Abstract
An analytical method based on sugaring-out assisted liquid-liquid extraction (SULLE) and HPLC-fluorescence detection has been developed for the determination of bisphenol A (BPA) and bisphenol B (BPB) in royal jelly. Experimental parameters of SULLE including the extraction solvent, amount of phase separation agent, initial concentration of acetonitrile (ACN), and sample size were investigated. The optimal SULLE procedure involves 0.3 g sample extracted by 2 mL ACN-water mixture (50%, v/v), followed by phase separation with the addition of 0.4 g glucose. The obtained extract was injected into the reversed-phase HPLC system without clean-up step. The chromatography separation of BPA and BPB from royal jelly matrix was achieved in the mobile phase composed of 40% ACN and 60% water with isocratic elution at 1.0 mL/min. Limits of quantification for BPA and BPB were 40 μg/kg and 45 μg/kg, respectively, and limits of detection for BPA and BPB were 16 μg/kg and 18 μg/kg, respectively. Average recoveries of BPA and BPB at three spiked levels were in the range of 88.32~93.59% and 95.14~97.48%, respectively. Precision expressed as relative standard deviations (RSDs) in the interday and intraday analysis were all lower than 5%. The developed method was applied to the analysis of ten commercial and eight raw royal jelly samples. Results indicated that none of BPA and BPB were detected in these 18 samples. The proposed method is simple, fast, and sensitive, and could be used for the monitoring of BPA and BPB contamination in royal jelly.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Royal jelly (RJ), a secretion of young worker bees to feed larvae and queen honeybee, has been widely used in functional foods resulted from its potential therapeutic properties (Cornara et al. 2017). RJ is regarded as the dietary induction of honeybee larvae development into queens, although the detailed mechanism remains controversial (Kamakura 2011; Buttstedt et al. 2016). Queens are fed with RJ through their larval development, whereas worker honeybees are fed with worker jelly only for the first 3 days. This diet difference causes queens develop into larger body size, shorter developmental time, and longer lifespan than worker honeybees (Winston 1991). The nutrition components in RJ include proteins, lipids, carbohydrates, minerals, and vitamins (Ramadan and Al-Ghamdi 2012). In addition to the nutrition value, health benefits of RJ have also been intensely studied (Cornara et al. 2017). For example, major royal jelly proteins, the most abundant dry matter protein in RJ, have shown several health promotion activities such as improvement of cognitive performance (Chen et al. 2017), immunomodulatory activities (Okamoto et al. 2003), and potential cytoprotective action on hepatocytes (Kamakura et al. 2001). The major fatty acid component in RJ, 10-hydroxy-2-decenoic acid, has been reported to possess neuromodulatory properties (Terada et al. 2011), metabolic syndrome preventing (Takikawa et al. 2013), and antidepressant effects (Ito et al. 2012).
China produces over 95% of the worldwide RJ production (Zheng et al. 2011a). Commercial RJ is produced by placing 1-day-old larvae in plastic artificial queen cups. After 3 days, raised by worker bees, production of RJ reaches the maximum amount and the optimum quality (Chen et al. 2002; Zheng et al. 2011b). Then RJ is scooped out of the cups and stored in dark and cold vessel to prevent deterioration (Chen and Chen 1995; Li et al. 2008).
Since RJ is produced in an open environment, the potential contaminants have been intensively studied to ensure its quality and safety. According to the original sources, contaminants can be divided into apicultural and environmental ones, which arised from beekeeping practices and environment, respectively (Bogdanov 2006). Determination methods of some apicultural contaminants in royal jelly have been reported. For example, analysis of tetracycline (Giannetti et al. 2010), chloramphenicol (Kawano et al. 2015), and quinolone (Lombardo-Agui et al. 2012) residues have been developed. In addition, multi-class pesticides and veterinary drug determination in RJ have been reported by using chromatography-coupled tandem mass spectrometry methods (Martinez-Dominguez et al. 2014; Zheng et al. 2018; Jin et al. 2017; Zhang et al. 2016). Though the determination methods of some antibiotics and pesticide residues in royal jelly have been developed, research on potential contaminants is still the key issue to ensure the food safety of RJ.
