Abstract
The effects of limonene, a mixture of limonene + carvone (1:1, v/v), and methyl jasmonate (MeJA) on diamondback moth (DBM) (Plutella xylostella L.) oviposition, larval feeding, and the behavior of its larval parasitoid Cotesia plutellae (Kurdjumov) with cabbage (Brassica oleracea L. ssp. capitata, cvs. Rinda and Lennox) and broccoli (B. oleracea subsp. Italica cv Lucky) were tested. Limonene showed no deterrent effect on DBM when plants were sprayed with or exposed to limonene, although there was a cultivar difference. A mixture of limonene and carvone released from vermiculite showed a significant repellent effect, reducing the number of eggs laid on the cabbages. MeJA treatment reduced the relative growth rate (RGR) of larvae on cv Lennox leaves. In Y-tube olfactometer tests, C. plutellae preferred the odors of limonene and MeJA to filtered air. In cv Lennox, the parasitoid preferred DBM-damaged plants with limonene to such plants without limonene. C. plutellae females were repelled by the mixture of limonene + carvone. In both cultivars, exogenous MeJA induced the emission of the sesquiterpene (E,E)-α-farnesene, the homoterpene (E)-4,8-dimethyl-1,3,7-nonatriene (DMNT), and green leaf volatile (Z)-3-hexenyl acetate + octanal. The attractive effect of limonene and MeJA predicts that these two compounds can be used in sustainable plant protection strategies in organic farming.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The diamondback moth (DBM), Plutella xylostella (L.) (Lepidoptera: Yponometidae) is economically the most important pest of cruciferous crops throughout the world (Talekar and Shelton, 1993). DBM feeds on all cruciferous crop plants, cole crops, and several greenhouse plants (Reddy et al., 2004). In this context, one of the most important biological control agents for DBM is the braconid Cotesia plutellae (Kurdjumov) (Hymenoptera: Braconidae). C. plutellae is a potential larval parasitoid that can parasitize in the first three instars of DBM larvae (Reddy et al., 2002; Zu-hua et al., 2002).
Plants can defend themselves against herbivores by direct and indirect defense mechanisms. Direct defense affects the insect herbivore through toxic or antinutritional compounds or through repellents or deterrents (Karban and Baldwin, 1997). In indirect defense, after herbivore damage, plants produce volatiles that can attract predators or parasitoids, as has been shown in many plants (Dickens, 1999; Shiojiri et al., 2001; Dicke et al., 2003; Vuorinen et al., 2004a,b). These volatile compounds permit insect parasitoids and predators to discriminate between intact and damaged plants (Reddy and Guerrero, 2004; Vuorinen et al., 2004a). The parasitoid C. plutellae prefers the odor of damaged cabbage plants to that of intact cabbage in Y-tube olfactometer tests (Vuorinen et al., 2004a).
In previous studies, limonene alone or with other monoterpenes is deterrent (Ntiamoah et al., 1996; Ntiamoah and Borden, 1996) or repellent (Peterson et al., 1994; Nehlin et al., 1994; Ibrahim et al., 2001) toward insects. Chenier and Philogene (1989), for example, reported that monoterpenes, including limonene, attracted predators of conifer bark beetles. Carvone has been reported to inhibit the feeding of pales weevil (Hylobius pales) on Pinus strobus seedlings (Salom et al., 1996), of pine weevil (Hylobius abietis, Coleoptera: Curculionidae) onScots pine (Pinus sylvestris) (Klepzig and Schlyter, 1999; Schlyter et al., 2004), and of the slug Arion lusitanicus on lettuce (Frank et al., 2002). Tripathi et al. (2003) reported feeding deterrence and contact and fumigant toxicity of carvone against stored product beetles, whereas Den Ouden et al. (1993) found a short-term oviposition repellence of carvone against cabbage root fly (Delia radicum).
Methyl jasmonate (MeJA) (a volatile derivative of jasmonic acid), which is involved in plant defense against herbivores, increases the activity of defense-related proteins (Thaler et al., 1996). MeJA was reported to protect genetically modified Arabidopsis plants (deficient in the jasmonate precursor linolenic acid) from attack by larvae of Bradysia impatiens (Diptera: Sciaridae) (McConn et al., 1997). The relative growth rate (RGR) of Spodoptera exigua larvae fed on MeJA-treated leaflets was found to be lower than that of those reared on control leaflets (Thaler et al., 1996). However, Oka et al. (1999) found that MeJA did not show promising results as a nematocide to protect tomato from root-knot nematodes.
