Abstract
The invasive Halyomorpha halys was accidentally introduced into Switzerland around 2004 and has recently established in the neighbouring countries of France and Italy. To better understand the population dynamics of this pest in Europe, the phenology, reproductive biology and temperature requirements of Swiss H. halys populations were investigated. Overwintered adults became active in April, but peak oviposition was not observed before early July. Individual females laid on average 79 eggs (maximum of 160). The oviposition period lasted from mid-June to end of September. Eggs laid in August and September did not result in offspring due to the low temperatures in autumn. Under natural fluctuating temperatures, development from egg to adult lasted between 60 and 131 days. The first new generation of adults did not occur before mid-August when the photoperiod was already below 15 h, which likely initiated diapause and suppressed the reproductive activity of new generation adults. Under controlled conditions of 20, 25 and 30 °C, Swiss H. halys populations developed within 75.8, 42.3 and 33.2 days from egg to adult, respectively. No development was possible at or below 15 and at or above 35 °C. The number of degree days required for completion of development from egg to adult was 588.24 DD. Under semi-natural conditions, total mortality of Swiss H. halys populations was 86.7 % with a net reproductive rate of 5.69, indicating growing populations. In Switzerland, H. halys is univoltine, but if it continues to spread into the Mediterranean area two generations per year could be expected.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Increased global trade and travel over the last century have resulted in the growing spread of invasive species. Among the alien insects intercepted by European phytosanitary services, nearly 40 % were found on commodities from Asia (Kenis et al. 2007) and several have developed into significant pests, e.g. Drosophila suzukii (Diptera: Drosophilidae) and Cydalima perspectalis (Lepidoptera: Crambidae) (Calabria et al. 2012; Kenis et al. 2013). Among the most harmful invasive insect pests is the brown marmorated stink bug, Halyomorpha halys Stål (Heteroptera: Pentatomidae), which is widely distributed throughout eastern China, Japan, Korea and Taiwan (Zhu et al. 2012). Invasive populations were first detected in Pennsylvania in the US, where it was most likely introduced in the mid-1990s (Hoebeke and Carter 2003) and from where it has since spread and/or became established in 40 states (Northeastern IPM Center 2013a) and one province in Canada (Gariepy et al. 2013a). The first report of invasive H. halys populations in Europe occurred in Switzerland in 2008 based on material collected in 2007 (Wermelinger et al. 2008), although there is photographic evidence that this species was already present in 2004 (Haye, unpublished data). Since its original introduction into Zurich, this pest is now present in at least ten Cantons (Wyniger and Kment 2010; Haye and Wyniger 2013). H. halys is often unintentionally transported to other locations by humans via vehicles, machinery, personal luggage, or other goods (Hoebeke and Carter 2003) and thus, movement of Swiss populations is likely responsible for the recently discovered H. halys populations in France (Callot and Brua 2013) and Italy (EPPO 2013) and records of single individuals from Germany (Heckmann 2012) and Liechtenstein (Arnold 2009). Although H. halys has been present in Switzerland since 2004, its spread is relatively slow compared to other invasive Heteroptera in Europe, such as the Western conifer seed bug, Leptoglossus occidentalis Heidemann (Heteroptera: Coreidae) (Werner 2011).
The highly polyphagous pest is able to develop on more than 100 hosts plants in the US and China, including a wide variety of economically important agricultural crops (Leskey et al. 2012c, Lee et al. 2013, Northeastern IPM Center 2013b). Damage is caused by nymphs and adults sucking on flower buds, fruits, or stems and injecting digestive enzymes to feed on the plants fluids. In 2010, severe outbreaks in the apple growing area of the Mid-Atlantic region of the US resulted in >$37 million in losses, demonstrating the destructive potential of the pest (United States Apple Association 2010). Although established in Switzerland for at least 9 years, H. halys is primarily considered an urban and household pest, since the stink bugs tend to overwinter in human built structures (Inkley 2012). To date only a single incidence of economic damage on pepper crops has been reported in Europe from the Canton Aargau in Switzerland (Sauer 2012), but private homeowners in Zurich have reported increased damage to backyard fruit and vegetable gardens over the years (Haye, unpublished data). In the US, it took nearly 14 years before H. halys became one of the most significant pests and thus, it seems possible that Europe may face the same situation in the future.
The biology of H. halys in its native range in Asia has been summarized by Lee et al. (2013), whereas the life history in the northeastern US was characterized by Nielsen and Hamilton (2009). In contrast, only little is known on its phenology and biology in Europe (Wermelinger et al. 2008). As invasive North American and European H. halys populations are genetically variable and likely originated from the Beijing region of China and an unknown area in Asia, respectively (Gariepy et al. 2013b; Xu et al. 2013), we investigated the phenology, survival, fecundity and temperature requirements of Swiss H. halys populations to gain a better understanding of the population dynamics in Europe.