Bisphenol A (BPA) is widely used as raw material for the production of polycarbonate and epoxy resins. Because of the estrogenic properties and the ubiquity presence in environment, potential effects of BPA on human health have attracted special attentions (Vandenberg et al. 2007). The major human exposure route of BPA is the dietary pathway, which resulted from the contact of foodstuff with food packing materials containing polycarbonate and epoxy resin materials (Geens et al. 2012). The accurate quantification method of BPA in food matrices is recommended for the reliable health risk assessment of BPA (Ballesteros-Gomez et al. 2009). A number of analytical methods including chromatography-based methods (Ballesteros-Gomez et al. 2009; Capriotti et al. 2013) and novel sensing methods (Ragavan et al. 2013) have been developed for the determination of BPA. To the best of our knowledge, there is no report on the analysis of BPA in RJ. The aim of this study was to develop a simple, rapid, and sensitive analytical method for the determination of BPA and its analogue bisphenol B (BPB) in RJ based on sugaring-out assisted liquid-liquid extraction (SULLE) combined with HPLC-fluorescence detection.
Sugaring-out is a novel phase transition phenomenon first observed in ACN-water mixture, that ACN can be separated from ACN-water mixture with the addition of sugars (Wang et al. 2008a). This phase separation can be attributed to the replacing of the hydrogen bonding between ACN and water molecules by sugar molecules (Wang et al. 2008b). Comparing with the traditional phase transition methods, sugaring-out shows advantages of rapid phase separation with more environmentally friendly (Dhamole et al. 2010). Recently, SULLE has been applied for the extraction of protein (Dhamole et al. 2010), lipid acid (Tu et al. 2018), and metal ions (Zhang et al. 2012) and for the analysis of drugs (Tsai et al. 2010; Zhang et al. 2013) and natural products (Cardoso et al. 2013). In the present work, SULLE was employed for the sample preparation of BPA and BPB from RJ sample. The crucial parameters of the SULLE procedure were investigated and optimized. The clean-up effect of dispersive solid-phase extraction (d-SPE) which is a flexible and efficient clean-up procedure (Anastassiades et al. 2003; Cruz-Vera et al. 2011) was also discussed. The proposed method was validated and applied for the analysis of BPA and BPB residues in 18 collected RJ samples.
Materials and Methods
Materials
ACN (HPLC grade) was obtained from Merck (Darmstadt, Germany); standards of BPA, BPB, and 4, 4′-cyclohexylidenebisphenol (> 99.0%) were purchased from Aladdin (Shanghai, China); other reagents including glucose, ethanol, methanol, and acetone were all of analytical grade and obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). C18 and primary secondary amine (PSA) were obtained from Sepax (Suzhou, China), Florisil from Sigma (Shanghai, China), and silica from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Ultrapure water (18.2 MΩ) was used through this article. Raw royal jelly samples were collected from apiaries in Hubei, China. Commercial royal jelly samples were purchased from local markets. The stock solutions of standards were prepared by dissolving 10 mg in 100 mL ACN at the concentration of 100 μg/mL. Working standard solutions were prepared by further dilution with ACN. All standard solutions were stored at 4 °C until used.
Optimization of Sugaring-out Assisted Liquid-Liquid Extraction
Effect of Organic Solvents
RJ sample (0.3 g) was mixed with 2 mL organic solvent (ACN, ethanol, methanol, or acetone) aqueous solution with concentration (v/v) of 30%, 40%, 50%, 60%, and 70%. After vortexing for 1 min, 0.4 g glucose was added to the mixture. After another 30-s vortex, the mixed solution was centrifuged at 6000 rpm for 5 min to observe the phase separation.
Effect of Sugar Concentration and Initial ACN Concentration
RJ sample (0.3 g) was spiked with 10 μL standard solution containing 100 μg/mL BPA and 100 μg/mL BPB. Then 10 μL internal standard (IS) solution (100 μg/mL) was added to the sample. This spiked sample was mixed with 2 mL ACN aqueous solution with concentration (v/v) of 30%, 40%, 50%, 60%, and 70%. After vortexing for 1 min, different amount of glucose (0.2 g, 0.3 g, 0.4 g, or 0.5 g) was added to the mixture. After another 30-s vortex, the mixed solution was centrifuged at 6000 rpm for 5 min. The upper phase was collected and analyzed by HPLC. Recovery was calculated by recovery (%) = (calculated amount of bisphenol in the upper phase/amount of bisphenol added) × 100.