Natural compounds originating from plants might be potential alternative pesticide (Lee et al., 2001; Ibrahim et al., 2004) that are not persistent in the environment and are safe to natural enemies, nontarget organisms, and human beings for use in sustainable agriculture (Lacey and Shapiro-Ilan, 2003). Therefore, our aim was to investigate the role of limonene, a mixture of limonene + carvone, and MeJA in DBM control and the effects of these compounds on the parasitoid C. plutellae.
Methods and Materials
Plant Material and Insects
Cabbage (Brassica oleracea L. ssp. capitata, cvs. Rinda and Lennox) and broccoli (B. oleracea subsp. Italica cv Lucky) seedlings, 4 to 5 wk‐old, grown at 24/18°C (day/night) and relative humidity (RH) of 60% were used. Diamondback moth P. xylostella L. and the parasitoid C. plutellae (Kurdjumov) were from our own mass rearings (Vuorinen et al., 2004a). Second and third instars of P. xylostella feeding on broccoli plants were offered to C. plutellae females for egg laying. The emerged adults of C. plutellae were collected and released into a clean insect cage, and a honey–water solution (1:1) was provided for feeding. One- to three-d-old C. plutellae females were used in the behavioral assay.
Test Compounds
(S)-(+)-Carvone and (R)-(+)-limonene are the major compounds in the essential oil of caraway seeds (Bouwmeester et al., 1995, 1998; Hannukkala et al., 2002). Recently, there has been some interest in the use of caraway oil for plant protection purposes (Iacobellis et al., 2005). (R)-(+)-Limonene has been found to be more effective than (S)-(−)-limonene against the pine processionary caterpillar, Thaumetopoea pityocampa (Tiberi et al., 1999). (R)-(+)-Limonene (97% purity) and (S)-(+)-carvone (96%) obtained from Aldrich Chemical Co. Ltd. (Milwaukee, WI, USA), and methyl jasmonate (96%) provided by Bedoukian Research Inc. (Danbury, CT, USA) were used in this study.
DBM Egg-Laying Experiments
The following three experiments were conducted for the egg-laying study.
Experiment 1: Cabbage plants sprayed with 3% limonene solution in a 5% ethanol (99.5% purity) solution in water were offered to DBMs for egg laying in a two-choice test where the moths were allowed to choose between two treatments (control and treated) 24 hr after spraying in an acrylic polyester gauze cage (60 × 33 × 33 cm, external dimensions). Plants sprayed with 5% ethanol in 95 ml of water were used as controls. The amount of solution reaching the plant surface was estimated to be 3–4 ml. The test was replicated 15 times.
Experiment 2: Cabbage plants were exposed to limonene released from vermiculite (phyllosilicate mineral). Fifteen milliliters of limonene were mixed into 300 ml of vermiculite by vigorous stirring, and 25 ml of the mixture were placed at the base of each plant. For controls, 25 ml of vermiculite without limonene were placed at the base of the plants. Thereafter, the plants were introduced into the cages individually in a no-choice test where there was only one plant in a cage. The two tests were conducted simultaneously in two separate growth chambers, and each test was replicated ten times.
Experiment 3: As previously, 7.5 ml of limonene and 7.5 ml of carvone were mixed into 300 ml of vermiculite, and thereafter 25 ml of the mixture were placed at the base of each plant. For controls, 25 ml of vermiculite without test compounds were placed at the base of each cabbage and broccoli plant. Each test was replicated ten times.
Ten moths (1:1 sex ratio) were released into the cage and allowed to lay eggs for 48 hr for all three experiments. The moths were then removed from the cage and the eggs were counted.
Feeding Experiment
Cabbage (cv Lennox and Rinda) plants sprayed with 3% limonene (prepared as previously) or 4.5 mM MeJA in 5% ethanol (99.5% purity) in water were used. Plants sprayed with 5% ethanol in water served as controls. Twenty-four hr after spraying, the fully developed leaves of the sprayed plants were cut, and the petiole was inserted into a 1.5-ml Eppendorf tube filled with tap water. Thereafter, the leaf was placed into a plastic container (250 ml) with a lid. Late second or early third instars of DBM larvae were introduced onto the leaf and allowed to feed for 48 hr. The initial and final weights of larvae were measured to calculate the RGR, using the following formula: ln(Wf) − ln(Wi), where Wf is the final weight and Wi the initial weight. The experiment was replicated 20 times for limonene and 15 times for MeJA treatments.