Materials and methods
Laboratory colony
Overwintered H. halys adults were collected from infested sweet cherry trees (Rosaceae: Prunus avium L.) in July 2012 in Zurich-Wollishofen, Switzerland. Colony individuals were maintained in 50 × 50 × 50 cm Bug Dorms (MegaView) on organic green beans (Phaseolus vulgaris L.) and corn (Zea mays L.) at 22 °C and a 16L: 8D photoperiod. Each cage contained no more than 50 adults. Food was replaced twice weekly. Depending on the time of the year, fresh branches of cherry trees (Prunus avium L.), tree of heaven (Ailanthus altissima (Mill.) Swingle), common ash (Sorbus aucuparia L.), or common ivy (Hedera helix L.), were offered as additional food source and oviposition substrate and changed if needed. Strips of fine mesh hanging down from the centre of the cages served as additional oviposition substrate. Eggs were collected from the adult cages every 3 days and maintained under the same conditions in separate nymphal rearing cages. Newly developed adults were regularly removed from the nymphal rearing cages and evenly distributed among the adult rearing cages.
Phenology, lifetime fecundity and mortality under natural temperatures
To study overwintering survival, adults migrating to their overwintering sites were collected in Zurich, Switzerland, in September and October 2012 (n = 87). Adults were kept in a bug dorm (30 × 30 × 30 cm) filled with 10 cm of leaf litter, two pieces of wood and five pieces of stacked bark for protection. The cage containing the adults was placed in an open wooden shelter. Overwintering mortality was measured in mid-April 2013 when the first adults became active again. Of the adults that successfully overwintered, seven mating pairs were placed in individual, ventilated 1L plastic rearing containers at constant 25 °C (16 h light) on April 23, 2013 and provided with beans to investigate how long it would take the overwintered females to start laying eggs at constant warm temperatures and long day conditions.
Additional overwintered adults (n = 59) were collected from private residences (primarily from balconies) in April 2013 in the city of Basel, Canton Basel-Stadt, Switzerland (N47°33.14917 E7°36.055). Adults were sexed and grouped in couples (n = 25), which were then placed in ventilated, sealed 1L plastic containers and provided with organic green beans and sweet corn as a food source. The rearing containers were transferred in a tightly sealed gauze cage, which was placed in an open wooden shelter, providing protection against direct sunlight and rain. From April 14th onwards, couples were monitored daily for mating, oviposition and adult mortality.
Four egg masses per week were randomly selected during the first month of the study to assess their development. After this first month, two egg masses per week were selected for the remainder of the study. Egg masses were placed individually into small sealed plastic dishes (5 cm diameter). When 1st instar nymphs had hatched and dispersed from the egg masses, ten individuals were selected per egg mass and transferred to a larger 0.5 L rearing container containing fresh beans as a food source. Development time and mortality were evaluated as described above and outdoor temperatures were recorded with HOBO® data loggers. At the final moult to adult, all individuals were sexed, and placed in pairs in rearing containers to investigate if newly emerged adults could produce a second generation under normal environmental conditions.
Parallel to the experimental phenology study, an area in Zurich-Wollishofen (N47°20.57783 E8°31.6845), which has hosted large H. halys populations for many years, was visited seven times over the summer months of 2013 to monitor the composition of nymphal stages and the presence of overwintered and newly developed adults. Nymphs and adults were collected from infested trees and shrubs in private gardens. On the first two visits (19 June, 14 July), additional overwintered adults were collected to study their lifetime fecundity as described above.
Since the first newly emerged adults at Zurich-Wollishofen were observed 8 days before the first adults developed from our experimental study, additional new generation adults were collected from our field site on August 19th, 2013, grouped in mating pairs (n = 9) and kept under outdoor conditions to investigate their oviposition behaviour.
In early September 2013, when the day length was already below 14 h light, nymphs (N3–N5) and new generation adults were collected at Zurich-Wollishofen and Basel to study the impact of day length on diapause and oviposition behaviour of H. halys. Adults and 5th instar nymphs were divided into two groups each, one kept in cages outside in an open wooden shelter, and the other in the laboratory at 22 °C and 16 h light. Since the number of the third and fourth instar nymphs found in the field was low, all nymphs were kept under laboratory conditions. When nymphs developed into adults, they were set up in couples and monitored daily for oviposition.
Construction of a life table
To estimate the generational mortality of H. halys in Switzerland, a life table was constructed using the data from the experiments described above. Since average temperatures varied throughout the oviposition and nymphal development period, eggs laid in the first or second half of each month were treated as separate cohorts. As we had selected egg masses (n = 30) on a weekly basis throughout the season to follow the development from egg to adult (see above), it was possible to estimate the mortality of eggs and nymphs for each of these cohorts. However, the selected egg masses were only representatives for each cohort, since it was not possible to follow all egg masses laid throughout the season. The observed mortalities of eggs and nymphs in the experimental study were then applied to the actual number of eggs laid during each time period (cohort). Accordingly, the life table referred to the total number of eggs (2,656) laid by 23 females throughout the summer 2013.