Clean-Up by Dispersive Solid-Phase Extraction
Dispersive solid-phase extraction was performed based on the typical QuEChERS method (Anastassiades et al. 2003). For the procedure, 0.5 mL of the SULLE extract was added to a tube containing 25 mg d-SPE sorbent (Florisil, C18, silica, or PSA). After vortex for 1 min, the sorbent was separated by centrifuged at 6000 rpm for 5 min. The solution was collected and analyzed by HPLC.
HPLC Analysis
HPLC analysis was performed on a Shimadzu system composed of LC-20AT pumps, SIL-20AC autosampler, CTO-AC column oven, and RF-20AXL fluorescence detector. Samples were separated by InertSustain (GL Sciences, Japan) C18 column (4.6 × 250 mm, 5 μm) under isocratic elution. The mobile phase consisted of water (60%) and ACN (40%). Flow rate was 1 mL/min, injection volume was 10 μL, column temperature was 35 °C, and the analysis time was 40 min. Excitation and emission wavelength of the fluorescence detector was set at 270 nm and 305 nm, respectively.
Method Validation
Five level calibration curves were constructed by standard solutions containing BPA (0.01, 0.05, 0.1, 0.2, and 0.4 μg/mL), BPB (0.02, 0.05, 0.1, 0.2, and 0.4 μg/mL), and IS (0.1 μg/mL). The ratio of peak area (analyte/IS) vs the ratio of weight (analyte/IS) was used to construct the calibration curves. The y-intercept was set to 0 and the linear fit was performed. Limits of detection (LOD) and limits of quantification (LOQ) were estimated in spiked RJ samples as three and ten times the signal-to-noise (S/N) ratio, respectively. Accuracy and precision studies were carried out by analyzing blank samples spiked at three concentration levels (1 × LOQ, 5 × LOQ, and 10 × LOQ). Accuracy was expressed in terms of recovery, and precision was measured as relative standard deviation to the mean recovery of both intra-day (n = 6) and inter-day (n = 18, three consecutive days) analysis.
Results and Discussion
Sample Preparation Procedure
ACN was selected as the extraction solvent in the present SULLE procedure. The extraction solvent in SULLE required to be miscible with water before the addition of sugar. The preferred solvent for the extraction of BPA is ACN, and others such as ethanol, methanol, and acetone also have been reported (Ballesteros-Gomez et al. 2009). Those solvents were tested for the sugaring-out extraction in RJ samples. In the tested solvent-water mixture which ranged from 30 to 70% (v/v), the phase separation was only occurred in ACN. The key issue in the sample preparation of RJ sample is the separation of target molecules from proteins, which are the main constituents of RJ. ACN as the extraction solvent would be helpful for removing the proteins because of its deproteinization property (Wang et al. 2011). In addition, ACN is compatible with the following reversed-phase HPLC system for the isolation of BPA. This would simplify the sample preparation procedure.
Amount of sugar and the initial concentration of ACN in ACN-H2O mixture are two crucial parameters of SULLE. It has been speculated that ACN is surrounded by water molecules through hydrogen bonding and dipole-dipole interaction, and these interactions between ACN and water may be replaced by sugars (Wang et al. 2008b). As a result, when more sugar molecules are introduced into the ACN-H2O solution, more ACN molecules are forced out from the water and the volume of upper ACN phase increased. Consequently, the extraction yields of target compounds will be significantly improved (Wang et al. 2008b; Tu et al. 2018). In addition, increasing the initial concentration of ACN in ACN-H2O mixture would also increase the volume of ACN phase and the extraction yields (Zhang et al. 2012; Tu et al. 2018). It means that these two parameters, amount of sugar and the initial concentration of ACN, would affect the extraction yields and the concentration of target compounds in the final extract. The latter would depend the sensitivity of the method. Therefore, in addition to the extraction yields, signal responses of BPA and BPB in the final extract were also investigated to optimize the parameters in SULLE.