Olfactometer Experiments
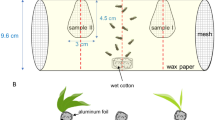
Cabbage plants from the above-mentioned cultivars and broccoli were used. Ten microliters of limonene, 10 μl of MeJA, and 2 μl of a mixture of limonene + carvone (1:1 v/v) were applied by pipetting on Whatman filter paper (42.5 mm2) and left in a fume hood for 10 min to evaporate. The filter paper with the elicitor was introduced into the glass container, as the odor source with a plant or without, depending on the test. The filter paper was placed on a piece of aluminum foil in the glass container. This experiment was conducted in a Y-tube olfactometer (main arm 10.5 cm, other arms 10 cm, inner diam 1.6 cm, angle between two arms ∼90°). Plants were placed into 1-l glass containers closed with Teflon-sealed lids with two inlets. Pressurized air was filtered through activated‐charcoal and passed through the glass container holding the plant with the odor source (with or without limonene, with or without limonene + carvone, and with or without MeJa), and then to one of the Y-tube arms. C. plutellae females were released individually into the opening of the main arm end of the Y tube and observed for 5 min or until they made the final choice. This choice was recorded as the insect passed into the end of the Y-tube arm. The Y tube was rotated 180° after each test run. The source of the odor, the glass container, the Y-tube device, and the lids of the containers were replaced after testing eight C. plutellae females in the limonene and MeJA experiments. Ten females were used for each test in the limonene + carvone assays, the filter papers with the mixture, and the Y tube being replaced after testing five females.
Collection of Volatile Compounds
Volatiles emitted from the foliage of the two cultivars of cabbage plants were collected using the headspace collection technique and analyzed by gas chromatography–mass spectrometry (GC-MS) as described by Vuorinen et al. (2004a) 1 d after spraying with 3% limonene in 5% ethanol in water or 4.5 mM MeJA in 5% ethanol in water. Five and six (Lennox and Rinda, respectively) randomly selected seedlings per treatment were used for volatile collections. The roots of each sample plant were washed and pruned slightly before being inserted into a 15 ml vial filled with water. Thereafter, the whole plant was enclosed in a 1.5 l (Lennox) or 1-l (Rinda) glass vessel. The glass vessels had two inlets, one for purified air and one for sampling. Charcoal-filtered air was led through Teflon tubing at a flow rate of 200 ml min−1 to the Tenax TA adsorbent tubes (150 mg), where the volatiles were collected for 30 min per sample. Samples were analyzed by GC-MS (Hewlett Packard GC type 6890, MSD 5973). Compounds collected on the Tenax TA adsorbent tubes were released by thermodesorption at 250°C for 10 min. Compounds were cryofocused in a cold trap at −30°C and subsequently injected onto an HP-5 capillary column (50.0 m × 0.2 mm i.d. × 0.50 μm film thickness). The column temperature was first held at 40°C for 1 min. Thereafter, the temperature was programmed to increase from 40°C to 210°C at 5°C min−1 and finally to 250°C at 20°C min−1. An interval of 30 to 300 m/z range was considered for the MS runs. Compounds were identified with different external standards, one for terpenoids and one for green leaf volatiles (GLVs), by comparing the mass spectra of a single compound with those of pure standards and those in the Wiley library. The amount of α-thujene, (E)-4,8-dimethyl-1,3,7-nonatriene (DMNT), and (E,E)-α-farnesene was calculated by assuming that the responses to them were the same as those to α-pinene, (Z)-ocimene, and (E)-β-farnesene, respectively. The shoot biomass of sample plants was determined to calculate the volatile emissions as nanograms per gram dry weight per hour (ng g DW−1 h−1). Volatiles were collected at a room temperature of 22°C and light intensity of 250 μmol m−2 s−1. Light intensity was measured with Quantum Sensor LI-185B (LI-COR, inc. Lincoln, NE, USA).
Statistics
Statistical analysis was performed using the SPSS 11.5 for Windows statistical package. One-way ANOVA and general linear models (GLM) procedures followed by Tukey’s or Dunnett’s T3 multiple comparison tests were used to determine the RGR of larvae and for normally distributed volatile compounds. Other compounds that were not normally distributed were tested with the nonparametric Kruskal–Wallis test. The Mann–Whitney test with Bonferroni correction was used to analyze differences between treatments. Data from the DBM egg-laying tests were analyzed with the independent-samples t test. The response of C. plutellae was analyzed with the nonparametric binomial test.
Results
Egg-Laying Experiments
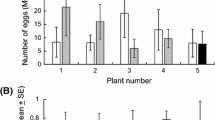
DBM females laid marginally significantly more eggs on cabbage (cv. Rinda) plants sprayed with limonene (Figure 1A) in the two-choice tests and when limonene was released from vermiculite (Figure 1B) in the no-choice test than on control plants. The number of eggs laid by females on cabbage plants treated with limonene + carvone released from vermiculite was lower in both cultivars in the no-choice tests (Figure 1C). On broccoli plants, the difference was not significant.