In the life table, mortality attributable to outdoor temperatures and background mortality related to other unknown factors (e.g. food quality) was expressed as apparent mortality, real mortality, intensity of mortality (k-values) and generational mortality, following the review by Bellows et al. (1992). As the study was conducted in containment under semi-natural conditions, the impact of natural enemies was not considered. The apparent mortality (q x ) is the fraction dying (mainly due to temperature) in a specific stage related to the number entering the same stage (l x ). Apparent mortality was calculated from the number of dead individuals (d x ) recorded from each life stage (x) of H. halys, i.e. q x = d x /l x . The real mortality (r x ) is the fraction of hosts dying in each stage (d x ) related to the number of H. halys eggs (l 0 = 2,656) at the beginning of the life table (r x = d x /l 0). The k x -value is the intensity of mortality and is a measure of mortality that is independent from individual numbers, i.e. k x = −log (1−m x ). The k x values are expressed as proportions of the generational mortality K G, which is the sum of all k x -values. The proportion of the total generational mortality by the mortality in a particular stage is further expressed as 100k x /K G. This value shows the impact of mortality in each stage on the generational mortality of H. halys. The population dynamics of H. halys is described by the net reproductive rate of increase (R 0), which shows the number of times the population increases or decreases from one generation to the next (Van Driesche et al. 2008). Growing populations have R 0 values greater than one, whereas R 0 values less than 1 show that the population is declining. R 0 was calculated from the realized progeny divided by the number of eggs in the first generation (l 0 = 2,656).
Temperature dependent development
The effect of temperature on developmental time and mortality of eggs and nymphs was examined at seven temperatures: 12.5, 15, 20, 25, 30 and 35 °C and at photoperiod 16:8 (L:D), following the methods used by Nielsen et al. (2008). For each temperature replicate, egg masses laid within 24 h were randomly chosen from the laboratory colony and placed individually into small Petri-dishes (5 cm diameter). Upon hatching, the number of emerged first instars was counted, and subsequently verified by counting the number of hatched eggs present on the egg mass. When the first instars began moving away from the egg mass, ten 1st instar nymphs were randomly selected and moved to larger 0.5 L plastic rearing containers, containing organic beans as a food source. Remaining nymphs were used to supplement the laboratory colony. The rearing containers were then placed inside environmental chambers at ±0.5 °C of the set temperature. Individuals were checked daily for development or mortality. Wherever possible, newly moulted adults within each temperature group were sexed, and couples were maintained at their respective developmental temperature in order to assess the preoviposition period of the new generation in their respective developmental temperature.
The development data from each set of laboratory temperatures were used to construct a degree-day model, which assumes that there is a linear relationship between development rate (1/days) and temperature above a minimum development threshold: 1/d = a + (bT), where 1/d is the rate of development and d the duration of development (days), a is the intercept and b is the slope of the linear function (Campbell et al. 1974). Although Nielsen et al. (2008) had pointed out that a linear regression model is not ideal to define the lower critical temperature for H. halys, it was still used to make the results comparable to previously published studies from Asia (Watanabe 1980; Fujiie 1985, Kiritani 1997). As suggested by Nielsen et al. (2008), we also applied the more suitable model by Briere et al. (1999) to investigate if thermal thresholds Swiss and US populations would differ: 1/d = aT (T−T 0) /T m−T)1/2, where T 0 is the lower threshold, T m is the upper threshold (=lethal temperature) and a is an empirical constant. Both models were developed using SYSTAT 13 (Systat Software, San Jose, CA, USA).
Results
Phenology, lifetime fecundity and mortality under natural temperatures
The first overwintered adults became active in early April 2013, when daily maximum temperatures occasionally exceeded 25 °C. 61 % of the overwintered adults (n = 87) were found alive. When moved to an incubator set at 25 °C and 16 light, it took on average 20.14 ± 1.47 days until females started laying eggs (n = 7 couples).
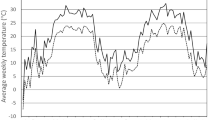
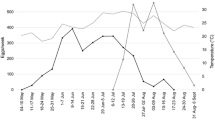
In the colonies kept outside (which consisted of H. halys mating pairs that were collected in April in Basel), mating was first observed on May 13, but the first egg mass was not laid until June 19. The peak oviposition period was observed in the 26th calendar week (June 24–30) (Fig. 1). In the following weeks, oviposition declined but smaller peaks were observed in early August, early September and end of September, when the average daily temperature exceeded 15 °C. The last egg mass was found on September 30. Mortality of overwintered adults in early spring was fairly low, but increased steadily from the second week of July onwards (Fig. 2). The last adults died in mid-November. In total, 88 % of the overwintered females laid eggs (n = 25). The number of egg masses laid by single fertile females varied from 1 to a maximum 6 egg masses. The average realized lifetime fecundity was 79.18 ± 8.77 (SE) eggs and the maximum number of eggs per female was 160 eggs (Table 1). Females collected at Zurich-Wollishofen on June 19 (n = 7) and July 14 (n = 6) had a realized lifetime fecundity of 84.00 ± 11.51(SE) eggs and 45.50 ± 8.20 (SE) eggs, respectively (Table 1). The time between single oviposition events were on average 17 ± 1 (SE) days, but ranged from 6 to 54 days (n = 49). Egg mortality from mid-June through September varied from 6.6 % (15–31 Aug.) to maximum 100 % in the second half of September, when average temperatures dropped below 15 °C (Table 2). Nymphs hatching from the very first eggs laid in mid-June developed into adults no earlier than August 27. Under natural fluctuating temperatures development from egg to adult lasted between 60 and 131 days (n = 64) at average temperatures of 15.4–19.9 °C (Fig. 3). In total, 9.4 % of the individuals (n = 64) moulted to the adult stage in the last week of August, but the majority became adults in September (70.3 %). Development of nymphs to the adult stage continued throughout October (18.7 %) until early November (1.6 %). Among eggs that were laid in the first 6 weeks of the oviposition period, the proportion of eggs developing successfully from egg to adult varied between 12.4 and 24.6 % (Table 2). However, none of the nymphs developing from eggs laid in August and September were able to complete their development (Table 2).
