its partial vapor pressure, but this value can be kept constant by dissolution in paraffin oil (Kafka, 1970). It was injected into the continuous air stream upon activation of a valve. The indifferent electrode, filled with a saline solution (Kaissling, 1995), was inserted into the head and the recording electrode, containing saline was brought into contact with the antennal tip. The EAG signal was recorded using a lab-built amplifier with high input impedance (1013 Ω) and low bias current (<10 pA). The signals were filtered (electronic high-pass with cornering frequency of 0.001 Hz), amplified (100 times), and digitized using Superscope II 3.0 Software (GW Instruments, Somerville, MA, USA) on a Macintosh computer. The EAG amplitudes were determined using PowerChrom v2.2.4 software (AD Instruments, Colorado Springs, CO, USA). The responses to the concentration series of each isothiocyanate were measured, from the lowest to the highest concentration; isothiocyanates were presented in a randomized order. We also tested the pure solvent in order to check that it did not elicit a strong olfactory response. Before and after running each concentration series, we recorded the response to 100 μg (E)-2-hexenal on filter paper (as described above). The data were analyzed by calculating the mean amplitude of the five responses to each tested combination of compound and concentration, then dividing this value by the average amplitude of the associated response to (E)-2-hexenal. This standardized the data to control for changes in preparation sensitivity over time.
Results
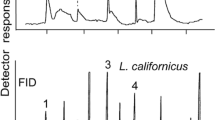
As previously reported, medium-pressure silica gel chromatography yielded two major active fractions that eluted with 30% chloroform in hexane (A) and 4% MeOH in chloroform (B). When these fractions were further separated by HPLC, fraction A revealed the presence of two major groups of UV-absorbing compounds that could not be completely separated, and, thus, no meaningful spectral data could be obtained. However, fraction B yielded clear peaks that were well separated (Fig. 2). Collection and assay of individual compounds and groups of compounds showed that the combined compounds eluting at 20.5–22.0 min as well as compounds 1 and 2 at 24.3 and 24.9 min were highly active in stimulating oviposition. Since the other groups of compounds could not be easily separated, further studies focused on compounds 1 and 2.
High vacuum separation using a sublimation apparatus showed that compounds 1 and 2 are volatile, whereas other constituents of fraction B remained in the nonvolatile residue. The UV spectra of 1 and 2 were similar, with absorption maxima at 227 and 245 nm, which is typical of isothiocyanates. The positive HREMS of compound 1 gave a quasimolecular ion at m/z 164.020383 (M + H)+ and its sodium adduct at 186.002327 (M + Na)+, suggesting a molecular formula (MF) of C5H9S2ON. The presence of sulfur was confirmed by a peak at m/z 166 (M + 2 + H)+ and its abundance in relation to m/z 164. In MS–MS, a molecular ion m/z 164 gave daughter ions at m/z 132, 130, 105, and 100. Similarly, the ion m/z 166 gave peaks at 134, 107, and 100. This suggested that fragments at 132 and 105 still retained at least one sulfur atom. On the basis of the fragmentation results, we identified this compound as iberin. This was further substantiated by comparing its NMR (in CDCl3) spectrum with reported literature values (Kore et al., 1993) and by comparing its retention times on GC and HPLC with those of an authentic sample of iberin. The MS fragmentation pattern also matched that of the authentic sample.
The mass spectrum of compound 2 gave a quasimolecular ion at m/z 178.02 for (M + H)+ in LRESIMS, and had a similar fragmentation pattern to that of 1. The combined MS and UV spectral properties suggested that this compound was sulforaphane. The identity of compound 2 was confirmed by comparing its MS, as well as retention times, on HPLC and GC with those of authentic sulforaphane.