Effects of initial concentration of ACN and glucose concentration on the response signal were investigated. It has been demonstrated that glucose is the better phase separation agent than other sugars in the ACN-H2O mixture (Wang et al. 2008a); thus, only glucose was investigated in the present work. When the initial concentration of ACN is 30% and 40% (v/v), phase separation cannot be observed with the addition of glucose up to 250 g/L. Whereas, the phase separation is evident under the concentration of 50 to 70%. As shown in Fig. 1, the response signal is dramatically decreased as the concentration of ACN increased from 50 to 70%. For instance, when the addition amount of sugar was 200 g/L, the response signal of BPA and BPB at 50% ACN are 2.0 and 2.1 times of the signal at 70% ACN, respectively. The higher initial concentration of ACN would lead to the increase of the volume of upper phase (Zhang et al. 2012; Tu et al. 2018). As the volume increasing, concentration of target compounds and consequently the signal response significantly reduced. It means that the detection sensitivity under the initial concentration of 50% would be significantly improved compared with the condition of 70% ACN.
Effect of glucose concentration on the signal response is also shown in Fig. 1. When the initial concentration of ACN was 50% (v/v), the signal response was relatively higher at the glucose concentration of 100 g/L. This may be attributed to the dramatically lower volume of the upper phase at this condition, which would lead to the high concentration of target compounds in the upper phase although the extraction yields are also in the low level (Tu et al. 2018). As the concentration of sugar increased from 150 to 250 g/L, the optimum response signal was achieved at 200 g/L. Under the initial ACN concentration of 60% and 70%, the response signals were increased as more glucose was added, and reached the plateau at 200 g/L. The increase of signal response could be attributed to the increase of extraction yields which was relatively higher than the increase of the volume of upper phase. But the increase of the upper phase volume would be more significantly than the increase of extraction yields when the concentration of glucose is above the critical concentration (Wang et al. 2008b). This would result in the slightly decrease of signal response when the concentration of glucose was larger than 200 g/L.
In addition, recoveries of BPA and BPB were investigated under different concentrations of glucose and ACN. The compound of 4, 4′-cyclohexylidenebisphenol which shows similar molecular structure with BPA and BPB was used as the internal standard based on the reported literature (Sun et al. 2001) to quantify the recovery of BPA and BPB in the upper phase. As shown in Fig. 2, when the concentration of ACN are 50% and 60% (v/v), recoveries of BPA and BPB are slightly increased as the amount of glucose increasing. It is valuable to notice that the value of recovery shows no significant differences (p > 0.05) between 200 and 250 g/L glucose at these two ACN concentrations. Moreover, the values of recoveries were all higher than 90% when the glucose concentration was ≥ 200 g/L. Although recoveries of BPA and BPB under the ACN concentration of 70% were slightly increased compared with ACN concentrations of 50% and 60%, the signal response was only half of that in 50% ACN (Fig. 1). Considering both the higher signal response and calculated recovery, 50% ACN (v/v) with the addition of 200 g/L glucose was selected as the sample preparation protocols to gain the better signal response and recovery.
Furthermore, the sample size of RJ has also been investigated. Test of sample size ranged from 0.1 to 0.5 g were extracted by ACN-water mixture (50%, v/v) with the addition of 0.4 g glucose. Results indicated that 0.3 g was the largest sample size that could be extracted without failure in phase separation.
HPLC Analysis
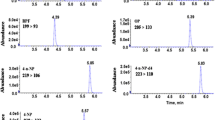
Reversed-phase HPLC was employed for the separation of BPA and BPB from RJ matrix. Isocratic elution with different ratios of ACN to water (v/v) ranged from 30 to 50% with increment of 5% were estimated. Good separation was achieved when the concentration of ACN was ≤ 40%. However, the analysis time was dramatically increased as the decrease of ACN concentration. Therefore, the mixture solution composed of 40% ACN was employed as the mobile phase. Under this elution condition, target compounds were well separated from matrix interferences in 40 min (Fig. 3). It is valuable to notice that clean-up effect of common sorbents including Florisil, C18, PSA, and silica was also investigated. As shown in Fig. S1 (supplementary material), the clean-up effect of these sorbents was not significant in the way of d-SPE (Anastassiades et al. 2003). Thus, the d-SPE clean-up procedure was unnecessary in the present work. This would make the proposed sample preparation procedure simple and fast.