P. xylostella ovipositioning results on (A) cabbage plants sprayed with 3% of limonene (N = 15) in 5% ethanol in water, (B) cabbage plants with limonene released from vermiculite (N = 10), and (C) cabbage (cv Lennox and Rinda) and broccoli plants exposed to the combination of limonene + carvone released from vermiculite (N = 10). The data were analyzed with independent samples t test, and error bars are from SE values. *P < 0.05.
Feeding Experiment
Limonene had no deterrent effect on DBM larvae when detached leaves from plants sprayed with this compound were offered to them. Larval weight was not influenced by limonene (Figure 2A), but was significantly lower in cv Lennox sprayed with MeJA (Figure 2B).
Detached leaves from cabbage plants (cv Rinda and Lennox) sprayed with (A) 3% of limonene in 5% ethanol in water or (B) 4.5 mM of MeJA in 5% ethanol (99.5%) in water were fed to the DBM larvae for 48 hr and their RGR was calculated. Data were analyzed with one-way ANOVA and error bars are from SE values. *P < 0.05.
Olfactometer Experiments
Females of C. plutellae preferred the odors of pure compounds (limonene and MeJA) to filtered air without plants in the Y-tube olfactometer tests (Figure 3A), but did not significantly discriminate between the limonene–carvone mixture and clean air. However, the number (N = 12) of C. plutellae females responding to any of the odor sources was lower (P < 0.001) than that of females (N = 38) that did not choose any of the odor source (Figure 3A). In another case, C. plutellae females showed a significant preference for the damaged plant with limonene over the damaged plant alone in cv Lennox (Figure 3B). In the tests with broccoli and with both cabbage cultivars, females preferred the damaged plant without limonene + carvone to the damaged plant with the mixture of limonene + carvone (Figure 3C). Females were not able to differentiate between intact cabbage plants with limonene and those without it (Figure 3D). In the MeJA treatment, females did not show significant preference for any of the odor sources when tested with intact or damaged plants (data not shown).
C. plutellae responses in Y-tube olfactometer tests to (A) the odors of limonene, MeJA, and limonene + carvone without plants (treatment) and clean air; (B) damaged plants by DBM larvae + limonene (treatment) or damaged plants without limonene as control; (C) damaged plants with limonene + carvone as treatment or damaged plants without limonene + carvone serving as controls; and (D) intact plants + limonene + treatment) or without limonene as control. B = Broccoli, L = Lennox, R = Rinda. Total = the total number of females used in the experiment. No choice = the number of females that did not choose any of the odor sources. The data were analyzed using binomial tests: *P < 0.05, **P < 0.01, ***P < 0.001.
Collection of Volatile Compounds
In both cultivars, emission of homoterpene DMNT and the sesquiterpene (E,E)-α-farnesene was induced in the MeJA treatment (Tables 1 and 2). In cv Rinda, the concentration of total monoterpenes was higher in the limonene treatment than in the other two treatments (Table 2). In cv Lennox, sabinene concentration was lower in the MeJA treatment. In both cultivars, the GLV (Z)-3-hexenyl acetate + octanal was higher in the MeJA treatment than in the other two treatments. In cv Rinda, the total GLVs were higher in the MeJA treatment.
Discussion
Limonene Attracts DBM and Its Parasitoid C. plutellae
We demonstrated for the first time that exogenous limonene alone attracts DBM females to cabbage cv Rinda for ovipositioning. Previously, Pivnick et al. (1994) showed that DBMs are highly sensitive to an uncharacterized combination of volatiles released by intact plants and probably dominated by terpenes. In this regard, limonene is one of the main volatile compounds in the headspace of intact cabbages (Shiojiri et al., 2001; Vuorinen et al., 2004b). However, DBM moths prefer the volatiles released from conspecific-damaged cabbage plants over undamaged plants (Shiojiri and Takabayashi, 2003). Vuorinen et al. (2004a) found that emission of total monoterpenes from cv Rinda was induced by the 48-hr feeding damage of DBM larvae. We found that the total monoterpene emission from limonene-treated cv Rinda was significantly higher than that in other treatments. This suggests that the volatile emission from exogenously limonene-treated cabbage cv Rinda resembles that of DBM-damaged cv Rinda.