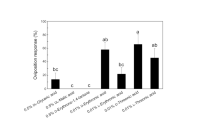
When authentic samples of iberin and sulforaphane were tested in bioassays, both isothiocyanates were highly active as stimulants. Comparative assays of a range of isothiocyanates were then conducted using allyl isothiocyanate (ANCS) as a standard. Tests of ANCS showed that this representative had limited activity when compared to iberin and sulforaphane. The comparative assays showed that methyl, ethyl, propyl, and phenyl isothiocyanates were considerably less active than ANCS (Fig. 3). Butyl and benzyl isothiocyanates had activity that was similar to that of ANCS, but sulforaphane, iberin, and iberverin were much more active than the standard (Fig. 3). Sulforaphane was previously identified as a major inducer of anticarcinogenic activity in mouse tissue (Zhang et al., 1992), and several isothiocyanate analogs with additional functional groups were subsequently found to have similar anticarcinogenic activity (Posner et al., 1994). One representative of these analogs, exo-2-acetyl-5-isothiocyanatonorbornane, was included in our oviposition assays, and this bicyclic ketal (referred to as Posner #23) also showed high activity when compared with ANCS (Fig. 3).
Electrophysiological recordings from DBM antennae provided EAGs that were in good agreement with behavioral responses. When tested at a medium dose of 10−3 to 10−2 mg, the most active isothiocyanates were the sulfur-containing iberin, sulforaphane, iberverin, and the bicyclic keto analog (Posner # 23). The least-active representatives were methyl and allyl isothiocyanates, whereas phenyl isothiocyanate gave an intermediate response, and phenethyl isothiocyanate was highly stimulatory (Fig. 4). However, at higher doses of 10−1 to 10−0 mg, saturation of olfactory neurons by some compounds was apparent (Fig. 5). The almost perfectly linear relationship of EAG response to increasing concentrations of Posner # 23 was particularly striking, although the highest dose (1 mg) of this compound was not available and could not be tested.
Discussion
Recognition of cabbage as a host plant by ovipositing DBM females is dependent on perception of a combination of chemicals at or near the surface of foliage. However, the results of this study show that highly active individual constituents may be just as effective as total extracts. Although nonvolatile constituents are involved (Renwick and Radke, 1990; Spencer, 1996), volatile compounds apparently play a key role in stimulating oviposition, either before or after contact with the leaf surface. Our identification of the volatile isothiocyanates, iberin and sulforaphane, as the most prominent volatile compounds in active fractions provides additional evidence to support the idea that these moths may already recognize host plants while in flight (Bukovinszky et al., 2004).
The involvement of isothiocyanates in host recognition is not surprising. However, the presence of these compounds at the leaf surface or their release from intact foliage has not been reported previously. Isothiocyanates are released as hydrolysis products of glucosinolates, which are characteristic glycosides in crucifers. However, this hydrolysis is believed to occur only when foliage is disrupted by insect feeding or mechanical damage (Städler, 2002). The concentrations of isothiocyanates in the headspace above undamaged crucifer plants are known to be extremely low (Finch, 1978; Tollsten and Bergström, 1988). However, host recognition by another crucifer specialist, the cabbage root fly, has been shown to involve detection of these low levels of volatiles (De Jong and Städler, 1999). The presence of iberin and sulforaphane in chloroform extracts of intact foliage would now suggest that they are readily available at or near the leaf surface and may in fact be released in small quantities from foliage in the field. However, we cannot discount the possibility that compounds may leak from the cut petioles of the leaves, or that some kind of chloroform-induced tissue damage occurs.
The apparent synergy between a glucosinolate and wax in stimulating oviposition by DBM (Spencer, 1996: Spencer et al., 1999) may now be explained by the likely presence of small quantities of isothiocyanate from spontaneous degradation of the glucosinolate, sinigrin. The allyl isothiocyanate released might be adsorbed to the wax, which could then act as a slow release substrate and result in increased oviposition. On a test substrate, in the absence of wax, the stimulatory isothiocyanate would evaporate before significant oviposition could occur.