Length of development (days) of H. halys from egg to adult as a function of average outdoors temperatures at Delémont, Switzerland, in 2013 (n = 65). White markers indicate individuals that developed from eggs laid from July 17 to 29 and developed to adults in the second half of October or early November. Black markers represent the eggs laid from 19 June to 15 July and developing to adults from late August to early October
The phenology of H. halys in Zurich-Wollishofen (Fig. 4) followed a similar pattern as observed in our experimental study. In mid-June only overwintered adults were found, whereas in mid-July the first 2nd instar nymphs were observed, suggesting that oviposition had occurred at the end of June. The first new generation adults were found in mid-August, which was slightly earlier than in our experimental trial. In September, mainly new generation adults and late instar nymphs were present, but a small number of 2nd instar nymphs in late September suggested that oviposition had occurred at least throughout August.
None of the newly developed adults showed any mating or oviposition behaviour under outdoor conditions (Table 1). The same was observed for all additional new generation adults which were collected at the field sites in Wollishofen and Basel. Field-collected new generation females that were subsequently maintained under controlled laboratory conditions at 22 °C and long day conditions (16 h light) showed no oviposition either. However, nearly all nymphs (N3–N5) that were collected in early September and kept under the same laboratory conditions developed into adults that started laying eggs (Table 1).
Life table analysis
Overall egg mortality (June–September) was 22.8 %, contributing 12.76 % to the generational mortality (Table 3). Among the nymphal instars, the highest mortality was observed for the 1st (25.8 %) and 5th instar (41.2 %), whereas mortality of the 3rd instar was lowest (7.3 %). The high mortality of 5th instar nymphs is explained by the large number of nymphs that developed from eggs laid in August, which were then only able to develop to the 5th instar due to colder temperatures in the following month. The second most important factor apart from 5th instar mortality was the overwintering mortality of adults (39 %), which contributed 24.49 % to the generational mortality. The total generational mortality was 86.7 %. Similarly, the realized net reproductive rates (R 0) of 5.69 indicated growing populations. However, the life table presented here only considers temperature as a mortality factor and excludes any potential mortality by natural enemies.
Temperature dependent development
At constant temperatures of 20, 25 and 30 °C, H. halys females completed development within 75.68 ± 0.94 (SE), 41.94 ± 0.77 (SE) and 34.57 ± 0.60 (SE) days, respectively (Table 4). At 15 and 35 °C, the development to the adult stage was not possible. The linear regression model resulted in the lower threshold temperature T 0 = 12.24 °C and the thermal constant K = 588.24. (y = 0.0017x−0.0208; R 2 = 0.9189) (Fig. 5). The Briere-1 model estimated T 0 = 12.97 °C and T m = 36.5 °C (R 2 = 0.9235) for the total development.
Average egg mortality was 100 and 82.7 % at 12.5 and 15 °C, respectively (Table 5). The lowest egg mortality was observed at 30 °C (1.3 %). Although a small proportion of eggs hatched at 15 °C (17.3 %), all 1st instar nymphs died shortly thereafter. Mortality among 1st instar nymphs was generally higher than among the other instars, but decreased with increasing temperatures. Mortality of 2nd and 3rd instars was always below 10 %. In the 4th instar, unusual high mortality (19.1 %) was observed at 25 °C. In the 5th instar, the opposite trend was observed as in the 1st instar. Mortality increased with increasing temperatures from 7.1 % at 20 °C to 14.4 % at 30 °C.
At constant 20 and 25 °C, (16 h light) newly emerged and mated females started oviposition on average after 25.17 ± 1.17 days (n = 6) and 12.17 ± 1.078 days (n = 6), respectively. At constant 30 °C, nearly all adults died before reaching the reproductive stage, and only a single was observed laying eggs after 13 days. When only maturing females at 20 and 25 °C were considered, degree day accumulation from emergence to first oviposition was calculated to be 117.65 DD.
Discussion
To successfully control an invasive insect pest such as H. halys, detailed knowledge on its phenology, the direct relationship of insect development to weather, is essential. If chemical or biological control is considered, applications of insecticides or releases of biological control agents must be timed to coincide with specific life stages of the pest, particularly if not all life stages are equally vulnerable to the control measures (Ascerno 1991). To date, knowledge on the phenology and population dynamics of invasive H. halys in Europe is mainly based on descriptive field observations (Wermelinger et al. 2008). Thus, the aim of the present study was to provide detailed baseline data on the phenology and reproductive biology of H. halys in Switzerland and contribute to a better understanding of the biology of this invasive pest in Europe.