Analytical Performance
The calibration curves for BPA and BPB were constructed with five levels of concentration from 0.01 to 0.4 μg/mL and 0.02 to 0.4 μg/mL, respectively. Good linearity was achieved with correlation coefficients higher than 0.997. The limits of detection (LOD, S/N = 3) and limits of quantification (LOQ, S/N = 10) for BPA in RJ sample were estimated to be 16 μg/kg and 40 μg/kg, respectively. For BPB, the LOD and LOQ were 18 μg/kg and 45 μg/kg, respectively. This sensitivity fulfills the migration limit for BPA from food contact plastic materials of 0.6 mg/kg set by the EU Commission (EC 2011).
To evaluate accuracy and precision, blank RJ sample was spiked with BPA and BPB standard at three different levels (1 × LOQ, 5 × LOQ, and 10 × LOQ). The obtained recoveries for BPA and BPB were between 88.32 and 93.59% and between 95.14 and 97.48%, respectively (Table 1). The interday and intraday precision for BPA and BPB was all less than 5% (Table 1). These results were all fulfilled the requirements of Association of Official Analytical Chemist (AOAC) (Taverniers et al. 2004).
Application of the Method
The validated method was applied to determine BPA and BPB residues in 18 royal jelly samples, including ten commercial samples collected from market and eight raw samples collected from apiaries. Results indicated that none of BPA and BPB was detected in the investigated samples.
Conclusions
In this paper, an analytical method for the determination of BPA and BPB residues in royal jelly sample has been developed. The sugaring-out sample preparation procedures were optimized and demonstrated to be high efficiency. LODs of 16 μg/kg and 18 μg/kg were achieved for BPA and BPB, respectively. The method produced good recovery and precision at different concentration levels. This is the first report of analytical method for the determination of BPA and BPB residues in royal jelly sample. The proposed method is simple, sensitive, low cost, and easy to perform, which could be used for regular monitoring of BPA and BPB contamination in royal jelly.
References
Anastassiades M, Lehotay SJ, Stajnbaher D, Schenck FJ (2003) Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J AOAC Int 86:412–430
Ballesteros-Gomez A, Rubio S, Perez-Bendito D (2009) Analytical methods for the determination of bisphenol A in food. J Chromatogr A 1216:449–469
Bogdanov S (2006) Contaminants of bee products. Apidologie 37:1–18
Buttstedt A, Ihling CH, Pietzsch M, Moritz RFA (2016) Royalactin is not a royal making of a queen. Nature 537:E10–E12
Capriotti AL, Cavaliere C, Colapicchioni V, Piovesana S, Samperi R, Laganà A (2013) Analytical strategies based on chromatography–mass spectrometry for the determination of estrogen-mimicking compounds in food. J Chromatogr A 1313:62–77
Cardoso GD, Mourao T, Pereira FM, Freire MG, Fricks AT, Soares CMF, Lima AS (2013) Aqueous two-phase systems based on acetonitrile and carbohydrates and their application to the extraction of vanillin. Sep Purif Technol 104:106–113
Chen CS, Chen SY (1995) Changes in protein-components and storage stability of royal jelly under various conditions. Food Chem 54:195–200
Chen SL, Su SK, Lin XZ (2002) An introduction to high-yielding royal jelly production methods in China. Bee World 83:69–77
Chen D, Liu F, Wan JB, Lai CQ, Shen LR (2017) Effect of major royal jelly proteins on spatial memory in aged rats: metabolomics analysis in urine. J Agric Food Chem 65:3151–3159
Cornara L, Biagi M, Xiao JB, Burlando B (2017) Therapeutic properties of bioactive compounds from different honeybee products. Front Pharmacol 8:412
Cruz-Vera M, Lucena R, Cárdenas S, Valcárcel M (2011) Sample treatments based on dispersive (micro)extraction. Anal Methods 3:1719–1728