Our results show that limonene has no deterrent effect on DBM larval feeding. This contrasts with the findings with other pest insects by Peterson et al. (1994), Nehlin et al. (1994), and Ntiamoah et al. (1996), who have shown that limonene deterred the oviposition of pickleworm moths (Diaphania nitidalis), carrot psyllids (Trioza apicalis), and onion maggots (Delia antiqua M.), respectively. In Y-tube olfactometer tests, C. plutellae females preferred infested plants with limonene to infested plants without limonene in cv Lennox. This observation agrees with our previous study, which indicated that limonene attracts the generalist predator Podisus maculiventris (Hemiptera: Pentatomidae) (Ibrahim and Holopainen, 2002).
Limonene + Carvone Improves the Repellent and Deterrent Effect
We found that a mixture of limonene and carvone reduces the number of eggs laid by DBMs on cabbage. These results are consistent with the findings of Ntiamoah et al. (1996) and those of Ntiamoah and Borden (1996), who have shown that a mixture of limonene, 3-carene, and p-cymene deters oviposition by the onion maggot (D. antique M.), and by the cabbage maggot (D. radicum L.), respectively. Similarly, limonene + carvone repels females of C. plutellae in Y-tube olfactometer tests. Although the use of this mixture in the Y-tube olfactometer has not been reported previously, our findings principally agree with those of Nehlin et al. (1994), Ntiamoah et al. (1996), and Ntiamoah and Borden (1996). This demonstrates that the deterrent effect of single monoterpene increases when it is combined with other terpenoids. In previous studies, Salom et al. (1996), Klepzig and Schlyter (1999), Frank et al. (2002), and Schlyter et al. (2004) reported a feeding deterrent effect of carvone on insects and slugs.
MeJA Induces the Emission of Volatiles
We found that MeJA treatment induces the emission of the sesquiterpene (E,E)-α-farnesene and the homoterpene DMNT, as previously reported by Rodriguez et al. (2001) for cotton plants treated with exogenous MeJA. Similarly, the exogenous application of MeJA on oilseed rape (Brassica rapa subsp. oleifera) increases the amount of these compounds (Loivamäki et al., 2004). In addition to constitutive monoterpenes (sabinene, limonene, β-pinene, myrcene, 1,8-cineole, α-thujene, and α-pinene), infested cabbage plants emit induced compounds such as DMNT, and (E,E)-α-farnesene, which are known to attract herbivores and their natural enemies (Dicke et al., 1999; Rodriguez et al., 2001; Vuorinen et al., 2004a,b). Although the mechanism whereby MeJA induces DMNT and (E,E)-α-farnesene is not fully understood, Mandujano-Chavez et al. (2000) found that MeJA can induce the expression of sesquiterpene cyclase genes in tobacco cell cultures. However, MeJA has limited ability to induce later steps in the sesquiterpene pathway (Mandujano-Chavez et al., 2000). On the other hand, MeJA induces the expression of lipoxygenase in common bean (Porta et al., 1999) and in maize (Kim et al., 2003), which suggests that MeJA affects the emission of DMNT and (E,E)-α-farnesene via lipoxygenase pathway by inducing the accumulation of endogenous jasmonic acid.
As reported (Bogahawatte and van Emden, 1996; Potting et al., 1999; Vuorinen et al., 2004a), the volatile compounds emitted from plants damaged by DBM allow C. plutellae to discriminate between intact and damaged plants. In the present study, we demonstrated that the parasitoid C. plutellae is able to discriminate between limonene, MeJA, and clean air. The response of C. plutellae varies with the plant species and probably with the cultivar (Liu and Jiang, 2003), and the volatiles induced from infested plants seem to be important cues to C. plutellae (Shiojiri et al., 2001; Shiojiri and Takabayashi, 2003). Reddy et al. (2002) have also shown that DBM parasitoids, including C. plutellae, are attracted to a variety of chemical cues related to their host.
In summary, our results suggest that limonene and MeJA can be used as attractants for natural enemies of insect herbivores, particularly those of DBM, in organic agriculture. A mixture of limonene with carvone can act as a deterrent on crop plants, but deterrents have negative effects on natural enemies of DBM. Limonene can perhaps be used as an attractant of DMB on trap crop plants.