In most parts of China, Japan and Korea, H. halys has been reported to have one or two generations (reviewed by Lee et al. 2013). However, Hoffmann (1931) suggested that H. halys can have as many as 4–6 generations per year in southern China. In the invaded region of the north eastern USA, H. halys has 1–2 generations (Nielsen and Hamilton 2009; Leskey et al. 2012d). The present study demonstrated that in Switzerland H. halys is strictly univoltine, with temperature and day length restricting populations to a single generation. Since overwintered females need 148 degree days prior to first oviposition (Nielsen et al. 2008), low average temperatures (<15 °C; Fig. 1) in spring resulted in a delay of the oviposition period until mid-June. Average monthly temperatures in April and May in the cities of Zurich and Basel, which currently host the largest H. halys populations in Switzerland, are 8 and 9 °C and 12 and 14 °C, respectively (http://www.climatemps.com). In comparison, the oviposition period in the Beijing province of China, where H. halys is bivoltine, begins a month earlier (i.e. mid-May) due to much higher average temperatures in April (14 °C) and May (20 °C) (Zhang et al. 1993). In Switzerland, average temperatures between 15 and 20 °C are only observed during 3 months (June–August), whereas in Beijing average temperatures are above 20 °C from May to September (http://www.climatemps.com). These warmer summer temperatures accelerate the nymphal development in China, such that new generation adults occur in mid-July and then produce a second generation. In Switzerland, the first new generation adults did not occur before mid-August, when the photoperiod was already decreasing (15 August: 14 h 13 min; www.timeanddate.com). As Yanagi and Hagihara (1980) estimated the critical day length for stimulating diapause in adults is about 15 h, the reproductive activity of new generation adults was consequently suppressed. To be able to produce a second generation, first generation adults would need to develop latest in the second or third week of July, when day length is still above 15 h. Since nymphs collected in September (13 h light) developed into reproductive adults when moved to long-day conditions, only the adult stage seems to be responsive to diapause cues.
In Switzerland, overwintered adults were very long-lived and followed a type I survivorship curve (Slobodkin 1962; Southwood and Henderson 2000) which indicates that risk of mortality is fairly low during the early adult stage. The oviposition period lasted three and a half months, from mid-June to the end of September. Accordingly, egg masses found in late season are not necessarily an indication for a second generation. A similarly long oviposition period of 12 weeks was observed for laboratory colonies in Japan, and it was speculated that under natural conditions it could have been even longer (Yanagi and Hagihara 1980). Oviposition of H. halys females was clearly stimulated by temperatures. All four oviposition peaks in 2013 were observed during periods when temperatures had continuously increased and exceeded 15 °C. This oviposition behaviour is well adapted to the temperature requirements of eggs and first instar nymphs, which develop poorly at 15 °C. The average realized fecundity of adults collected in Zurich in mid-June (84 eggs) was similar to those collected in April in Basel (79 eggs), suggesting that the females collected in Zurich had not started laying eggs prior to collection. Females collected in mid-July had a much lower fecundity (45 eggs), indicating that at this time females had already laid approximately half of their eggs. Under outdoor conditions, the majority of eggs was laid within the first 3 weeks post-ecdysis in July, which agrees with laboratory observations by Nielsen et al. (2008). Compared to the realized fecundity of Swiss populations (averaging approximately 80 eggs per female), the potential lifetime fecundity of US and Japanese H. halys populations under optimal laboratory conditions was much higher, averaging 212 eggs and 486 eggs, respectively (Nielsen et al. 2008; Kawada and Kitamura 1983). Although we did not measure the potential fecundity of Swiss populations, it seems likely that under outdoor conditions H. halys females were not able to reach their full reproductive potential. Whether the diet used in the experiments (beans) had an influence on the fecundity remains unknown.
Development time defines how many generations of a pest insect fit in the available growing season and is accordingly an important contributor to fitness of an insect pest in that seasonality can also affect the number of offspring (Bradshaw et al. 2004). Although invasive H. halys populations in North America and Switzerland are genetically distinct and originate from different areas in Asia (Gariepy et al. 2013b), their temperature requirements differed only slightly. At 20, 25 and 30 °C, Swiss H. halys populations developed within 75.8, 42.3 and 33.2 days from egg to adult, whereas US populations needed 81.2, 44.9 and 33.4 days, respectively (Nielsen et al. 2008). Both the invasive populations further showed that complete development would not be possible at the temperature extremes of 15 and 35 °C. The critical temperature threshold (12.2 °C) and the number of degree days (DD) required for completion of development of Swiss H. halys populations (588.24 DD) fall in the range that has been reported for Asian (T 0: 11–12.9 °C; DD: 580–649; Watanabe 1980; Fujiie 1985; Kiritani 1997) and US populations (T 0: 12.0 °C, DD: 537.63; Nielsen et al. 2008). Only the values of a Japanese population (T 0: 13.9 °C, DD: 471) reported by Yanagi and Hagihara (1980) were noticeably different. The similarity of the temperature requirements of Swiss and US populations was further demonstrated, when the more suitable Briere-1 model was applied. The estimated thresholds for the complete development of Swiss populations were T 0 = 12.97 °C and T m = 36.5 °C, whereas the values estimated for US populations were T 0 = 14.17 °C and T m = 35.76 °C (Nielsen et al. 2008). Although the Briere-1 model provided a better estimate of the thresholds, laboratory development studies by Nielsen et al. (2008) as well as the present study suggest that the “real” thresholds are more likely in the range of 17–33 °C. The previous studies on H. halys development were mostly conducted at constant laboratory temperatures, which do not take fluctuating temperatures under field conditions into consideration. In the present study, development time and average temperatures were calculated for 65 individuals that successfully completed development under outdoor conditions. Average temperatures fell in a very narrow range of 15.3–19.9 °C due to the fact that most adults completed development within the same time period (early July–early September). The last individuals that were able to complete development came from eggs that were laid from July 17 to 29, and moulted into adults in the second half of October to early November. The average temperatures measured during this period varied from 15.3 to 16.6 °C, which are noticeably near the assumed lower threshold.