Dhamole PB, Mahajan P, Feng H (2010) Sugaring out: a new method for removal of acetonitrile from preparative RP-HPLC eluent for protein purification. Process Biochem 45:1672–1676
European Commission (2011) Regulation (EU) No. 10/2011 of 14 January 2011 on plastic materials and articles intended to come into contact with food. Off J Eur Union L12:1–89
Geens T, Aerts D, Berthot C, Bourguignon J-P, Goeyens L, Lecomte P, Maghuin-Rogister G, Pironnet A-M, Pussemier L, Scippo M-L, Loco JV, Covaci A (2012) A review of dietary and non-dietary exposure to bisphenol-A. Food Chem Toxicol 50:3725–3740
Giannetti L, Longo F, Buiarelli F, Russo MV, Neri B (2010) Tetracycline residues in royal jelly and honey by liquid chromatography tandem mass spectrometry: validation study according to commission decision 2002/657/EC. Anal Bioanal Chem 398:1017–1023
Ito S, Nitta Y, Fukumitsu H, Soumiya H, Ikeno K, Nakamura T, Furukawa S (2012) Antidepressant-like activity of 10-hydroxy-trans-2-decenoic acid, a unique unsaturated fatty acid of royal jelly, in stress-inducible depression-like mouse model. Evid-Based Compl Alt 2012:139140
Jin Y, Zhang JZ, Zhao W, Zhang WW, Wang L, Zhou JH, Li Y (2017) Development and validation of a multiclass method for the quantification of veterinary drug residues in honey and royal jelly by liquid chromatography-tandem mass spectrometry. Food Chem 221:1298–1307
Kamakura M (2011) Royalactin induces queen differentiation in honeybees. Nature 473:478–483
Kamakura M, Suenobu N, Fukushima M (2001) Fifty-seven-kDa protein in royal jelly enhances proliferation of primary cultured rat hepatocytes and increases albumin production in the absence of serum. Biochem Biophys Res Commun 282:865–874
Kawano S, Hayakawa Y, Hashi Y, Lin JM (2015) Quantitative analysis of chloramphenicol in royal jelly by column-switching LC-MS/MS using a pretreatment column with a higher-pressure capability. Anal Sci 31:445–450
Li JK, Feng M, Zhang L, Zhang ZH, Pan YH (2008) Proteomics analysis of major royal jelly protein changes under different storage conditions. J Proteome Res 7:3339–3353
Lombardo-Agui M, Garcia-Campana AM, Gamiz-Gracia L, Cruces-Blanco C (2012) Determination of quinolones of veterinary use in bee products by ultra-high performance liquid chromatography-tandem mass spectrometry using a QuEChERS extraction procedure. Talanta 93:193–199
Martinez-Dominguez G, Romero-Gonzalez R, Garrido-Frenich A (2014) Multi-class pesticide determination in royal jelly by gas chromatography coupled to triple quadrupole tandem mass spectrometry. Anal Methods 6:5376–5386
Okamoto I, Taniguchi Y, Kunikata T, Kohno K, Iwaki K, Ikeda M, Kurimoto M (2003) Major royal jelly protein 3 modulates immune responses in vitro and in vivo. Life Sci 73:2029–2045
Ragavan KV, Rastogi NK, Thakur MS (2013) Sensors and biosensors for analysis of bisphenol-A. Trends Anal Chem 52:248–260
Ramadan MF, Al-Ghamdi A (2012) Bioactive compounds and health-promoting properties of royal jelly: a review. J Funct Foods 4:39–52
Sun Y, Wada M, Kuroda N, Hirayama K, Nakazawa H, Nakashima K (2001) Simultaneous determination of phenolic xenoestrogens by solid-phase extraction and high-performance liquid chromatography with fluorescence detection. Anal Sci 17:697–702
Takikawa M, Kumagai A, Hirata H, Soga M, Yamashita Y, Ueda M, Ashida H, Tsuda T (2013) 10-Hydroxy-2-decenoic acid, a unique medium-chain fatty acid, activates 5′-AMP-activated protein kinase in L6 myotubes and mice. Mol Nutr Food Res 57:1794–1802
Taverniers I, Loose MD, Bockstaele EV (2004) Trends in quality in the analytical laboratory. II. Analytical method validation and quality assurance. Trends Anal Chem 23:535–552