References
C. N. L. Bogahawatte H. F. Emden Particlevan (1996) ArticleTitleThe influence of the host plant of diamondback moth (Plutella xylostella) on the plant preferences of its parasitoid Cotesia plutellae in Sri Lanka Physiol. Entomol. 21 93–96
H. J. Bouwmeester J. A. R. Davies H. Toxopeus (1995) ArticleTitleEnantiomeric composition of carvone, lomonene and carveols in seeds of dill and annual and biennial caraway varieties J. Agric. Food Chem. 43 3057–3064 Occurrence Handle10.1021/jf00060a013
H. J. Bouwmeester J. Gershenzon M. C. J. M. Konings R. Croteau (1998) ArticleTitleBiosynthesis of the monoterpenes limonene and carvone in the fruit of caraway Plant Physiol. 117 901–912 Occurrence Handle10.1104/pp.117.3.901 Occurrence Handle9662532
J. V. R. Chenier B. J. R. Philogene (1989) ArticleTitleField responses of certain forest coleopteran to conifer monoterpenes and ethanol J. Chem. Ecol. 15 1729–1745 Occurrence Handle10.1007/BF01012261
H. Ouden ParticleDen J. H. Visser D. P. W. Alkema J. J. Vlieger Particlede P. S. M. Derks (1993) ArticleTitleExperiments with volatile substances in slow release formulations causing repellency for oviposition of cabbage root fly, Phorbia brassicae Bché. (Dipt., Anthomyiidae) J. Appl. Entomol. 115 307–312
M. Dicke R. Gols D. Ludeking M. A. Posthumus (1999) ArticleTitleJasmonic acid and herbivory differentially induce carnivore-attracting plant volatiles in lima bean plants J. Chem. Ecol. 25 1907–1922 Occurrence Handle10.1023/A:1020942102181
M. Dicke R. M. P. Poecke Particlevan J. G. Boer Particlede (2003) ArticleTitleInducible indirect defence of plants: from mechanisms to ecological functions Basic Appl. Ecol. 4 27–42 Occurrence Handle10.1078/1439-1791-00131
J. C. Dickens (1999) ArticleTitlePredator–prey interactions: olfactory adaptations of generalist and specialist predators Agric. For. Entomol. 1 47–54 Occurrence Handle10.1046/j.1461-9563.1999.00007.x
T. Frank K. Bieri B. Speiser (2002) ArticleTitleFeeding deterrent effect of carvone, a compound from caraway seeds, on the slug Arion lusitanicus Ann. Appl. Biol. 141 93–100
Hannukkala, A., Keskitalo, M., Laamanen, J., and Rastas, M. 2002. Control of potato late blight with Caraway and Dill extracts, pp. 279–280, in H. T. A. M. Schepers and C. E. Westerdijk (eds.). Proceedings of the Sixth Workshop of a European Network for Development of an Integrated Control Strategy of Potato Late Blight, Edinburgh, Scotland, 26–30 September 2001. PPO-Special Report 8.
N. S. Iacobellis Cantore Particlelo P. Capasso F. Senatore (2005) ArticleTitleAntibacterial activity of Cuminum cyminum L. and Carum carvi L. essential oils J. Agric. Food Chem. 53 57–61 Occurrence Handle10.1021/jf0487351 Occurrence Handle15631509
Ibrahim, M. A. and Holopainen, J. K. 2002. Podisus maculiventris (Hemiptera: Pentatomidae), apotential biocontrol agent of Plutella xylostella is not repelled with limonene treatments on cabbage, pp. 200–202, in A. A. Kirk and D. Bordat (eds.). Improving Biocontrol of Plutella xylostella. Proceedings of the International Symposium, CIRAD 2004.
M. A. Ibrahim P. Kainulainen A. Aflatuni K. Tiilikkala J. K. Holopainen (2001) ArticleTitleInsecticidal, repellent, antimicrobial activity and phytotoxicity of essential oils: With special reference to limonene and its suitability for control of insect pests Agric. Food Sci. Finl. 10 243–259
M. A. Ibrahim E. J. Oksanen J. K. Holopainen (2004) ArticleTitleEffects of limonene on growth and physiology of cabbage and carrot plants J. Sci. Food Agric. 84 1319–1326 Occurrence Handle10.1002/jsfa.1819
R. Karban I. T. Baldwin (1997) Induced Responses to Herbivory University of Chicago Press Chicago, IL
E. S. Kim E. Choi Y. Kim K. W. Cho A. Lee J. Shim R. Rakwal G. K. Agrawal O. S. Han (2003) ArticleTitleDual positional specificity and expression of non-traditional lipoxygenase induced by wounding and methyl jasmonate in maize seedlings Plant Mol. Biol. 52 1203–1213 Occurrence Handle10.1023/B:PLAN.0000004331.94803.b0 Occurrence Handle14682619