Although empirical field data on population dynamics of H. halys in Switzerland are lacking, the recent spread into other European countries and the increased number of reports of H. halys in private residences in Zurich (Mueller et al. 2011) indicate growing populations, which also was predicted by the present life table. However, the life table only represents the year 2013 and mortality may vary between years. In nature, H. halys adults often invade houses and other human-made structures to overwinter in protected environments (Inkley 2012), a behaviour which may result in reduced overwintering mortality. In the present study, mortality of H. halys adults overwintered in a wooden shelter was indeed relatively low (39 %) compared to other insects overwintering in the adult stage in Europe, such as Meligethes aeneus (Coleoptera: Nitidulidae) (85–92 % mortality, Hokkanen 1993) and Ceutorhynchus obstrictus (Coleoptera: Curculionidae) (95–98 %, Haye et al. 2010). Since the number of overwintered adults followed in this study was low and only little is known about the actual conditions at natural overwintering sites of H. halys, the calculated overwintering mortality may not be a true representation of the environmental overwintering mortality, as such, further investigations will be needed. The impact of natural enemies was not included due to lack of information, yet it is likely that this factor would decrease the net reproductive rate. Particularly toward the end of the season, mortality of eggs and nymphs was primarily related to temperature (Table 2), but probably not exclusively, since background mortality related to unknown factors (e.g. food quality, cage size) remained unknown. Remarkably, eggs laid in August and September (32.1 % of all eggs) did not develop into offspring due to decreasing temperatures in early autumn. Years with unusual warm temperatures late in the season would allow more nymphs to complete development, which could then further facilitate population growth. Zhu et al. (2012) predicted that H. halys could potentially establish in nearly all parts of Europe, but southern Europe would be the most favourable. Recently, H. halys populations were found in Northern Italy, and it is likely that they will continue to spread throughout the Mediterranean area. Temperature conditions in southern Europe would clearly be more favourable for development, which could result in two generations per year in some regions.
Integrated pest management strategies for H. halys that combines various control measures such as chemical and biological control are currently being investigated in the US (Leskey et al. 2012a). In Switzerland, nearly 70 % of all eggs were laid in June and July, consequently, control measures against eggs and adults would be most effective during this time period. A wide variety of insecticides have been tested against H. halys, with some broad spectrum insecticides showing a high level of efficacy, e.g. dimethoate, malathion and bifenthrin (Leskey et al. 2012b). The use of these chemicals, however, has resulted in the disruption of ongoing IPM programs, causing outbreaks of secondary pests which were previously well controlled by natural enemies (Leskey et al. 2012c). As an alternative option to chemical control, a classical biological control strategy involving the introduction of natural enemies from the pest’s native range should be considered. In China and Japan, egg parasitoids in the genus Trissolcus (Hymenoptera: Scelionidae) are considered the most effective natural enemies of H. halys (Arakawa and Namura 2002; Yang et al. 2009; Lee et al. 2013). Among these egg parasitoids, Trissolcus japonicus (Ashmead) (syn. Trissolcus halyomorphae Yang; Talamas et al. 2013) is one of the most promising biological control agents, causing an average annual parasitism of 50 % in Chinese fruit orchards (Yang et al. 2009). If an additional 50 % egg mortality is considered in the present life table analysis, the net reproductive rate would decrease from 5.69 to 1.85, thereby reducing the population growth by a factor of 3. As mentioned above, the impact of natural enemies on H. halys populations in Europe is unknown, but laboratory tests with common European pentatomid egg parasitoids, e.g. Trissolcus semistriatus Nees, Trissolcus flavipes (Thomson) and Telenomus chloropus Thomson, suggest that H. halys is not a suitable host (Haye and Gariepy, unpublished data). However, given the diversity of parasitoids associated with the Pentatomidae, this does not exclude the possibility that other native parasitoid species could be used for augmentative biological control. To date, H. halys is not considered an economically important pest in Europe; nonetheless, its spread and the development of existing populations should be carefully monitored so that European countries are prepared in case H. halys populations reach damaging levels as already observed in the northeastern United States.