Preliminary behavioral assays of authentic isothiocyanates indicated intermediate activity for allyl isothiocyanate. When this representative was used as a standard for comparison, it was clear that iberin, sulforaphane, and iberverin were extremely active, whereas the alkyl representatives, methyl, ethyl, and propyl, were less active than the standard. The most active compounds are characterized by the presence of sulfur, as thio, sulfinyl, or sulfonyl, in the side chain. When the bicyclic ketoisothiocyanate (Posner # 23) was tested, this analog had high activity that was comparable to that of the sulfur-containing representatives. This compound is one of several bifunctional analogs of sulforaphane that were found to be potent inducers of anticarcinogenic detoxication enzymes in mouse tissues and murine hepatoma cells (Posner et al., 1994). These authors found that the most potent anticarcinogenic analogs were those isothiocyanates in which the isothiocyanate group was separated from a methyl sulfonyl or an acetyl group by three or four carbons. It appears, therefore, that the structure–activity relationship for DBM stimulation and for anticarcinogenic activity is remarkably similar.
EAG recordings in response to the various isothiocyanates verified the fact that olfaction is the mode of perception of these stimulants. Independent experiments have shown that volatiles from differently fertilized Brassica plants play an important role in host selection of ovipositing DBM females (Marazzi and Städler, 2004). Furthermore, the most active stimulants in our study evoked the highest levels of electrophysiological activity. The high activity of sulforaphane, iberin, iberverin, and Posner #23 mirrored the behavioral responses to these compounds. The response to phenyl isothiocyanate, which was relatively inactive in behavioral assays, and to phenethyl isothiocyanate (not available for behavioral assays) may be explained by the fact that EAG responses may be positive or negative. In addition, EAG responses are likely to be higher in response to more volatile members of the series. No attempt was made to compensate for differences in volatility of the different compounds. The most active compounds in behavioral tests are actually among the least volatile of the group. It is likely, therefore, that the EAG results would be more dramatic if the actual dose reaching the antenna were to be more precisely controlled. Lower activity of the most volatile compounds in behavioral assays might be expected as a result of rapid dissipation during the test period, but no evidence of reduced activity after a 15 hr exposure was observed.
The correlation between structural features required for oviposition stimulating activity and anticarcinogenic activity is remarkable. The extensive structure–activity experiments for anticarcinogenic activity that were conducted by the Johns Hopkins Group (Posner et al., 1994) showed the superiority of the sulfur-containing compounds that, we now find, are most active as oviposition stimulants. Although only one of the synthetic bicyclic analogs was available for our experiments, the behavioral and electrophysiological responses to this compound appear to confirm that the same molecular characteristics are required for activity. The close similarities in requirements for the two types of biological responses suggest that the receptors involved have features in common.
Our study was restricted to a continuation of previous work on cabbage, B. oleracea. However, subsequent investigations have shown that similar soaking of foliage of several other crucifers in chloroform provides highly stimulatory extracts. Extracts of those species that produce glucosinolates with sulfur in the side chain appear to be particularly active (Renwick, unpublished data). Since the glucosinolate composition of crucifers varies widely, it is likely that other isothiocyanates are responsible for most of the activity in other plants. Recent studies on the attractiveness of Barbarea vulgaris to DBM have focused on the possible use of this plant as a trap crop, since the hatching larvae do not survive (Badenez-Perez et al., 2005). Although the involvement of volatiles has been suggested (Lu et al., 2004), the compounds responsible for high levels of oviposition and the types of isothiocyanates released from this plant have yet to be determined. Glucosinolate analyses of Barbarea vulgaris have shown that phenethyl is the only glucosinolate that would yield an isothiocyanate upon hydrolysis (Agerbirk et al., 2001: Windsor et al., 2005), and autolysis of foliage results in the release of traces of phenethyl as well methyl thioalkyl and allyl isothiocyanates (Cole, 1976). However, we have no evidence to indicate that the isothiocyanates released into chloroform are dependent on the glucosinolate composition of the plant.