K. D. Klepzig F. Schlyter (1999) ArticleTitleLaboratory evaluation of plant-derived antifeedants against the pine weevil Hylobius abietis (Coleoptera: Curculionidae) J. Econ. Entomol. 92 644–650
L. A. Lacey D. I. Shapiro-Ilan (2003) ArticleTitleThe potential role for microbial control of orchard insect pests in sustainable agriculture J. Food Agric. Environ. 1 326–331
B. H. Lee W. S. Choi S. E. Lee B. S. Park (2001) ArticleTitleFumigant toxicity of essential oils and their constituent compounds towards the rice weevil, Sitophilus oryzae (L.) Crop Prot. 20 317–320 Occurrence Handle10.1016/S0261-2194(00)00158-7
S. S. Liu L. H. Jiang (2003) ArticleTitleDifferential parasitism of Plutella xylostella (Lepidoptera: Plutellidae) larvae by the parasitoid Cotesia plutellae (Hymenoptera: Braconidae) on two host plant species Bull. Entomol. Res. 93 65–72 Occurrence Handle10.1079/BER2002208 Occurrence Handle12593684
M. Loivamäki J. K. Holopainen A.-M. Nerg (2004) ArticleTitleChemical changes induced by methyl jasmonate in oilseed rape grown in the laboratory and in the field J. Agric. Food Chem. 52 7607–7613 Occurrence Handle10.1021/jf049027i Occurrence Handle15675811
A. Mandujano-Chavez M. A. Schoenbeck L. F. Ralston E. Lozoya-Gloria J. Chappell (2000) ArticleTitleDifferential induction of sesquiterpene metabolism in tobacco cell suspension cultures by methyl jasmonate and fungal elicitor Arch. Biochem. Biophys. 381 285–294 Occurrence Handle10.1006/abbi.2000.1961 Occurrence Handle11032417
M. McConn R. A. Creelman E. Bell J. E. Mullet J. Browse (1997) ArticleTitleJasmonate is essential for insect defense Arabidopsis Proc. Natl. Acad. Sci. USA 94 5473–5477 Occurrence Handle10.1073/pnas.94.10.5473 Occurrence Handle11038546
G. Nehlin I. Valterova A. K. Borg-Karlsson (1994) ArticleTitleUse of conifer volatiles to reduce injury caused by carrot psyllid, Trioza apicalis, Förster (Homoptera, Psylloidea) J. Chem. Ecol. 20 771–783 Occurrence Handle10.1007/BF02059612
Y. A. Ntiamoah J. H. Borden (1996) ArticleTitleMonoterpene oviposition deterrents for cabbage maggots, Delia radicum (L.) (Diptera: Anthomyiidae) Can. Entomol. 128 351–352
Y. A. Ntiamoah J. H. Borden H. D. Pierce SuffixJr. (1996) ArticleTitleIdentity and bioactivity of oviposition deterrents in pine oil for the onion maggot, Delia antiqua Entomol. Exp. Appl. 79 219–226 Occurrence Handle10.1007/BF00343342
Y. Oka Y. Cohen Y. Spiegel (1999) ArticleTitleLocal and systemic induced resistance to the root-knot nematode in tomato by dl-beta-amino-n-butyric acid Phytopathology 89 1138–1143
J. K. Peterson R. J. Horvat K. D. Elsey (1994) ArticleTitleSquash leaf glandular trichome volatiles: Identification and influence on behavior of female pickleworm moth (Diaphnia nitidalis Stoll.) (Lepidoptera: Pyralidae) J. Chem. Ecol. 20 2099–2109 Occurrence Handle10.1007/BF02066246
K. A. Pivnick B. J. Jarvis G. P. Slater (1994) ArticleTitleIdentification of olfactory cues used in host-plant finding by Diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae) J. Chem. Ecol. 20 1407–1427 Occurrence Handle10.1007/BF02059870
H. Porta P. Rueda-Benitez F. Campos J. M. Colmenero-Flores M. J. Carmona A. A. Covarrubias M. Rocha-Sosa (1999) ArticleTitleAnalysis of lipoxygenase mRNA accumulation in the common bean (Phaseolus vulgaris L.) during development and under stress conditions Plant Cell Physiol. 40 850–858 Occurrence Handle10555305
R. P. J. Potting G. M. Poppy T. H. Schuler (1999) ArticleTitleThe role of volatiles from cruciferous plants and pre-flight experience in the foraging behaviour of the specialist parasitoid Cotesia plutellae Entomol. Exp. Appl. 93 87–95 Occurrence Handle10.1023/A:1003822208843
G. V. P. Reddy A. Guerrero (2004) ArticleTitleInteractions of insect pheromones and plant semiochemicals Trends Plant Sci. 9 253–261 Occurrence Handle10.1016/j.tplants.2004.03.009 Occurrence Handle15130551