References
Arakawa R, Namura Y (2002) Effects of temperature on development of three Trissolcus spp. (Hymenoptera: Scelionidae), egg parasitoids of the brown marmorated stink bug, Halyomorpha halys (Hemiptera: Pentatomidae). Entomol Sci 5:215–218
Arnold K (2009) Halyomorpha halys (Stål, 1855), eine für die europäische Fauna neu nachgewiesene Wanzenart (Insecta: Heteroptera: Pentatomidae: Cappaeini). Mitt Thüringer Entomol 16:19
Ascerno ME (1991) Insect phenology and integrated pest management. J Arboric 17:13–15
Bellows TS Jr, Van Driesche RG, Elkinton JS (1992) Life-table construction and analysis in the evaluation of natural enemies. Annu Rev Entomol 37:587–612
Bradshaw WE, Zani PA, Holzapfel CM (2004) Adaptation to temperate climates. Evolution 58:1748–1762
Briere JF, Pracros P, Le Roux AY, Pierre JS (1999) A novel rate model of temperature-dependent development for arthropods. Environ Entomol 28:22–29
Calabria G, Máca J, Bächli G, Serra L, Pascual M (2012) First records of the potential pest species Drosophila suzukii (Diptera: Drosophilidae) in Europe. J Appl Entomol 136:139–147
Callot H, Brua C (2013) Halyomorpha halys (Stål, 1855), la Punaise diabolique, nouvelle espèce pour la faune de France (Heteroptera Pentatomidae). L’Entomologiste 69:69–71
Campbell A, Frazer BD, Gilbert N, Gutierrez AP, Mackauer M (1974) Temperature requirements of some aphids and their parasites. J Appl Ecol 11:431–438
EPPO (2013) EPPO Reporting Service No 5—Pest & Diseases, pp 10–11
Fujiie A (1985) Seasonal life cycle of Halyomorpha mista. Bull Chiba Agric Exp Stn 26:87–93
Gariepy TD, Fraser H, Scott-Dupree CD (2013a) Brown marmorated stink bug (Hemiptera: Pentatomidae) in Canada: recent establishment, occurrence, and pest status in Southern Ontario. Can Entomol. doi:10.3752/cjai.2013.24
Gariepy TD, Haye T, Zhang J (2013b) Occurrence, genetic diversity, and potential pathways of entry of Halyomorpha halys in newly-invaded areas of Canada and Switzerland. J Pest Sci. doi:10.1007/s10340-013-0529-3
Haye T, Wyniger D (2013) Current distribution of Halyomorpha halys in Europe. http://www.halyomorphahalys.com. Accessed Nov 2013
Haye T, Mason PG, Dosdall L, Kuhlmann U (2010) Mortality factors affecting the cabbage seedpod weevil, Ceutorhynchus obstrictus (Marsham), in its area of origin: a life table analysis. Biol Control 54:331–341
Heckmann R (2012) Erster Nachweis von Halyomorpha halys (STÅL, 1855) (Heteroptera: Pentatomidae) für Deutschland. Heteropteron 36:17–18
Hoebeke ER, Carter ME (2003) Halyomorpha halys (Stål) (Heteroptera: Pentatomidae): a polyphagous plant pest from Asia newly detected in North America. P Entomol Soc Wash 105:225–237
Hoffmann WE (1931) A pentatomid pest of growing bean in south China. Peking Nat Hist Bull 5:25–26
Hokkanen HMT (1993) Overwintering survival and spring emergence in Meligethes aeneus: effects of body weight, crowding, and soil treatment with Beauveria bassiana. Entomol Exp Appl 67:241–246
Inkley DB (2012) Characteristics of home invasion by the brown marmorated stink bug (Hemiptera: Pentatomidae). J Entomol Sci 47:125–130
Kawada H, Kitamura C (1983) The reproductive behaviour of the brown marmorated stink bug, Halyomorpha mista Uhler (Heteroptera: Pentatomidae). I. Observation of mating behavior and multiple copulation. Appl Entomol Zool 18:234–242
Kenis M, Rabitsch W, Auger-Rozenberg MA, Roques A (2007) How can alien species inventories and interception data help us prevent insect invasions? Bull Entomol Res 97:489–502
Kenis M, Nacambo S, Leuthardt FLG, di Domenico F, Haye T (2013) The box tree moth, Cydalima perspectalis, in Europe: Horticultural pest or environmental disaster? Alien 33:38–41
Kiritani K (1997) The low development threshold temperature and the thermal constant in insects, mites, and nematodes in Japan. Misc Publ Nat Inst Agro-Environ Sci 21:1–72
Lee DH, Short BD, Joseph SV, Bergh JC, Leskey TC (2013) Review of the biology, ecology, and management of Halyomorpha halys (Hemiptera: Pentatomidae) in China, Japan, and the Republic of Korea. Environ Entomol 42:627–641
Leskey TC, Hamilton GC, Nielsen AL et al (2012a) Pest status of the brown marmorated stink bug, Halyomorpha halys in the USA. Outlooks Pest Manag 23:218–226
Leskey TC, Lee DH, Short BD, Wright SE (2012b) Impact of insecticides on the invasive Halyomorpha halys (Hemiptera: Pentatomidae): analysis of insecticide lethality. J Econ Entomol 105:1726–1735