The involvement of sulfur-rich compounds in DBM oviposition has been further indicated by fertilization experiments. Brassica napus plants that received higher rates of sulfur fertilization were preferred for oviposition by the moths. Methanolic surface extracts of the B. napus foliage produced EAG responses that were higher in the case of fertilized plants (Marazzi et al., 2004), although isothiocyanates were not detected within the threshold limits of the GC-MS system that was used.
Future studies on chloroform extracts of foliage from Barbarea vulgaris and other highly attractive crucifers are likely to reveal additional isothiocyanates that play a role in host recognition by the DBM. However, the involvement of sulforaphane and related compounds that have important physiological activity in mammals would suggest that comparative results from assays might reveal structure–activity relationships that could have wider biological applications. Additional work on the DBM will be required to identify nonvolatile constituents of host plants involved in the sequence of events leading to host selection by this important worldwide pest.
References
Agerbirk, N., Olsen, C. E., and Nielsen, J. K. 2001. Seasonal variation in leaf glucosinolates and insect resistance in two types of Barbarea vulgaris ssp. arcuata. Phytochemistry 58:91–100.
Badenez-Perez, F. R., Shelton, A. M., and Nault, B. A. 2005. Using yellow rocket as a trap crop for diamondback moth (Lepidoptera: Plutellidae). J. Econ. Entomol. 98:884–890.
Bukovinszky, T., Trefas, H., Van Lenteren, J. C., Vet, L. E. M., and Fremont, J. 2004. Plant competition in pest-suppressive intercropping systems complicates evaluation of herbivore responses. Oikos 109:435–446.
Cole, R. A. 1976. Isothiocyanates, nitriles and thiocyanates as products of autolysis of glucosinolates in Cruciferae. Phytochemistry 15:559–762.
De Jong, R. and Städler, E. 1999. The influence of odour on the oviposition behaviour of the cabbage root fly. Chemoecology 9:151–154.
Finch, S. 1978. Volatile plant chemicals and their effect on host plant finding by the cabbage root fly (Delia brassicae). Entomol. Exp. Appl. 24:350–359.
Guerin, P. M. and Visser, J. H. 1980. Electroantennogram responses of the carrot fly, Psila rosae, to volatile plant components. Physiol. Entomol. 5:111–119.
Gupta, P. D. and Thorsteinson, A. J. 1960. Food plant relationships of the diamond-back moth (Plutella maculipennis (Curt.)) II. Sensory regulation of oviposition of the adult female. Entomol. Exp. Appl. 3:241–250.
Hughes, P. R., Renwick, J. A. A., and Lopez, K. D. 1997. New oviposition stimulants for the diamondback moth in cabbage. Entomol. Exp. Appl. 85:281–283.
Kafka, W. A. 1970. Molekulare Wechselwirkung bei der Erregung einzelner Riechzellen. Z. Vergl. Physiol. 70:105–143.
Kaissling, K.-E. 1995. Single unit and electroantennogram recordings in insect olfactory organs, pp. 361–377, in A. I. Spielman and J. G. Brandl (eds.). Experimental Cell Biology of Taste and Olfaction: Current Techniques and Protocols. CRC Boca Raton.
Kore, A. M., Spencer, G. F., and Walligll, M. A. 1993. Purification of the O-(methylsulfiny1) alkyl glucosinolate hydrolysis products: 1-Isothiocyanato-3-(methylsulfinyl) propane, 1-isothiocyanato-4-(methylsulfinyl) butane, 4-(methylsulfinyl) butanenitrile, and 5-(methylsulfinyl) pentanenitrile from Broccoli and Lesquerella fendleri. J. Agric. Food Chem. 41:89–95.
Lu, J. H., Liu, S. S., and Shelton, A. M. 2004. Laboratory evaluations of a wild crucifer Barbarea vulgaris as a management tool for the diamondback moth Plutella xylostella (Lepidoptera: Plutellidae). Bull. Entomol. Res. 94:509–516.