G. V. P. Reddy J. K. Holopainen A. Guerrero (2002) ArticleTitleOlfactory responses of Plutella xylostella natural enemies to host pheromone, larval frass, and green leaf cabbage volatiles J. Chem. Ecol. 28 131–143 Occurrence Handle10.1023/A:1013519003944 Occurrence Handle11871395
G. V. P. Reddy E. Tabone M. T. Smith (2004) ArticleTitleMediation of host selection and oviposition behavior in the diamondback moth Plutella xylostella and its predator Chrysoperla carnea by chemical cues from cole crops Biol. Cont. 29 270–277 Occurrence Handle10.1016/S1049-9644(03)00162-2
S. C. Rodriguez B. S. J. Crafts P .W. Pare T. J. Henneberry (2001) ArticleTitleExogenous methyl jasmonate induces volatile emissions in cotton plants J. Chem. Ecol. 27 679–695 Occurrence Handle10.1023/A:1010393700918 Occurrence Handle11446293
S. M. Salom J. A. Gray A. R. Alford M. Mulesky C. J. Fettig S. A. Woods (1996) ArticleTitleEvaluation of natural products as antifeedants for the pales weevil (Coleoptera: Curculionide) and as fungitoxins for Leptographium procerum J. Entomol. Sci. 31 453–465
F. Schlyter O. Smitt K. Sjödin H.-E. Högberg J. Löfqvist (2004) ArticleTitleCarvone and less volatile analogues as repellent and deterrent antifeedants against the pine weevil, Hylobius abietis J. Appl. Entomol. 128 610–619 Occurrence Handle10.1111/j.1439-0418.2004.00889.x
K. Shiojiri J. Takabayashi (2003) ArticleTitleEffects of specialist parasitoids on oviposition preference of phytophagous insects: encounter-dilution effects in a tritrophic interaction Ecol. Entomol. 28 573–578 Occurrence Handle10.1046/j.1365-2311.2003.00539.x
K. Shiojiri J. Takabayashi S. H. Yano (2001) ArticleTitleInfochemically mediated tritrophic interaction webs on cabbage plants Popul. Ecol. 43 23–29
N. S. Talekar A. M. Shelton (1993) ArticleTitleBiology, ecology and management of the diamondback moth Annu. Rev. Entomol. 38 275–301 Occurrence Handle10.1146/annurev.en.38.010193.001423
J. S. Thaler M. J. Stout R. Karban S. S. Duffey (1996) ArticleTitleExogenous jasmonates simulate insect wounding in tomato plants (Lycopersicon esculentum) in the laboratory and field J. Chem. Ecol. 22 1767–1781
R. Tiberi A. Niccoli M. Curini F. Epifano M. C. Marcotullio O. Rosati (1999) ArticleTitleThe role of the monoterpene composition in Pinus spp. needles, in host selection by the pine processionary caterpillar, Thaumetopoea pityocampa Phytoparasitica 27 263–272
A. K. Tripathi V. Prajapati S. Kumar (2003) ArticleTitleBioactivities of l-carvone, d-carvone, and dihydrocarvone toward three stored product beetles J. Econ. Entomol. 96 1594–1601 Occurrence Handle14650536
T. Vuorinen A.-M. Nerg M. A. Ibrahim G. V. P. Reddy J. K. Holopainen (2004) ArticleTitleEmission of Plutella xylostella-induced compounds from cabbages grown at elevated CO2 and orientation behaviour of the natural enemies Plant Physiol. 135 1984–1992 Occurrence Handle10.1104/pp.104.047084 Occurrence Handle15299116
T. Vuorinen G. V. P. Reddy A.-M. Nerg J. K. Holopainen (2004) ArticleTitleMonoterpene and herbivore-induced emissions from cabbage plants grown at elevated atmospheric CO2 concentration Atmos. Environ. 38 675–682 Occurrence Handle10.1016/j.atmosenv.2003.10.029
S. H. I. Zu-Hua L. I. U. Shu-Sheng L. I. Yuan-Xi (2002) ArticleTitleCotesia plutellae parasitizing Plutella xylostella: Host-age dependent parasitism and its effect on host development and food consumption Biocontrol 47 499–511 Occurrence Handle10.1023/A:1016577406820
Acknowledgments
This study was funded by the Jenny and Antti Wihuri Foundation, the Finnish Cultural Foundation and the European Commission (contract MC-RTNCT-2003-504720 ‘ISONET’). The English text was revised by V. Michael Paganuzzi, MA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ibrahim, M.A., Nissinen, A. & Holopainen, J.K. Response of Plutella xylostella and its Parasitoid Cotesia plutellae to Volatile Compounds. J Chem Ecol 31, 1969–1984 (2005). https://doi.org/10.1007/s10886-005-6071-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-005-6071-x