Leskey TC, Short BD, Butler BR, Wright SE (2012c) Impact of the invasive brown marmorated stink bug, Halyomorpha halys (Stål), in mid-Atlantic tree fruit orchards in the United States: case studies of commercial management. Psyche 2012:1–14
Leskey TC, Wright SE, Short BD, Khrimian A (2012d) Development of behaviorally based monitoring tools for the brown marmorated stink bug, Halyomorpha halys (Stål) (Heteroptera: Pentatomidae) in commercial tree fruit orchards. J Entomol Sci 47:76–85
Mueller G, Luescher IL, Schmidt M (2011) New data on the incidence of household arthropod pests and new invasive pests in Zurich (Switzerland). Proceedings of the Seventh International Conference on Urban Pests, pp.99–104
Nielsen AL, Hamilton GC (2009) Life history of the invasive species Halyomorpha halys (Hemiptera: Pentatomidae) in Northeastern United States. Ann Entomol Soc Am 102:608–616
Nielsen AL, Hamilton GC, Matadha D (2008) Developmental rate estimation and life table analysis for Halyomorpha halys (Hemiptera: Pentatomidae). Environ Entomol 37:348–355
Northeastern IPM Center (2013a) Where is BMSB? State by state. http://www.stopbmsb.org/where-is-bmsb/state-by-state/. Accessed 21 Nov 2013
Northeastern IPM (2013b) Host plants of the brown marmorated stink bug in the U.S. http://www.stopbmsb.org/where-is-bmsb/host-plants/. Accessed Jan 2014
Sauer C (2012) Die Marmorierte Baumwanze tritt neu im Deutschschweizer Gemüsebau auf. Extension Gemüsebau, Forschungsanstalt Agroscope Changins-Wädenswil, Gemüsebau Info 28(12):4–5
Slobodkin LB (1962) Growth and regulation of population animal populations. Holt, Rinehart and Winston, New York
Southwood TRE, Henderson PA (2000) Ecological methods. Blackwell, Oxford
Talamas EJ, Buffington M, Hoelmer K (2013) New synonymy of Trissolcus halyomorphae Yang. J Hymenopt Res 33:113–117
United States Apple Association (2010) Asian pest inflicting substantial losses, raising alarm in eastern apple orchards. Apple News 41:488
Van Driesche R, Hoddle M, Center T (2008) Control of pests and weeds by natural enemies: an introduction to biological control. Blackwell Publishing, Malden
Watanabe M (1980) Study of the life cycle of the brown marmorated stink bug, Halyomorpha mista, by observing ovary development. Insectarium 17:168–173
Wermelinger B, Wyniger D, Forster B (2008) First records of an invasive bug in Europe: Halyomorpha halys Stal (Heteroptera: Pentatomidae), a new pest on woody ornamentals and fruit trees? Mitt Schweiz Entomol Ges 81:1–8
Werner DJ (2011) Die Amerikanische Koniferen-Samen- Wanze Leptoglossus occidentalis (Heteroptera: Coreidae) als Neozoon in Europa und in Deutschland: Ausbreitung und Biologie. Entomol heute 23:31–68
Wyniger D, Kment P (2010) Key for the separation of Halyomorpha halys (Stål) from similar-appearing pentatomids (Insecta: Heteroptera: Pentatomidae) occurring in Central Europe, with new Swiss records. Mitt Schweiz Entomol Ges 83:261–270
Xu J, Fonseca DM, Hamilton GC, Hoelmer KA, Nielsen AL (2013) Tracing the origin of US brown marmorated stink bugs, Halyomorpha halys. Biol Invasions. doi:10.1007/s10530-013-0510-3
Yanagi T, Hagihara Y (1980) Ecology of the brown marmorated stink bug. Plant Prot 34:315–321
Yang ZQ, Yao YX, Qiu LF, Li ZF (2009) A new species of Trissolcus (Hymenoptera: Scelionidae) parasitizing eggs of Halyomorpha halys (Heteroptera: Pentatomidae) in China with comments on its biology. Ann Entomol Soc Am 102:39–47
Zhang CT, Li DL, Su HF, Xu GL (1993) A study on the biological characteristics of Halyomorpha picus and Erthesina fullo. For Res 6:271–275
Zhu G, Bu W, Gao Y, Liu G (2012) Potential geographic distribution of brown marmorated stink Bug invasion (Halyomorpha halys). PLoS ONE 7:e31246
Acknowledgments
The authors are grateful to all Swiss homeowners who kindly allowed us to collect specimens on their property. We would like to thank Alyson Carter and Léna Durocher-Granger for technical assistance in the field and in the laboratory.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Traugott.
Rights and permissions
About this article
Cite this article
Haye, T., Abdallah, S., Gariepy, T. et al. Phenology, life table analysis and temperature requirements of the invasive brown marmorated stink bug, Halyomorpha halys, in Europe. J Pest Sci 87, 407–418 (2014). https://doi.org/10.1007/s10340-014-0560-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-014-0560-z