Marazzi, C. and Städler, E. 2004. Influence of plant sulphur nutrition on oviposition and larval performance of the diamondback moth. Entomol. Exp. Appl. 111:225–232.
Marazzi, C., Patrian, B., and Städler, E. 2004. Secondary metabolites of the leaf surface affected by sulphur fertilisation and perceived by the diamondback moth. Chemoecology 14:81–86.
Palaniswamy, P. C., Gillot, C., and Slater, G. P. 1986. Attraction of diamondback moths, Plutella xylostella (L.) (Lepidoptera: Plutellidae), by volatile compounds of canola, white mustard, and faba bean. Can. Entomol. 118:1279–1285.
Pivnick, K. A., Jarvis, B. J., Slater, G. P., Gillot, C., and Underhill, E. W. 1990. Attraction of the diamondback moth (Lepidoptera: Plutellidae) to volatiles of oriental mustard: The influence of age, sex and prior exposure to mates and host plants. Environ. Entomol. 19:704–709.
Posner, G. H., Cho, C.-C., Green, J. V., Zhang, Y., and Talalay, P. 1994. Design and synthesis of bifunctional isothiocyanate analogs of sulforaphane: Correlation between structure and potency as inducers of anticarcinogenic detoxication enzymes. J. Med. Chem. 37:170–176.
Reed, D. W., Pivnick, K. A., and Underhill, E. W. 1989. Identification of chemical oviposition stimulants from the diamondback moth, Plutella xylostella, present in three species of Brassicaceae. Entomol. Exp. Appl. 53:227–286.
Renwick, J. A. A. and Radke, C. D. 1990. Plant constituents mediating oviposition by the diamondback moth, Plutella xylostella (Lepidptera: Plutellidae). Phytophaga 3:37–46.
Spencer, J. L. 1996. Waxes enhance Plutella xylostella oviposition in response to sinigrin and cabbage homogenates. Entomol. Exp. Appl. 81:165–173.
Spencer, J. L., Pillai, S., and Bernays, E. A. 1999. Synergism in the oviposition behavior of Plutella xylostella: Sinigrin and wax compounds. J. Insect Behav. 12:483–500.
Städler, E. 2002. Plant chemical cues important for egg deposition by herbivorous insects, pp. 171–204, in M. Hilker and T. Meiners (eds.). Chemoecology of Insect Eggs and Egg Deposition. Blackwell, Berlin.
Talekar, N. S. and Shelton, A. M. 1993. Biology, ecology and management of the diamondback moth. Annu. Rev. Entomol. 38:275–301.
Tollsten, L. and Bergström, G. 1988. Headspace volatiles of whole plants and macerated plant parts of Brassica and Sinapis. Phytochemistry 27:2073–2077.
Windsor, A. J., Reichelt, M., Figuth, A., Svatos, A., Kroymann, J., Kliebenstein, D. J., Gershenzon, J., and Mitchell-Olds, T. 2005. Geographic and evolutionary diversification of glucosinolates among near relatives of Arabidopsis thaliana (Brassicaceae). Phytochemistry 66:1321–1333.
Zhang, Y., Talalay, P., Cho, C.-G., and Posner, G. H. 1992. A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc. Natl. Acad. Sci. USA 89:2399–2403.
Acknowledgment
We are indebted to Gary H. Posner of the Johns Hopkins School of Medicine for providing a sample of the bicyclic ketone. We thank Athula Attygalle for help with mass spectral analyses. We also thank two anonymous reviewers for helpful comments. This work was supported in part by USDA NRI Agreement No. 97-35302-4225 to J.A.A.R and by Swiss National Science Foundation Grant 31-65016.01 to E.S.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Renwick, J.A.A., Haribal, M., Gouinguené, S. et al. Isothiocyanates Stimulating Oviposition by the Diamondback Moth, Plutella xylostella . J Chem Ecol 32, 755–766 (2006). https://doi.org/10.1007/s10886-006-9036-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-006-9036-9