Terada Y, Narukawa M, Watanabe T (2011) Specific hydroxy fatty acids in royal jelly activate TRPA1. J Agric Food Chem 59:2627–2635
Tsai WH, Chuang HY, Chen HH, Wu YW, Cheng SH, Huang TC (2010) Application of sugaring-out extraction for the determination of sulfonamides in honey by high-performance liquid chromatography with fluorescence detection. J Chromatogr A 1217:7812–7815
Tu XJ, Sun FY, Wu SY, Gao ZS, Huang SK, Chen WB (2018) Comparison of salting-out and sugaring-out liquid-liquid extraction methods for the partition of 10-hydroxy-2-decenoic acid in royal jelly and their co-extracted protein content. J Chromatogr B 1073:90–95
Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV (2007) Human exposure to bisphenol A (BPA). Reprod Toxicol 24:139–177
Wang B, Ezejias T, Feng H, Blaschek H (2008a) Sugaring-out: a novel phase separation and extraction system. Chem Eng Sci 63:2595–2600
Wang B, Feng H, Ezeji T, Blaschek H (2008b) Sugaring-out separation of acetonitrile from its aqueous solution. Chem Eng Technol 31:1869–1874
Wang M, Cai ZW, Xu L (2011) Coupling of acetonitrile deproteinization and salting-out extraction with acetonitrile stacking in chiral capillary electrophoresis for the determination of warfarin enantiomers. J Chromatogr A 1218:4045–4051
Winston ML (1991) The biology of the honey bee, 1st edn. Harvard University Press, London
Zhang C, Huang K, Yu PH, Liu HZ (2012) Sugaring-out three-liquid-phase extraction and one-step separation of Pt (IV), Pd (II) and Rh (III). Sep Purif Technol 87:127–134
Zhang J, Myasein F, Wu HA, El-Shourbagy TA (2013) Sugaring-out assisted liquid/liquid extraction with acetonitrile for bioanalysis using liquid chromatography-mass spectrometry. Microchem J 108:198–202
Zhang YQ, Liu XM, Li X, Zhang JJ, Cao YZ, Su M, Shi YH, Sun HW (2016) Rapid screening and quantification of multi-class multi-residue veterinary drugs in royal jelly by ultra performance liquid chromatography coupled to quadrupole time-of-flight mass spectrometry. Food Control 60:667–676
Zheng HQ, Wei WT, Hu FL (2011a) Beekeeping industry in China. Bee World 88:41–44
Zheng HQ, Hu FL, Dietemann V (2011b) Changes in composition of royal jelly harvested at different times: consequences for quality standards. Apidologie 42:39–47
Zheng W, Park JA, Abd El-Aty AM, Kim SK, Cho SH, Jm C, Yi H, Cho SM, Ramadan A, Jeong JH, Shim JH, Shin HC (2018) Development and validation of modified QuEChERS method coupled with LC-MS/MS for simultaneous determination of cymiazole, fipronil, coumaphos, fluvalinate, amitraz, and its metabolite in various types of honey and royal jelly. J Chromatogr B 1072:60–69
Funding
This work was supported by the Natural Science Foundation of China [No. 31201861, No. 51202030].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Xijuan Tu declares no conflict of interest. Siyuan Wu declares no conflict of interest. Weiyi Liu declares no conflict of interest. Zhaosheng Gao declares no conflict of interest. Shaokang Huang declares no conflict of interest. Wenbin Chen declares no conflict of interest.
Ethical Statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Not applicable.
Electronic supplementary material
Fig S1
(PDF 228 kb)
Rights and permissions
About this article
Cite this article
Tu, X., Wu, S., Liu, W. et al. Sugaring-Out Assisted Liquid-Liquid Extraction Combined with High-Performance Liquid Chromatography-Fluorescence Detection for the Determination of Bisphenol A and Bisphenol B in Royal Jelly. Food Anal. Methods 12, 705–711 (2019). https://doi.org/10.1007/s12161-018-1398-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-018-1398-4